Abstract
Methyl deficiencies have been implicated in metabolic programming during the periods of oocyte and embryo development. Semisynthetic methyl-deficient diets (MD) with no folic acid, 0.05% choline, and approximately one-half the recommended content of methionine were fed to female rats for 3 wk prior to mating and for the first 5 d of gestation. During the period of MD feeding, plasma homocysteine concentrations were approximately twice those of rats fed the complete (CON) diet. From d 5, both groups received a complete semipurified AIN diet until birth. On d 8, plasma homocysteine concentrations did not differ between the 2 groups. Thereafter, dams and offspring were fed a nonpurified diet for the remainder of the experiment. At 6 mo of age, the homeostatic model assessment (HOMA) index of the male MD offspring tended to be 32% higher (P = 0.053) and peak insulin during an oral glucose tolerance test (oGTT) was 39% higher (P < 0.05) compared with the male CON offspring. There was no difference in the response to an oGTT in the female offspring at 6 mo of age. The increased HOMA index of male MD offspring persisted to 12 mo of age. The peak glucose concentration during oGTT was 23% higher (P < 0.05) in MD compared with the CON males despite 39% greater (P < 0.05) peak insulin concentrations. This study shows that in rats, a physiologically relevant methyl-deficient diet fed during the period of oocyte maturation and preimplantation development programs gender-specific changes in glucose handling by the offspring.
Introduction
It is becoming apparent that the programming of glucose metabolism and the subsequent susceptibility to age-related diseases in adult humans is influenced by the mother’s nutritional status during gestation (1). Indeed, there is both epidemiological and experimental evidence to suggest that there is an important window of sensitivity during the periods of oocyte maturation and early preimplantation development (2, 3). In human populations, prenatal exposure to famine, especially during mid or early gestation, was related to impaired glucose tolerance at age 58 y due to an altered disposition index (4). Animal studies also suggest that programming can take place during the very early stages of development, e.g. embryos derived from sheep fed a diet limiting in methyl donors [methyl deficient (MD)8] transferred to surrogate ewes produced adult offspring that were both heavier and fatter and showed early signs of type 2 diabetes compared with those from donors fed a complete diet (5). The laboratory rat has been widely used as an experimental model to study the early origins of disease and the present experiments were undertaken to examine glucose metabolism in the offspring of rats exposed to nutritional deficiencies during the periods of oocyte and embryo development.
Epigenetic modifications are a putative mechanism by which imbalances or deficiencies in the maternal diet reprogram gene expression through changes in methyl metabolism during the preimplantation period (6, 7). For example, the addition of excess folic acid, methionine, and choline to the diet of pregnant Agouti mice suppresses expression of the Agouti gene in offspring through increased methylation of the long-terminal repeat sequence that controls its expression (8, 9). However, these diets containing excess methyl groups are unusual and are unlikely to be encountered outside an experimental setting. Human diets are more likely to be deficient in methionine and folate, leading to a low availability of methyl groups. Therefore, methyl-deficient diets were chosen for this study. The impact of feeding restricted diets for 3 wk prior to conception (during oocyte maturation) and during the first 5 d of gestation (preimplantation development) was assessed by measuring postnatal growth, body composition, glucose tolerance, and insulin responsiveness in offspring at 6 and 12 mo of age.
Methods
Experimental diets.
The composition of the complete (CON) and methyl-deficient (MD) diets used in this study (Supplemental Table 1) was based on those previously described (10). A pilot study suggested that choline-free diets adversely affected pregnancy rate (data not shown), so the MD diet contained 0.05% choline. On d 5 of gestation, rats were offered a complete diet (NRM) based on the AIN-93G formula except that maize oil was used in the preparation (11).
Rats.
All experiments were performed using rats of the Rowett Hooded strain bred in the Institute’s closed colony maintained under specific pathogen-free conditions and a 12–h-light cycle. All experimental procedures were approved by the ethical review committee of the Rowett Research Institute and conducted in accordance with the U.K. Animals (Scientific Procedures) Act, 1986. Power calculations based on our previous study (12) suggested that a minimum of 16 litters/treatment would be required. A total of 96 females was assigned to the experimental diets. To allow for the stringent mating regime, this experiment was carried out in 2 phases at 3 mo apart. Each phase of the study was conducted over a 3-wk period with 16 rats assigned to diet each week. The rats consumed the CON or MD diets ad libitum for a 3-wk adaptation period before being mated with males of the same strain. Mating was confirmed by detection of a vaginal plug and this was denoted d 0. The dams were maintained on the same diets for the first 5 d of pregnancy after which they were switched to the NRM diet. Food consumption of the dams was measured daily following the confirmation of pregnancy and for the first 2 wk of pregnancy. To reduce stress, weight and food intake data were not collected from the dams prior to birth.
