Abstract
Observational studies of breakfast frequency in children and adults suggest an inverse (protective) association between the frequency of eating breakfast and the risk for obesity and chronic diseases such as type 2 diabetes. More prospective studies with stronger designs are needed, as are experimental studies on this topic. In addition, above and beyond breakfast frequency, the roles of dietary quality and composition need to be studied in the context of eating or skipping breakfast. Experimental studies are also necessary to rigorously test causality and biological mechanisms. Therefore, we conducted 2 pilot experimental studies to examine some of the effects of breakfast skipping and breakfast composition on blood glucose and appetite in children and adults. Our results suggest that breakfast frequency and quality may be related in causal ways to appetite controls and blood sugar control, supporting the hypothesis that the breakfast meal and its quality may have important causal implications for the risk of obesity and type 2 diabetes.
Introduction
Scientists continue to struggle to understand the etiology of obesity and attempts to prevent obesity at the individual or population level have been largely futile. One area of research that may have broad public health applications in obesity prevention is the role of breakfast habits in terms of the frequency and quality of the meal. Breakfast skipping has a high prevalence, with 12–34% of youth regularly skipping breakfast (1–6). A working definition of breakfast for research has been proposed as “the first meal of the day, eaten before or at the start of daily activities (e.g. errands, travel, work), within 2 hours of waking, typically no later than 10:00 in the morning, and of an energy level between 20 and 35% of total daily energy needs.” (7) Throughout this article, we refer to breakfast frequency, which is defined as the number of days per week consuming a breakfast meal, with a maximum value set at 7 d so that multiple breakfasts on a single day would only be counted once for that day.
The purpose of this paper is to describe 2 new experimental pilot studies of the possible effects of breakfast frequency and quality on appetite and blood sugar in adults and children. Two recent review articles have described the physiological mechanisms that may explain why meal skipping, and breakfast skipping in particular, may lead to upregulation of appetite, possibly leading to weight gain over time, and deleterious changes in risk factors for diabetes and cardiovascular disease, as well as the observational and clinical research on this topic in humans (7, 8). We observed that breakfast skipping has also been linked to poorer overall dietary quality. Conversely, those who eat breakfast on a daily basis may benefit further in terms of obesity and disease prevention through, e.g., nutrient- and fiber-rich meals such as whole grain cereals (7–13).
Observational studies
Most cross-sectional studies that have examined the association between breakfast habits and measures of obesity (i.e. BMI) in adults report an inverse association, even with adjustment for potential confounding factors (14–17). Studies also tend to find that breakfast eaters report reduced intake of dietary fat and cholesterol (15, 18, 19) and increased fiber (15) relative to those who skip breakfast. Thus, the role of the quality of the breakfast meal, aside from its frequency, may have implications for daily energy, body weight changes, and chronic disease risk through a variety of mechanisms.
Only a few prospective studies have examined the associations between breakfast habits and body weight (20–22). In 1 study, increased meal frequency (meals per week) was associated with a 45% reduced risk for obesity [odds ratio = 0.55 (95% CI = 0.33, 0.91)] in adults (22), whereas skipping breakfast appeared to be associated with a significant increase in risk of developing obesity (22). Among male health professionals, inverse associations were observed between breakfast cereal intake and BMI and between breakfast cereal intake and weight gain (21). In addition, those who consumed ≥1 serving/d (equal to a slice of bread, ~30 g) of either whole (P-trend < 0.0001) or refined (P-trend < 0.005) grain cereals had lower body weights than infrequent cereal consumers.
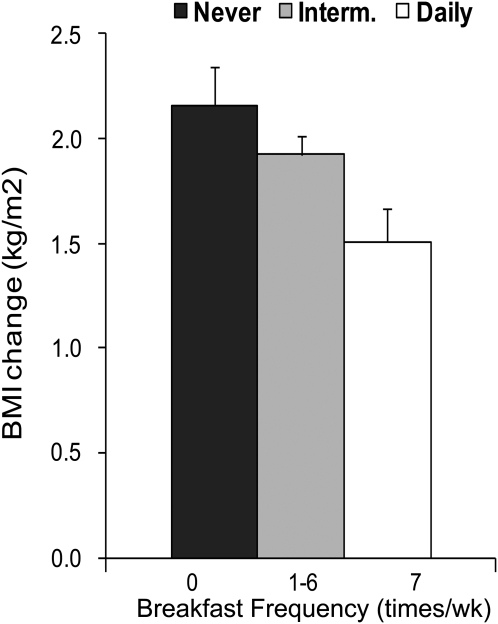
Recently, Timlin et al. (20) reported a prospective analysis of breakfast frequency and 5-y body weight change in the Project Eating Among Teens cohort of 2216 adolescents. Surveys were completed at baseline and 5 y later, with BMI, breakfast habits, and lifestyle and demographics measured over time. Frequency of breakfast (days per week in 3 categories: – 0, 1–6, 7 d) was inversely associated with weight gain and appeared to be a dose-response association (P < 0.01) (Fig. 1). Interestingly, adjustment for weight-related variables such as dieting and weight concerns partially attenuated this association. Future studies should further examine the role of breakfast habits among youth who are particularly concerned about their weight.