Pups were delivered naturally and on postnatal d 1 the litters were culled to 8 pups/dam (4 males and 4 females where possible) and litters of <8 pups were excluded. Once they had given birth, the dams consumed ad libitum a commercial nonpurified stock diet (CRM (P) breeder and grower diet 801722, Special Diet Services, Witham Essex UK; Supplemental Table 1) until weaning was complete. The offspring were weaned onto the same stock diet, which they consumed ad libitum for the remainder of the experiment. The offspring were weighed twice weekly to monitor growth. Representative rats from each litter were randomly allocated to 2 cohorts of offspring studied at 6 and 12 mo of age. Only males were assessed at 12 mo of age, because female offspring did not differ at 6 mo.
TABLE 1.
Plasma homocysteine concentrations in rat dams fed CON or MD diets during the peri-conception period1
| Time point | CON | n | MD | n |
| μmol/L | μmol/L | |||
| Day of mating | 8.2 ± 1.2 | 19 | 19 ± 1.3* | 18 |
| d 8 gestation | 6.0 ± 0.2 | 19 | 6.1 ± 0.2 | 18 |
| Day of weaning | 1.8 ± 0.1 | 14 | 1.5 ± 0.1 | 9 |
Values are means ± SEM. *Different from CON, < 0.05.
Plasma homocysteine.
Total homocysteine concentrations were determined in plasma by isotope dilution GC-MS. Briefly, 0.04 g of plasma was added to 0.05 g of a 10.0 nmol/g [1-13C]-homocystine solution (a gift from Dr. N. O’Kennedy, Rowett Institute of Nutrition and Health). Samples were treated with dithiothreitol and deproteinized with sulphosalycylic acid. After centrifugation, the supernatant was purified by ion exchange chromatography and derivatized with N-heptafluorobutyryl imidazole (Sigma-Aldrich) (13). Separation was effected by capillary column GC and negative chemical ionization selective ion monitoring MS was used to detect and quantify the N-heptafluorobutyl derivative of homocysteine.
Body composition.
Whole body composition (as total fat and lean) of conscious rats was quantified by MRI (EchoMRI) as previously described (14). The 6-mo cohort (male and female offspring) was measured at 23 wk of age and the 12-mo cohort (male offspring) was measured at 30, 39, and 49 wk of age.
Oral glucose tolerance tests.
Oral glucose tolerance tests (oGTT) were performed on the offspring as previously described (15). Briefly, rats were deprived of food for 12 h overnight and a baseline blood sample used to measure insulin and glucose was taken following venesection of the tail vein. A single dose of glucose (200 mg/100 g body weight) dissolved in 1 mL of water/100 g of body weight was administered by gavage. Over the following 120 min, 6 blood samples of ∼150 μL were taken. All blood samples were collected in potassium EDTA-treated tubes. Red cells were removed by centrifugation and plasma glucose was estimated using glucose oxidase, peroxidase, and 4-amino-antipyrine (Sigma-Aldrich) (16). Plasma insulin was measured by enzyme-linked immunoassay (Mercodia) following the manufacturer’s instructions. The homeostatic model assessment (HOMA) index was calculated from blood samples taken after overnight feed deprivation using the formula HOMA = (G0 × I0)/22.5, where I0 is insulin expressed as pmol/L and G0 is glucose expressed as mmol/L (17). Area the under curve (AUC) values for both plasma insulin and glucose were calculated using the PK2 add-in for Microsoft Excel (Joel Usansky and Atul Desai, Department of Pharmacokinetics and Drug Metabolism, Allergan, Irvine, CA). At the end of the oGTT, the offspring were killed and their tissues were carefully dissected and weighed.
Statistics.