FIGURE 1.
Association between change in BMI and change in breakfast frequency in 2216 adolescent boys and girls from the Project Eating Among Teens cohort study. Data are means ± SEM adjusted for baseline BMI, baseline breakfast frequency, age, and gender. Adapted with permission from (20).
Potential mechanisms.
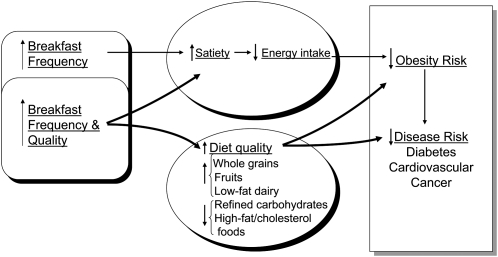
Breakfast frequency (days per week) and quality may contribute to appetite regulation, quality of diet, and prevention of obesity and chronic disease through a variety of mechanisms (Fig. 2) (7). Many studies have reported that ready-to-eat breakfast cereal (14, 23–25) and other fiber-rich foods (13, 14, 23, 26, 27) are associated with lower risk of obesity (11, 14, 28, 29). Intake of fiber-rich breakfast foods may improve blood sugar control and possibly prevent low blood sugar between meals (28, 30, 31). In terms of appetite, some studies report enhanced feelings of satiation (32, 33) and satiety (34, 35) following ingestion of these types of foods but not after breakfasts low in fiber and/or high in fat (36). Perhaps these effects are explained by release or activity of gut hormones, including cholecystokinin (37–39) or other incretin hormones (40–46). Additionally, resistant starches are susceptible to colonic fermentation that may lead to the production of SCFA which, upon entering the circulation, may attenuate hepatic glucose output and serum FFA (47, 48) and stimulate glucagon-like peptide-1 secretion (49, 50). These effects could modulate insulin sensitivity and secretion patterns in important ways that relate to satiety and reduced risk of type 2 diabetes and possibly of cardiovascular disease and cancer. Preliminary breakfast feeding studies suggest that eating, rather than skipping, breakfast may reduce fasting total and LDL cholesterol (51, 52), oxidized LDL (53), and serum triglycerides (54). Furthermore, slow absorption and digestion of starch at one meal (i.e. breakfast) may improve carbohydrate tolerance at the following meal (12, 28, 30, 31, 55, 56).
FIGURE 2.
Theoretical model of breakfast frequency and quality in the development of obesity and chronic diseases. Reproduced with permission from (7).
In summary, the literature suggests that regular consumption of breakfast, and especially whole-foods, fiber-rich breakfasts, may be protective against obesity and chronic diseases. Results from a few, small, short-term randomized trials of breakfast behavior provide somewhat inconsistent results, with some positive and some negative findings, but overall they do provide some support for the hypothesis that regular consumption of a breakfast meal may reduce the risk of obesity and chronic disease (52, 57–59). More definitive randomized controlled trials will provide answers to these important public health questions regarding pathways of dietary behaviors, dietary composition, and risk of obesity and related chronic diseases.
Pilot experimental studies in adults and children
We recently conducted 2 pilot studies, in adults and children, to compare the effects of eating compared with skipping breakfast (breakfast frequency) and of breakfast type [particularly differences in fiber, glycemic index (GI),8 and macronutrients] on perceived hunger, perceived energy levels, and glycemia and insulinemia. Study 1 examined the acute effects of breakfast frequency and composition (type of foods and beverages) on glycemia and perceived appetite and energy in adults. Study 2 compared the effects of breakfast frequency and composition on perceived appetite, energy levels, and mood in children. These studies were approved by the Human Subjects Committee of the University of Minnesota Internal Review Board, and all participants signed informed consent documents to participate in this research.
Study 1.