Growth data were analyzed by Residual Maximum Likelihood, in Genstat 13th Edition (VSN International). Rat and time within rat were assumed as random and diet, study phase, time, and their interactions were regarded as fixed effects. An appropriate model to account for dependency on previous time points was investigated by comparing deviances, and this resulted in a first-order power model (i.e. dependency on previous time points depends on the number of days between time points) for the effect of time within animal. When the effect of diet was significant, diet means for 1 d at a time were compared by post hoc t test. Maternal plasma homocysteine concentrations were compared using the 2-way ANOVA function of Genstat where maternal diet and study phase were factors. Data from the offspring, including organ weights, body composition, and responses to oral glucose infusion, were compared by variance component analysis using mixed linear restricted maximum likelihood (REML) algorithms where, in a series of analyses, study phase and the interactions between maternal diet and offspring sex or age formed the fixed effects and dam formed the random effect. Data are presented as predicted means with a SEM.
Results
Maternal characteristics.
By the end of the initial 3-wk dietary period prior to mating, female rats fed the MD diet were ∼5% lighter (P < 0.05) than those fed the control diet (CON) (Supplemental Fig. 1A). There was no difference in food consumption, with rats in both groups consuming ∼14. 5g of diet/d. Within 24 h of mating, food intake increased in both groups (Supplemental Fig. 2). However, although mean food intake over the first 13 d of gestation did not differ between the CON (19.5 ± 0.4 g/d; n = 19) and MD (20.1 ± 0.4 g/d; n = 18) groups, the MD dams ate substantially less on d 1 and tended to (P = 0.07) eat less on d 2 of gestation. The change in intake was reflected by reduced body weight gain of these dams (Supplemental Fig. 1B). After switching to the complete (NRM) diet on d 5, the body weight of the dams in the 2 groups started to converge, differing by ∼5% on postnatal d 1 (P < 0.02). The length of gestation was ∼22.5 d in both groups and was similar to rats of the same strain fed commercial nonpurified diet (data not shown). Dams in the MD group gave birth to litters with fewer offspring (10.7 ± 0.6) compared with dams in the CON group (12.9 ± 0.6; P < 0.05). The ratio of males:females within litters did not differ. Dams were offered the commercial nonpurified diet during lactation and the body weight of dams did not differ between the groups by the time that the pups were weaned (Supplemental Fig. 1C).
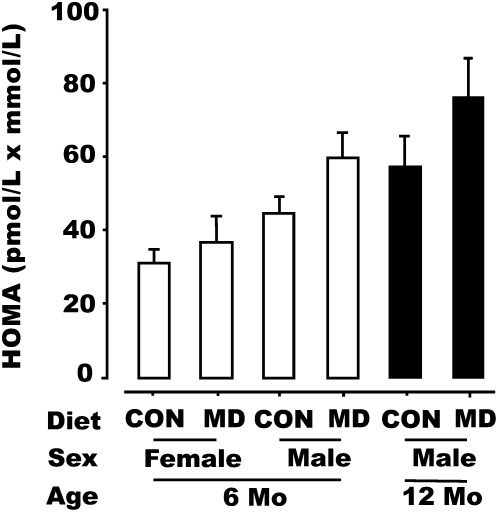
FIGURE 1.
HOMA in male and female offspring of rats fed the CON or MD diet during the peri-conception period at 26 wk and in the male offspring at 51 wk of age. One male and one female pup from each litter was assessed at 26 wk (CON male, n = 18; CON female, n = 16; MD male, n = 17; MD female, n = 16). A second male pup from each litter was assessed at 51 wk (CON, n = 12; MD, n = 12). Data were analyzed with residual maximum likelihood, where the interactions between maternal diet and offspring sex or age formed the fixed effects and dam formed the random effect. Data are presented as predicted means ± SEM. At 6 mo, male offspring > female offspring (P < 0.001) and MD > CON (P = 0.053). For male offspring, 12 mo > 6 mo (P = 0.039) and MD > CON (P = 0.020).
Plasma homocysteine concentrations.
On the day before mating, the plasma homocysteine concentrations in dams fed the MD diet were ∼1.3 times higher (P < 0.001) than those fed the CON diet (Table 1). By d 8 of gestation, 3 d after the switch to NRM diet on d 5 of gestation, this difference had disappeared and plasma homocysteine concentrations were similar in both groups.
Growth of the offspring.
The body mass of the offspring 24 h after birth did not differ between the CON (5.79 ± 0.13 g; n = 19) and MD (5.86 ± 0.13 g; n = 18) groups. At weaning, male offspring were heavier than female offspring, but there was no effect of the MD diet on offspring body mass (Table 2). As anticipated, the body mass of the offspring at the end of the study was influenced by sex (P < 0.001) and age (P < 0.001); however, there was also an effect of the maternal diet, with the MD offspring heavier than the CON offspring (P = 0.023). This pattern was also present in the incremental area under the growth curve (data not shown; P < 0.001).
TABLE 2.
Growth, body composition, and organ weight of male and female offspring of rats fed CON or MD diet during the peri-conception period at 26 wk and the male offspring at 51 wk1
| 26 wk |
51 wk |
||||||||||
| Female |
Male |
Male |
Significance2 |
||||||||
| CON | MD | CON | MD | CON | MD | A3 | S4 | D | A × D3 | S × D4 | |
| Body mass | |||||||||||
| At weaning, g | 38.1 ± 0.95 | 38.3 ± 0.91 | 39.6 ± 0.92 | 40.0 ± 0.90 | 39.5 ± 1.05 | 40.2 ± 1.01 | — | 0.003 | — | — | — |
| When killed, g | 288 ± 7.6 | 292 ± 7.3 | 536 ± 7.2 | 560 ± 7.1 | 572 ± 11.2 | 598 ± 10.8 | <0.001 | <0.001 | 0.023 | — | — |
| Body composition and organ weight | |||||||||||
| Fat, % body mass | 11.4 ± 0.7 | 11.7 ± 0.6 | 13.4 ± 0.6 | 13.5 ± 0.6 | 18.9 ± 0.9 | 18.3 ± 0.9 | <0.001 | <0.001 | — | — | — |
| Lean, % body mass | 78.9 ± 0.6 | 78.4 ± 0.4 | 77.4 ± 0.9 | 77.2 ± 0.5 | 69.3 ± 0.9 | 69.1 ± 0.8 | — | <0.001 | — | — | — |
| Liver, g | 8.1 ± 0.28 | 8.2 ± 0.27 | 15.6 ± 0.27 | 16.4 ± 0.27 | 17.2 ± 0.39 | 17.7 ± 0.38 | <0.001 | <0.001 | 0.077 | — | 0.077 |
| Heart, g | 0.86 ± 0.03 | 0.89 ± 0.03 | 1.34 ± 0.03 | 1.41 ± 0.03 | 1.34 ± 0.04 | 1.42 ± 0.03 | — | <0.001 | 0.080 | — | — |
| Kidney, g | 0.92 ± 0.03 | 0.93 ± 0.03 | 1.63 ± 0.03 | 1.70 ± 0.03 | 1.59 ± 0.05 | 1.72 ± 0.05 | — | <0.001 | 0.037 | — | — |
| Soleus, mg | 142 ± 4.9 | 149 ± 4.7 | 226 ± 4.6 | 237 ± 4.9 | 226 ± 5.4 | 238 ± 5.2 | — | <0.001 | 0.023 | — | — |
| Plantaris, mg | 255 ± 7.9 | 263 ± 7.7 | 398 ± 7.6 | 435 ± 7.5 | 404 ± 9.6 | 419 ± 9.4 | — | <0.001 | < 0.001 | — | 0.017 |
Values are predicted means ± SEM, = 16 (1 pup /litter for each sex and age). Abbreviations: A, Age; S, Sex, and D, Dietary treatment.
Where no value is shown, > 0.1.
Age comparisons and A×D interactions pertain to male offspring only.
Sex comparisons and S×D interactions pertain to 26-wk-old offspring only.
Body fat as a percentage of body mass was greater (P < 0.001) in male than female offspring and in the older than the younger males. There was no effect, however, of maternal diet (Table 2). Liver mass was also greater (P < 0.001) in male than female offspring and in the older than younger rats. Liver mass tended to be greater (P = 0.077) in MD than CON offspring. Kidney mass and soleus mass were also both greater (P < 0.05) in MD than CON offspring, as was plantaris mass (P < 0.001; Table 2). However, it should be noted that the male MD offspring were heavier than those in the corresponding CON group (P = 0.023) and there were no differences in the organ weights when the data were expressed relative to body weight (data not shown).
Plasma glucose and insulin.