In Study 1, in adult participants, we investigated the effects of breakfast meals varying in GI and carbohydrate amount on postprandial serum glucose and insulin concentrations as assessed by 120-min incremental area under the curve (AUC). Glycemic load was altered by manipulating the amount of carbohydrate and type (GI) in the meal. A randomized, within-person crossover study of 9 overweight young adults aged 20–40 y of age received 1 of 4 breakfast meals or water. Exclusion criteria included those who participated in regular physical activity, meeting or exceeding government recommendations, having a chronic or acute illness, taking medications, cigarette smoking, moderate to high alcohol intake, or allergies or aversions to any of the foods provided in the study. Participants were recruited through advertisements at the university and surrounding community. The breakfast meals were composed of either a high or low amount of carbohydrate and high- or low-GI foods. The composition of the test meals is shown in Table 1 and Supplemental Table 1 and were matched on energy content. Appetite (“How hungry do you feel at this moment?”), palatability (“How much did you like the meal?”) and mood/energy levels (e.g. “How energetic do you feel at this moment?”) were answered by selecting a number from a Likert scale administered just before eating and at 30 min following the breakfast meal and up to 5 h. Scales included the terms “not at all” on the low end and “extremely” on the high end. Postprandial glucose and insulin concentrations were measured over 5 h, with blood samples taken i.v. every 30 min. Samples were assayed for glucose by an enzymatic heterogeneous sandwich immunoassay using the VITROS 950 chemistry system (Ortho-Clinical Diagnostics) according to the supplier’s instructions. Insulin concentrations were measured by a commercial double-antibody RIA that shows little cross-reactivity (<0.2%) with proinsulin and a within-assay CV of 4% (Linco Research). The study took place at the General Clinical Research Center of the University of Minnesota.
TABLE 1.
Macronutrient composition of 5 breakfast meals in Study 1: adults1
| Meal | Protein | Fat | Carbohydrate | Dietary fiber | Diet GI | Diet GL |
| % energy | g | |||||
| HCHG | 15.1 | 19.5 | 65.5 | 3.96 | 66.3 | 58.6 |
| HCLG | 15.8 | 20.0 | 64.2 | 17.33 | 42.2 | 36.0 |
| LCHG | 15.0 | 40.7 | 44.4 | 2.50 | 64.0 | 38.3 |
| LCLG | 15.3 | 40.6 | 44.1 | 14.23 | 41.0 | 24.3 |
| Water | 0 | 0 | 0 | 0 | 0 | 0 |
GI, glycemic index (using white bread as 100%); GL, glycemic load (GI x grams of carbohydrate).
Incremental areas under the response curves (AUC) for glucose insulin from 0 to 2 h after breakfast were calculated using the trapezoidal rule, ignoring the area beneath the premeal value (54, 7). Specific time points of palatability, appetite, and mood were also investigated, and AUC was also calculated for appetite and mood. We used SAS software version 8.2 for all statistical analyses (SAS Institute) (55). The PROC MIXED program was used to perform repeated-measures regression to examine the effects of the breakfast meal on the 2-h glucose and insulin AUC. The independent variable was the breakfast treatment expressed as a 5-level class variable. The 5 levels were: 1) high carbohydrate and high GI (HCHG); 2) high carbohydrate and low GI (HCLG); 3) low carbohydrate and high GI (LCHG); 4) low carbohydrate and low GI (LCLG); and 5) water only. Estimate statements were written to test the main hypotheses of the study, comparing the AUC between high- and low-GI meals, holding constant the carbohydrate amount, and the AUC between high- and low-carbohydrate amount, holding GI constant. Gender and BMI were examined in the models as covariates that may influence the effects. Results are presented as the mean differences between treatments and their SE and 2-sided P-values for the hypothesis tests. As an additional secondary analysis, we examined the potential impact of the different meals on reactive hypoglycemia at the 5-h postprandial point. The 5-h glycemia was computed as the difference between glucose concentration at 5 h and the initial prebreakfast baseline glucose value. A P-value of < 0.05 was considered significant.
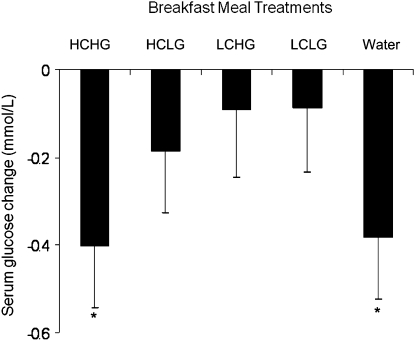
We found that the mean serum glucose 120-min AUC was significantly higher following the high- GI breakfast meals compared with the low-GI breakfast meals independent of carbohydrate amount (P = 0.005) (Fig. 3). Glucose AUC did not differ between the high-carbohydrate and low-carbohydrate breakfast meals that had similar GI values. Insulin AUC did not differ between the high-GI and low-GI breakfast meals that had similar amounts of carbohydrate. However, the postprandial 5-h drop in blood glucose was similar following the HCHG meal to that following the skipping breakfast/water-only condition, presumably an effect of the hyperinsulinemia produced by the high-glycemic load condition. In conclusion from this study, lowering the GI of a breakfast meal, but not necessarily total carbohydrate amount, reduced postprandial glucose concentrations in healthy, overweight young adults. Interestingly, a high-glycemic load diet, rich in high-GI foods, may lead to reactive hypoglycemia such that the drop in blood sugar 5 h after the meal may resemble that experience after skipping the breakfast meal altogether. Thus, consuming low-GI breakfast foods as part of whole meals may reduce hyperglycemia in healthy individuals and prevent reactive hypoglycemic between meals, important effects for the prevention or management of diabetes mellitus.