Following overnight feed deprivation, basal plasma glucose concentrations were unaltered by offspring sex and maternal diet, but plasma insulin concentrations were greater (P < 0.001) in male than female offspring at 26 wk and in MD than CON offspring (P = 0.019) at either 26 or 51 wk (Table 3). The HOMA index of insulin resistance was greater (P < 0.001) in male than female offspring at 26 wk and in MD offspring than in CON offspring at both wk 26 (males and females; P = 0.053) and wk 51 (male offspring only; P = 0.020) (Fig. 1). The HOMA index was also greater (P = 0.039) for male offspring at 12 compared with at 6 mo.
TABLE 3.
Basal metabolism and oGTT in male and female offspring of rats fed CON or MD diet during the peri-conception period at 26 and 51 wk of age1
| 26 wk |
51 wk |
||||||||||
| Female |
Male |
Male |
Significance2 |
||||||||
| CON | MD | CON | MD | CON | MD | A3 | S4 | D | A × D3 | S × D4 | |
| Plasma glucose, mmol/L5 | 6.0 ± 0.2 | 6.3 ± 0.2 | 6.1 ± 0.2 | 6.5 ± 0.2 | 6.6 ± 0.2 | 6.9 ± 0.2 | 0.088 | — | — | — | — |
| Plasma insulin, nmol/L5 | 0.11 ± 0.02 | 0.13 ± 0.02 | 0.16 ± 0.02 | 0.21 ± 0.02 | 0.19 ± 0.02 | 0.25 ± 0.02 | 0.095 | <0.001 | 0.019 | — | — |
| tAUC glucose, mol/L⋅2h | 1.10 ± 0.03 | 1.13 ± 0.03 | 1.14 ± 0.03 | 1.12 ± 0.03 | 1.09 ± 0.04 | 1.17 ± 0.04 | — | — | — | 0.051 | — |
| tAUC insulin, nmol/L⋅2h | 41.1 ± 5.0 | 39.6 ± 4.8 | 66.8 ± 4.7 | 83.0 ± 4.7 | 67.7 ± 6.5 | 64.8 ± 6.2 | 0.052 | <0.001 | — | 0.070 | 0.028 |
Values are predicted means ± SEM, = 12–16 (1 pup/litter for each sex and age). Abbreviations: A, Age; S, Sex, and D, Dietary treatment.
Where no value is shown, > 0.1.
Age comparisons and A×D interactions pertain to male offspring only.
Sex comparisons and S×D interactions pertain to 26-wk-old offspring only.
Following overnight food deprivation.
Glucose tolerance tests.
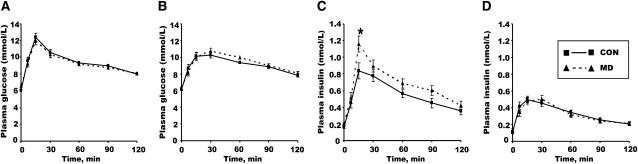
Glucose tolerance tests were carried out on the offspring at 26 wk (Fig. 1) and 51 wk (Fig. 2) of age. At 26 wk of age, neither peak glucose concentration nor AUC (Table 3) were affected by preconception diet in either male (Fig. 2A) or female (Fig. 2B) offspring. However, peak insulin concentrations of the male MD offspring (Fig. 2C) were 39% greater (P < 0.05) than that of male CON offspring. There was also an interaction (P = 0.028) between sex and maternal diet at 26 wk of age on total AUC for plasma insulin, confirming that the insulin response to oral glucose challenge was limited to male offspring.
FIGURE 2.
Plasma glucose (A,B) and insulin concentrations (C,D) during OGTT in 26-wk-old male (A,C) and female (B,D) offspring of rats fed a CON or MD diet during the peri-conception period. Values are means ± SEM, n = 16–18. *Different from CON, P < 0.05.