FIGURE 3.
Changes in the serum glucose concentrations of adults between 0 and 300 min after ingestion of 5 test breakfast meals differing in amount and type of carbohydrate (Study 1). Values represent the mean − SEM, n = 9. *Concentrations changed over time, P ≤ 0.01. The conditions did not differ from one another, P > 0.05.
Study 2.
In Study 2, we hypothesized that children will be less hungry, more energetic, and in a better mood when a breakfast was consumed compared with when a breakfast was skipped. Further, this study assessed the effects of a balanced breakfast of whole grains, fruit, and milk (whole) compared with a breakfast high in refined carbohydrates of pastry items and fruit juice (refined). We therefore further hypothesized that eating a whole grain breakfast may also result in children being less hungry, more energetic, and in a better mood compared with eating a refined breakfast. We used a crossover experimental design to compare the effects of 3 separate breakfast conditions on appetite and mood in children ages 9–13 y. Each child received the 3 breakfast conditions, 1 time for each condition, over a span of 2 wk, with at least 1 washout day between each condition. Following the breakfast meal, participants were tested throughout the morning to assess appetite, mood, and perceived energy levels. Twenty-eight participants completed the study during the summer of 2006. Participants were recruited to the study, approved by the University of Minnesota Human Subjects Committee, from the Minneapolis Park and Rec Plus+ summer daycare programs at 4 participating community centers, where the study took place with approval from the Minneapolis park system and signed consents from the children and their parents. Twenty-eight children (14 boys and 14 girls) ages 9–13 y (mean age 10.4 y) starting the 5th to 7th grades who were in good health without chronic disease or food allergies were enrolled in the study. The mean BMI was 20.2 kg/m2 and the sample was ethnically diverse with 17 Caucasians, 5 African Americans, 3 Native Americans, 1 Hispanic, 1 Asian, and 1 “other.”
The 3 breakfast conditions, given in random order to each child on separate days, were: 1) a balanced meal of whole grains (bread or cereal found to be acceptable to children in preliminary testing), milk, and fruit (whole condition); 2) a refined carbohydrate breakfast of a pastry and fruit drink (refined condition); and 3) water, artificially sweetened flavored water, or diet drink (skipped condition). All breakfast meals were matched in energy content at ~550 kJ/meal. Children were encouraged but not pressured to eat the entire meal and a visual estimate of plate waste was recorded by the cook when the children were finished eating. Standardized scales were adapted from the literature and are currently being used in other dietary interventions to assess appetite and mood on Likert scales (20, 21). Children in the skipped group were given a granola bar and fruit drink to prevent any adverse affects from not eating for the entire length of the testing period per the requirement of parents and the Human Subjects Committee. At 3.5 h after eating breakfast, the Appetite and Mood Questionnaire and Cognitive assessments were repeated.
As in Study 1, we used SAS software version 8.2 for all statistical analyses (SAS Institute) (55). The PROC MIXED program was used to perform repeated-measures regression to examine the effects of the breakfast meal on the dependent variables of appetite, perceived energy, and mood ratings. The independent variable was the breakfast treatment, expressed as a 3-level class variable representing the 3 conditions described above. Estimate statements were written to test the main hypotheses of the study similar to those for Study 1 above. Gender and BMI were examined in the models as covariates that may influence the effects, but these had no meaningful impact on the results (data not shown). Results are presented as the mean differences between treatments and their SE and 2-sided P-values for the hypothesis tests. P < 0.05 was considered significant.
The results indicate that the children equally enjoyed the whole food and refined foods breakfasts and that both breakfasts had a similar effect on satiation compared with skipping breakfast. Of the 28 participants, 4 had no plate waste for either breakfast condition. Eleven children ate everything during the refined condition but left some food or beverage unconsumed during the whole condition. The most common source of plate waste was leaving milk in the cereal bowl. At 2 h, the differences in hunger levels between skipping and eating breakfast were very strong (P < 0.0001). The difference was gone at 3.5 h because of the snack that was required for those who skipped breakfast. Hunger levels appeared to increase over time for the whole and refined conditions but did not significantly differ from each other. At 2 h after breakfast, the variable “How tired do you feel right now?” had a significantly higher score for skipped compared with eating the whole food breakfast (P = 0.02) and skipped compared with eating any breakfast (P = 0.03). Although not significant, there were some trends toward feeling more tired and lazy for skipped compared with eating the refined foods breakfast 2 h following breakfast. At 3.5 h after breakfast, no significant differences or trends were noted.
Again, recall that the children were given a mid-morning snack during the skipped breakfast condition. At 2 h after breakfast, the values for feeling tired were (mean ± SE): whole foods = 1.6 ± 0.27, refined foods = 1.9 ± 0.28, skipped breakfast = 2.4 ± 0.27. The last differed from the value for eating any breakfast (P = 0.03). At 2 h following breakfast, the mean values for feeling lazy were: whole foods = 1.5 ± 0.32, refined foods = 2.0 ± 0.33, skipped breakfast = 1.9 ± 0.33. The values for whole foods and refined foods tended to differ (P = 0.09).
One limitation of this study was that children received each breakfast treatment only 1 time. It would be desirable to give children each breakfast condition for an extended period of time to capture a better assessment of the effects of the separate conditions. In addition, a larger study with more participants, possibly involving students in a multi-school arrangement, would bring more power to the results found. Another limitation was that a standard amount of each breakfast type was given to all children, independent of a child’s individual factors such as size, weight, or usual breakfast habits and intake levels. Although no children in the current study requested more food at breakfast, there should be a provision for those who normally eat more at breakfast and want more than what was provided to truly assess children in this category. Finally, it was felt that a mid-morning snack was necessary for the no breakfast group to avoid any ill effects from not eating for an entire morning. This changed the eating condition, but was an ethically necessary decision. Strengths of this study include that children were assessed in a real-life situation rather than a clinical setting, where behaviors and reactions may be affected by unfamiliar surroundings. In addition, children were given ordinary foods that are routinely found in the grocery store, rather than unfamiliar items such as glucose drinks or fiber enhancers, to clinically establish nutrient intake. Another strength of this study is that it targeted an important age group, one that is at risk for increased breakfast skipping and its associated implications as they get older.
Conclusions
Breakfast consumption is associated with lower BMI in adults in a number of cross-sectional studies. The few prospective studies on this topic are generally supportive of the cross-sectional findings. Pilot experimental studies in adults and children suggest that both breakfast frequency (skipping compared with eating) and the composition of the meal may have important effects on a variety of factors related to appetite control and control of blood sugar and insulin. The results suggest that breakfast frequency (especially daily consumption) and quality (foods such as fiber- and nutrient-rich whole grains, fruit, and low-fat dairy) may be related in causal ways to appetite controls and blood sugar control, supporting the hypothesis that the breakfast meal and its quality may have important causal implications for the risk of obesity and type 2 diabetes. Future studies on this topic should further address these potential mechanisms, particularly in larger and longer trials. Because a large percentage of the U.S. population skips breakfast, effects of regular breakfast on public health may be significant. It may therefore be important to place more emphasis on breakfast habits, especially among youth, when behavioral patterns are developing.
Supplementary Material
Acknowledgments
M.A.P. designed the research, analyzed data, wrote the paper, had primary responsibility for final content. E.E. contributed to data collection, data analysis, and paper writing, and approved the final manuscript. P.M. collected data, contributed to writing the paper, and approved the final manuscript. K.S. collected data, contributed to writing the paper, and approved the final manuscript. S.K.R. assisted in study design, data collection, writing the paper, and approved the final manuscript. L.A.L. assisted in study design, data collection, analysis and interpretation, writing the paper, and approved the final manuscript. A.D.P. assisted in study design, data collection, analysis, and interpretation, writing the paper, and approved the final manuscript. All authors read and approved the final manuscript.
Footnotes
Published as a supplement to The Journal of Nutrition. Presented as part of the symposium entitled “Eating Patterns and Energy Balance: A Look at Eating Frequency, Snacking, and Breakfast Omission” given at the Experimental Biology 2009 meeting, April 19, 2009, in New Orleans, LA. This symposium was sponsored by the American Society for Nutrition Energy and Macronutrient Metabolism RIS, and was supported by an unrestricted educational grant from the Ingestive Behavior Research Center at Purdue University. The symposium was chaired by Megan A. McCrory and Wayne W. Campbell. Guest Editor for this symposium publication was Anna Maria Siega-Riz. Guest Editor disclosure: No conflicts to disclose.
Supported by the Minnesota Obesity Center, the Minnesota Medical Foundation, and the General Clinical Research Center supported by the NIH.
Supplemental Table 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AUC, area under the curve; GI, glycemic index; HCHG, high-carbohydrate, high-glycemic index treatment; HCLG, high-carbohydrate, low-glycemic index treatment; LCHG, low-carbohydrate, high-glycemic index treatment; LCLG, low-carbohydrate, low-glycemic index treatment.