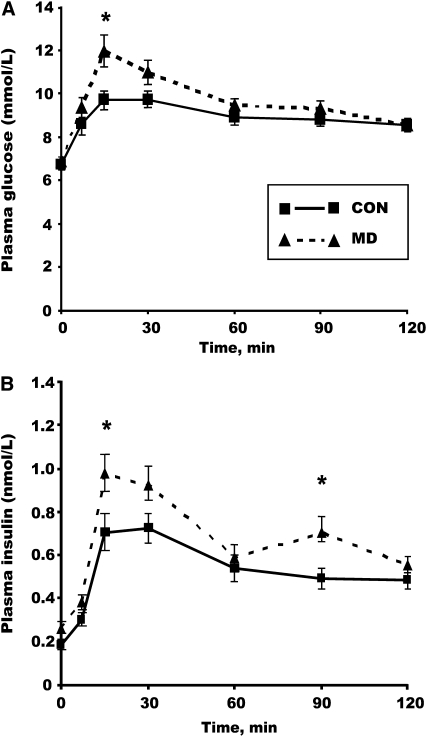
In male offspring tested at 51 wk of age, peak glucose concentrations were 23% greater (P < 0.05) and peak insulin concentrations 39% greater (P < 0.05) in MD offspring compared with CON offspring (Fig. 3A,B). The interaction (P = 0.051) between offspring age and maternal diet for glucose total AUC (Table 3) supports the concept of a breakdown in glucose tolerance following oral challenge in 51-wk-old male offspring.
FIGURE 3.
Plasma glucose (A) and insulin (B) concentrations during the OGTT in 51-wk-old male offspring of rats fed CON or MD diet during the peri-conception period. Values are means ± SEM, n = 12 (1 pup/litter). *Different from CON, P < 0.05.
Discussion
To our knowledge, this study demonstrates for the first time that feeding a diet deficient in methyl donors during the pre- and peri-conception period permanently programs glucose homeostasis in the male (but not female) offspring of the laboratory rat. At 6 mo of age, insulin release in response to a glucose load increased in male MD offspring. By 12 mo of age, male MD offspring had a peak plasma glucose concentration ∼2.5 mmol/L higher than their control counterparts even though they released more insulin in response to a glucose challenge. Furthermore, there was an increase in the HOMA index, suggesting that there was also insulin resistance. Previous studies have shown that similar MD diets reduce plasma folate by ∼50% and liver folate by up to 80% (10). It should be noted that the range of plasma homocysteine concentrations produced by feeding these experimental diets span the range found in normal human populations (4.30–22.35 μmol/L) (18). Thus, insulin resistance and glucose intolerance develop with age following pre- and peri-conception exposure to diets, which produce metabolic changes comparable to those in humans.
The phenotype with respect to glucose metabolism in the aged offspring is similar to other established models of programming, notably protein deficiency [low-protein (LP diet)]. The offspring of dams fed an LP diet, like the male offspring of the MD dams, had impaired glucose tolerance by 12–15 mo of age, despite an increase in insulin release (19, 20). There are, however, some important differences, notably in the gender specificity, where male offspring were affected by the MD diets, whereas females were more sensitive to the LP diets (15, 21). Although there were changes in growth, the MD diets did not alter the accumulation of fat. In the offspring of dams fed the LP diet, the effects on fat content were variable and appeared to depend on the degree of programming such that an increase in body fat content was observed only where there was extensive programming of the insulin axis, i.e. 2- to 3-fold difference in peak insulin concentrations following the glucose dose (15, 21). The relatively modest increase of only 39% in the peak insulin concentrations following a similar dose of glucose given to offspring of dams fed the MD diet may explain the lack of change in body fat at 6 and 12 mo of age in the present study.
The changes to glucose metabolism in the male offspring of dams fed the MD diet were similar to those in the male offspring of sheep fed a diet that restricted the supply of vitamin B-12, folate, and methionine. In both models, there was an increase in the insulin response to infused glucose and signs of insulin resistance that were independent of differences in adiposity (5). The MD diets affected muscle mass in the male offspring in both studies. However, in the sheep, MD males had proportionately less muscle mass [although absolute muscle mass (kg) was unaffected], whereas in rats it was unchanged on a proportional basis but increased on an absolute basis. It is therefore unlikely that insulin resistance is simply an effect of changes in the relative mass of muscle. The increase in the HOMA index infers that the tissues may be exhibiting insulin resistance; however, this analysis does not show whether the alteration in the glucose tolerance curve results from a change in the response of the liver or of peripheral tissues (muscle or adipose tissue).