Literature Cited
- 1.Skinner JD, Salvetti NN, Ezell JM, Penfield MP, Costello CA. Appalachian adolescents' eating patterns and nutrient intakes. J Am Diet Assoc. 1985;85:1093–9 [PubMed] [Google Scholar]
- 2.Sampson AE, Dixit S, Meyers AF, Houser RJ. The nutritional impact of breakfast consumption on the diets of inner-city African-American elementary school children. J Natl Med Assoc. 1995;87:195–202 [PMC free article] [PubMed] [Google Scholar]
- 3.Nicklas TA, Bao W, Webber LS, Berenson GS. Breakfast consumption affects adequacy of total daily intake in children. J Am Diet Assoc. 1993;93:886–91 [DOI] [PubMed] [Google Scholar]
- 4.Nicklas TA, Reger C, Myers L, O'Neil C. Breakfast consumption with and without vitamin-mineral supplement use favorably impacts daily nutrient intake of ninth-grade students. J Adolesc Health. 2000;27:314–21 [DOI] [PubMed] [Google Scholar]
- 5.Graham MV, Uphold CR. Health perceptions and behaviors of school-age boys and girls. J Community Health Nurs. 1992;9:77–8 [DOI] [PubMed] [Google Scholar]
- 6.Siega-Riz AM, Popkin BM, Carson T. Trends in breakfast consumption for children in the United States from 1965–1991. Am J Clin Nutr. 1998;67:S748–56 [DOI] [PubMed] [Google Scholar]
- 7.Timlin MT, Pereira MA. Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr Rev. 2007;65:268–81 [DOI] [PubMed] [Google Scholar]
- 8.Rampersaud GC, Pereira MA, Girard BL, Adams J, Metzl JD. Breakfast habits, nutritional status, body weight, and academic performance in children and adolescents. J Am Diet Assoc. 2005;105:743–60; quiz 761–42 [DOI] [PubMed] [Google Scholar]
- 9.Siega-Riz AM, Popkin BM, Carson T. Differences in food patterns at breakfast by sociodemographic characteristics among a nationally representative sample of adults in the United States. Prev Med. 2000;30:415–24 [DOI] [PubMed] [Google Scholar]
- 10.Pereira MA, Ebbeling CB, Pawlak DB, Leidig MM, Ludwig DS. Whole grain consumption and body weight regulation. : Marquart L, Slavin JL, Fulcher RG, Whole grain foods in health and disease. St. Paul: American Association of Cereal Chemists; 2002. p. 233–42 [Google Scholar]
- 11.Pereira MA, Jacobs DJ, Pins JJ, Marquart L, Keenan J. Whole grains, cereal fiber, and chronic disease: the epidemiologic evidence. : Spiller GA, editor CRC Handbook of dietary fiber in human disease. 3rd ed New York: CRC Press; 2001. p. 481–98 [Google Scholar]
- 12.Pereira MA, Jacobs DR, Jr, Pins JJ, Raatz SK, Gross MD, Slavin JL, Seaquist ER. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr. 2002;75:848–55 [DOI] [PubMed] [Google Scholar]
- 13.Pereira MA, Jacobs DR, Jr, Slattery M, Hilner J, Kushi LH. The association between whole grain intake and fasting insulin in a bi-racial cohort of young adults: The Cardia Study. CVD Prevention. 1998;1:231–42 [PMC free article] [PubMed] [Google Scholar]
- 14.Cho S, Dietrich M, Brown CJ, Clark CA, Block G. The effect of breakfast type on total daily energy intake and body mass index: results from the Third National Health and Nutrition Examination Survey (NHANES III). J Am Coll Nutr. 2003;22:296–302 [DOI] [PubMed] [Google Scholar]
- 15.Song WO, Chun OK, Obayashi S, Cho S, Chung CE. Is consumption of breakfast associated with body mass index in US adults? J Am Diet Assoc. 2005;105:1373–82 [DOI] [PubMed] [Google Scholar]
- 16.Summerbell CD, Moody RC, Shanks J, Stock MJ, Geissler C. Relationship between feeding pattern and body mass index in 220 free-living people in four age groups. Eur J Clin Nutr. 1996;50:513–9 [PubMed] [Google Scholar]
- 17.Wyatt HR, Grunwald GK, Mosca CL, Klem ML, Wing RR, Hill JO. Long-term weight loss and breakfast in subjects in the National Weight Control Registry. Obes Res. 2002;10:78–82 [DOI] [PubMed] [Google Scholar]
- 18.Morgan KJ, Zabik ME, Stampley GL. The role of breakfast in diet adequacy of the U.S. adult population. J Am Coll Nutr. 1986;5:551–63 [DOI] [PubMed] [Google Scholar]
- 19.Stanton JL, Jr, Keast DR. Serum cholesterol, fat intake, and breakfast consumption in the United States adult population. J Am Coll Nutr. 1989;8:567–72 [DOI] [PubMed] [Google Scholar]
- 20.Timlin MT, Pereira MA, Story M, Neumark-Sztainer D. Breakfast eating and weight change in a 5-year prospective analysis of adolescents: Project EAT (Eating Among Teens). Pediatrics. 2008;121:e638–45 [DOI] [PubMed] [Google Scholar]
- 21.Bazzano LA, Song Y, Bubes V, Good CK, Manson JE, Liu S. Dietary intake of whole and refined grain breakfast cereals and weight gain in men. Obes Res. 2005;13:1952–60 [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Bertone ER, Stanek EJ, Reed GW, Hebert JR, Cohen NL, Merriam PA, Ockene IS. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol. 2003;158:85–92 [DOI] [PubMed] [Google Scholar]
- 23.Maskarinec G, Novotny R, Tasaki K. Dietary patterns are associated with body mass index in multiethnic women. J Nutr. 2000;130:3068–72 [DOI] [PubMed] [Google Scholar]
- 24.Barton BA, Eldridge AL, Thompson D, Affenito SG, Striegel-Moore RH, Franko DL, Albertson AM, Crockett SJ. The relationship of breakfast and cereal consumption to nutrient intake and body mass index: the National Heart, Lung, and Blood Institute Growth and Health Study. J Am Diet Assoc. 2005;105:1383–9 [DOI] [PubMed] [Google Scholar]
- 25.Affenito SG, Thompson DR, Barton BA, Franko DL, Daniels SR, Obarzanek E, Schreiber GB, Striegel-Moore RH. Breakfast consumption by African-American and white adolescent girls correlates positively with calcium and fiber intake and negatively with body mass index. J Am Diet Assoc. 2005;105:938–45 [DOI] [PubMed] [Google Scholar]
- 26.Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. 2004;159:1150–9 [DOI] [PubMed] [Google Scholar]
- 27.McKeown NM, Meigs JB, Liu S, Wilson PW, Jacques PF. Whole-grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham Offspring Study. Am J Clin Nutr. 2002;76:390–8 [DOI] [PubMed] [Google Scholar]
- 28.Liljeberg HG, Akerberg AK, Bjorck IM. Effect of the glycemic index and content of indigestible carbohydrates of cereal-based breakfast meals on glucose tolerance at lunch in healthy subjects. Am J Clin Nutr. 1999;69:647–55 [DOI] [PubMed] [Google Scholar]
- 29.Bellisle F, McDevitt R, Prentice AM. Meal frequency and energy balance. Br J Nutr. 1997;77 Suppl 1:S57–70 [DOI] [PubMed] [Google Scholar]
- 30.Nestler JE, Barlascini CO, Clore JN, Blackard WG. Absorption characteristic of breakfast determines insulin sensitivity and carbohydrate tolerance for lunch. Diabetes Care. 1988;11:755–60 [DOI] [PubMed] [Google Scholar]
- 31.Clark CA, Gardiner J, McBurney MI, Anderson S, Weatherspoon LJ, Henry DN, Hord NG. Effects of breakfast meal composition on second meal metabolic responses in adults with type 2 diabetes mellitus. Eur J Clin Nutr. 2006;60:1122–9 [DOI] [PubMed] [Google Scholar]
- 32.Burley VJ, Leeds AR, Blundell JE. The effect of high and low-fibre breakfasts on hunger, satiety and food intake in a subsequent meal. Int J Obes. 1987;11 Suppl 1:87–93 [PubMed] [Google Scholar]
- 33.Holt SH, Delargy HJ, Lawton CL, Blundell JE. The effects of high-carbohydrate vs high-fat breakfasts on feelings of fullness and alertness, and subsequent food intake. Int J Food Sci Nutr. 1999;50:13–28 [DOI] [PubMed] [Google Scholar]
- 34.Pasman WJ, Blokdijk VM, Bertina FM, Hopman WP, Hendriks HF. Effect of two breakfasts, different in carbohydrate composition, on hunger and satiety and mood in healthy men. Int J Obes Relat Metab Disord. 2003;27:663–8 [DOI] [PubMed] [Google Scholar]
- 35.Pai S, Ghugre PS, Udipi SA. Satiety from rice-based, wheat-based and rice-pulse combination preparations. Appetite. 2005;44:263–71 [DOI] [PubMed] [Google Scholar]
- 36.Johnstone AM, Ryan LM, Reid CA, Stubbs RJ. Breakfasts high in monoglyceride or triglyceride: no differential effect on appetite or energy intake. Eur J Clin Nutr. 1998;52:603–9 [DOI] [PubMed] [Google Scholar]
- 37.Pereira MA, Ludwig DS. Dietary fiber and body-weight regulation. Observations and mechanisms. Pediatr Clin North Am. 2001;48:969–80 [DOI] [PubMed] [Google Scholar]
- 38.Holt S, Brand J, Soveny C, Hansky J. Relationship of satiety to postprandial glycaemic, insulin and cholecystokinin responses. Appetite. 1992;18:129–41 [DOI] [PubMed] [Google Scholar]
- 39.Bourdon I, Yokoyama W, Davis P, Hudson C, Backus R, Richter D, Knuckles B, Schneeman BO. Postprandial lipid, glucose, insulin, and cholecystokinin responses in men fed barley pasta enriched with beta-glucan. Am J Clin Nutr. 1999;69:55–63 [DOI] [PubMed] [Google Scholar]