Feeding the MD diet increased homocysteine concentrations in maternal plasma by at least 2-fold for the duration of oocyte maturation and preimplantation development. This difference disappeared within 3 d of switching the rats to a complete diet, suggesting that methyl metabolism was normal for the remainder of gestation. It is likely that homocysteine concentrations in the microenvironment surrounding both the developing oocyte and early embryo increased during this period as previously reported for embryo donor sheep fed MD diets (5). Homocysteine concentrations in the follicular fluid of humans are also modified by the folic acid content of the diet (22). In the sheep, elevated homocysteine concentrations during the periconceptional period were associated with specific changes in DNA methylation at numerous loci within the fetal liver. Supraphysiological levels of methyl donors in the diet of rodents have also been associated with altered DNA methylation in the offspring (23). However, although epigenetic changes induced during oocyte maturation and peri-implantation development remain a potentially important mechanism, it is important to note that the MD diet also produces extensive changes in the metabolism of amino acids (10, 24), an increase in liver triacylglycerols, a decrease in phosphocholine concentrations, and changes in the expression of a number of transcriptional activators in the liver, including sterol response element binding protein-1c, PPARα, and PPARγ (24). The impact of these metabolic changes on the development of the oocyte and early embryo is unknown.
The subtle change in food intake and growth of the dam during the first couple of days of gestation may also be an important part of the programming mechanism. The marked increase in food intake that normally occurs during the first 2 d of gestation was not as pronounced in the dams fed the MD diet. Furthermore, the MD group tended to increase their intake over and above that of the CON dams when they were fed the NRM diet on d 5. The reason for the change in intake is unknown but may be related to the imbalance of sulfur amino acids in the MD diet causing changes in appetite and satiety signals. There are parallels between this change in intake and studies where rats have been switched to a LP diet during preimplantation development, a model that has also been shown to produce long-term changes in both gene expression and phenotype (25, 26). Increased growth of the dams during this early period of development could have a lasting effect on the development of the placenta, leading to a change in the nutrient supply throughout the remainder of gestation.
This study shows that the nutritional status of the dam at the time of conception has the capacity to program insulin action in offspring. In humans, folic acid supplementation is often prescribed after a pregnancy is confirmed (normally after ~12 d). These findings in rats suggest that there may be a window for programming prior to this period in humans. The present findings imply that it is essential that women have appropriate folate/choline and methionine status prior to conception and perhaps improvements in this status could lead to improved glucose metabolism in offspring.
Supplementary Material
Acknowledgments
We thank Susan Anderson and Graham Calder for technical assistance and Dr. G. Holtrop (Biomathematics and Statistics, Scotland) for advice on the statistical analysis. C.A.M., W.D.R., K.S., and L.E.Y. designed research; C.A.M., S.M.H., and W.D.R. conducted research; C.A.M., K.S., and W.D.R. analyzed data; C.A.M., W.D.R., and K.S. wrote the paper; and W.D.R. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by the NIH (U01 HD044638 to K.D.S.) as a component of the National Institute of Child Health and Human Development Cooperative Program on Female Health and Egg Quality and by the Scottish Government Rural and Environmental Research and Analysis Directorate as part of the core funding of the Rowett Institute of Nutrition and Health (W.D.R.).
Supplemental Figures 1 and 2 and Table 1 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: tAUC, total area under curve; CON, control diet; HOMA, homeostatic model assessment; LP, low–protein diet; MD, methyl-deficient diet; NRM, complete diet; oGTT, oral glucose tolerance test.
Literature Cited
- 1.Hales CN, Ozanne SE. For debate: fetal and early postnatal growth restriction lead to diabetes, the metabolic syndrome and renal failure. Diabetologia. 2003;46:1013–9 [DOI] [PubMed] [Google Scholar]
- 2.Fleming TP, Kwong WY, Porter R, Ursell E, Fesenko I, Wilkins A, Miller DJ, Watkins AJ, Eckert JJ. The embryo and its future. Biol Reprod. 2004;71:1046–54 [DOI] [PubMed] [Google Scholar]
- 3.Duranthon V, Watson AJ, Lonergan P. Preimplantation embryo programming: transcription, epigenetics, and culture environment. Reproduction. 2008;135:141–50 [DOI] [PubMed] [Google Scholar]
- 4.de Rooij SR, Painter RC, Phillips DIW, Osmond C, Michels RPJ, Godsland IF, Bossuyt PMM, Bleker OP, Roseboom TJ. Impaired insulin secretion after prenatal exposure to the Dutch famine. Diabetes Care. 2006;29:1897–901 [DOI] [PubMed] [Google Scholar]
- 5.Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA. 2007;104:19351–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr. 2007;27:363–88 [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5:401–8 [DOI] [PubMed] [Google Scholar]
- 8.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–57 [PubMed] [Google Scholar]
- 9.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132:S2393–400 [DOI] [PubMed] [Google Scholar]
- 10.Maloney CA, Hay SM, Rees WD. Folate deficiency during pregnancy impacts on methyl metabolism without affecting global DNA methylation in the rat fetus. Br J Nutr. 2007;97:1090–8 [DOI] [PubMed] [Google Scholar]
- 11.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51 [DOI] [PubMed] [Google Scholar]
- 12.Maloney CA, Hay SM, Rees WD. The effects of feeding rats diets deficient in folic acid and related methyl donors on the blood pressure and glucose tolerance of the offspring. Br J Nutr. 2009;101:1333–40 [DOI] [PubMed] [Google Scholar]
- 13.Wilson FA, van den Borne JJ, Calder AG, O'Kennedy N, Holtrop G, Rees WD, Lobley GE. Tissue methionine cycle activity and homocysteine metabolism in female rats: the impact of dietary methionine and folate plus choline. Am J Physiol Endocrinol Metab. 2009;296:E702–13 [DOI] [PubMed] [Google Scholar]
- 14.Lobley GE, Bremner DM, Holtrop G, Johnstone AM, Maloney C. Impact of high-protein diets with either moderate or low carbohydrate on weight loss, body composition, blood pressure and glucose tolerance in rats. Br J Nutr. 2007;97:1099–108 [DOI] [PubMed] [Google Scholar]
- 15.Maloney CA, Lilley C, Czopek A, Hay SM, Rees WD. Interactions between protein and vegetable oils in the maternal diet determine the programming of the insulin axis in the rat. Br J Nutr. 2007;97:912–20 [DOI] [PubMed] [Google Scholar]
- 16.Maloney CA, Gosby AK, Phuyal JL, Denyer GS, Bryson JM, Caterson ID. Site specific changes in the expression of fat partitioning genes in weanling rats exposed to a low protein diet in utero. Obes Res. 2003;11:461–8 [DOI] [PubMed] [Google Scholar]
- 17.Muniyappa R, Chen H, Muzumdar RH, Einstein FH, Yan X, Yue LQ, Barzilai N, Quon MJ. Comparison between surrogate indexes of insulin sensitivity/resistance and hyperinsulinemic euglycemic clamp estimates in rats. Am J Physiol Endocrinol Metab. 2009; 297: E1023–E1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy MM, Scott JM, Arija V, Molloy AM, Fernandez-Ballart JD. Maternal homocysteine before conception and throughout pregnancy predicts fetal homocysteine and birth weight. Clin Chem. 2004;50:1406–12 [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin signalling protein expression in 21 month-old female rat offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R368–73 [DOI] [PubMed] [Google Scholar]
- 20.Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN. Diabetes in old male offspring of rat dams fed a reduced protein diet. Int J Exp Diabetes Res. 2001;2:139–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rees WD, Hay SM, Cruickshank M, Reusens B, Remacle C, Antipatis C, Grant G. Maternal protein intake in the pregnant rat programs the insulin axis and body composition in the offspring. Metabolism. 2006;55:642–9 [DOI] [PubMed] [Google Scholar]
- 22.Boxmeer JC, Brouns RM, Lindemans J, Steegers EAP, Martini E, Macklon NS, Steegers-Theunissen RPM. Preconception folic acid treatment affects the microenvironment of the maturing oocyte in humans. Fertil Steril. 2008;89:1766–70 [DOI] [PubMed] [Google Scholar]
- 23.Dolinoy DC. Epigenetic gene regulation: early environmental exposures. Pharmacogenomics. 2007;8:5–10 [DOI] [PubMed] [Google Scholar]
- 24.McNeil CJ, Hay SM, Rucklidge G, Reid M, Duncan G, Maloney CA, Rees WD. Disruption of lipid metabolism in the liver of the pregnant rat fed folate deficient and methyl donor deficient diets. Br J Nutr. 2008;99:262–71 [DOI] [PubMed] [Google Scholar]
- 25.Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–202 [DOI] [PubMed] [Google Scholar]
- 26.Kwong WY, Miller DJ, Wilkins AP, Dear MS, Wright JN, Osmond C, Zhang J, Fleming TP. Maternal low protein diet restricted to the preimplantation period induces a gender-specific change on hepatic gene expression in rat fetuses. Mol Reprod Dev. 2007;74:48–56 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.