- 40.Jenkins DJ, Wolever TM, Ocana AM, Vuksan V, Cunnane SC, Jenkins M, Wong GS, Singer W, Bloom SR, et al. Metabolic effects of reducing rate of glucose ingestion by single bolus versus continuous sipping. Diabetes. 1990;39:775–81 [DOI] [PubMed] [Google Scholar]
- 41.Tseng CC, Kieffer TJ, Jarboe LA, Usdin TB, Wolfe MM. Postprandial stimulation of insulin release by glucose-dependent insulinotropic polypeptide (GIP). Effect of a specific glucose-dependent insulinotropic polypeptide receptor antagonist in the rat. J Clin Invest. 1996;98:2440–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Dijk G, Thiele TE. Glucagon-like peptide-1 (7–36) amide: a central regulator of satiety and interoceptive stress. Neuropeptides. 1999;33:406–14 [DOI] [PubMed] [Google Scholar]
- 43.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord. 2001;25:781–92 [DOI] [PubMed] [Google Scholar]
- 45.D'Alessio DA, Kahn SE, Leusner CR, Ensinck JW. Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J Clin Invest. 1994;93:2263–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7–36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–22 [DOI] [PubMed] [Google Scholar]
- 47.Thorburn A, Muir J, Proietto J. Carbohydrate fermentation decreases hepatic glucose output in healthy subjects. Metabolism. 1993;42:780–5 [DOI] [PubMed] [Google Scholar]
- 48.Venter CS, Vorster HH, Cummings JH. Effects of dietary propionate on carbohydrate and lipid metabolism in healthy volunteers. Am J Gastroenterol. 1990;85:549–53 [PubMed] [Google Scholar]
- 49.Reimer RA, Thomson AB, Rajotte RV, Basu TK, Ooraikul B, McBurney MI. A physiological level of rhubarb fiber increases proglucagon gene expression and modulates intestinal glucose uptake in rats. J Nutr. 1997;127:1923–8 [DOI] [PubMed] [Google Scholar]
- 50.Reimer RA, McBurney MI. Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology. 1996;137:3948–56 [DOI] [PubMed] [Google Scholar]
- 51.Farshchi HR, Taylor MA, Macdonald IA. Beneficial metabolic effects of regular meal frequency on dietary thermogenesis, insulin sensitivity, and fasting lipid profiles in healthy obese women. Am J Clin Nutr. 2005;81:16–24 [DOI] [PubMed] [Google Scholar]
- 52.Farshchi HR, Taylor MA, Macdonald IA. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am J Clin Nutr. 2005;81:388–96 [DOI] [PubMed] [Google Scholar]
- 53.Jenkins DJ, Kendall CW, Vidgen E, Vuksan V, Jackson CJ, Augustin LS, Lee B, Garsetti M, Agarwal S, et al. Effect of soy-based breakfast cereal on blood lipids and oxidized low-density lipoprotein. Metabolism. 2000;49:1496–500 [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto R, Kawamura T, Wakai K, Ichihara Y, Anno T, Mizuno Y, Yokoi M, Ohta T, Iguchi A, et al. Favorable life-style modification and attenuation of cardiovascular risk factors. Jpn Circ J. 1999;63:184–8 [DOI] [PubMed] [Google Scholar]
- 55.Wolever TM, Jenkins DJ, Ocana AM, Rao VA, Collier GR. Second-meal effect: low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am J Clin Nutr. 1988;48:1041–7 [DOI] [PubMed] [Google Scholar]
- 56.Jenkins DJ, Jenkins AL, Wolever TM, Vuksan V, Rao AV, Thompson LU, Josse RG. Low glycemic index: lente carbohydrates and physiological effects of altered food frequency. Am J Clin Nutr. 1994;59 Suppl 3:S706–9 [DOI] [PubMed] [Google Scholar]
- 57.Keim NL, Van Loan MD, Horn WF, Barbieri TF, Mayclin PL. Weight loss is greater with consumption of large morning meals and fat-free mass is preserved with large evening meals in women on a controlled weight reduction regimen. J Nutr. 1997;127:75–82 [DOI] [PubMed] [Google Scholar]
- 58.Kleemola P, Puska P, Vartiainen E, Roos E, Luoto R, Ehnholm C. The effect of breakfast cereal on diet and serum cholesterol: a randomized trial in North Karelia, Finland. Eur J Clin Nutr. 1999;53:716–21 [DOI] [PubMed] [Google Scholar]
- 59.Schlundt DG, Hill JO, Sbrocco T, Pope-Cordle J, Sharp T. The role of breakfast in the treatment of obesity: a randomized clinical trial. Am J Clin Nutr. 1992;55:645–51 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.