Abstract
After menopause blood pressure (BP) increases in women. However, the underlying mechanisms responsible for postmenopausal hypertension are not completely understood. This study was conducted to determine the role that the renin angiotensin system plays in postmenopausal hypertension. Post estrous cycling (postmenopausal) spontaneously hypertensive rats or young female controls were treated with losartan, an angiotensin type 1 receptor blocker for 25 days. Mean arterial pressure was recorded continuously by radio-telemetry. Losartan significantly decreased blood pressure in postmenopausal rats and young female controls; but failed to normalize blood pressure in postmenopausal rats to levels found in young controls. Plasma renin activity and plasma angiotensinogen were significantly elevated and intrarenal angiotensin AT1 receptor and renin mRNA expression were significantly down-regulated in postmenopausal rats. Therefore, the renin angiotensin system only partially contributes to hypertension in postcycling spontaneously hypertensive rats, whereas hypertension in young females is mediated mainly by the renin-angiotensin system. The data suggest that other mechanisms besides activation of the renin angiotensin system are likely involved in postmenopausal hypertension.
Keywords: postmenopausal hypertension, aging, kidney, blood pressure
INTRODUCTION
Men are at greater risk for cardiovascular and renal disease than are women of similar age. Men also have higher blood pressure (BP) than women 1, 2. However, these sex differences change following menopause, when the risk for cardiovascular disease and prevalence of hypertension increases in women 3.
There are few clinical data regarding effectiveness of different antihypertensive therapy in controlling BP in postmenopausal hypertensive women. The Women’s Health Initiative (WHI) report was based on nearly 100,000 women, ages 50 to 79 years, and was the largest and best-characterized cohort of postmenopausal women in the United States. This report showed that although older hypertensive women (aged 70 to 79 years) were as likely to be on treatment for hypertension (63.2%) as the younger women (64.2%), a substantially smaller percentage of them had their blood pressures under control (29.3% versus 41.3% for the older versus younger women, respectively) 4. Similar findings were observed when the results from the National Health and Nutrition Estimation Survey (NHANES) III data set (ending 1994) were compared with NHANES IV data set (ending 2004) 5. Thus the best therapeutic options for treatment of postmenopausal hypertension are unclear.
A key system for modulating BP and body fluid volume is the renin angiotensin system (RAS) 6. Angiotensin (Ang) II causes vasoconstriction though activation of Angiotensin type 1 receptor (AT1R). Increases in Ang II and dysregulation (upregulation or activation) of the vasoconstrictor arm of the RAS have been implicated as adverse factors in cardiovascular pathophysiology.
Postmenopausal women exhibit an increase in plasma renin activity 7, 8, but the use of RAS blockers, angiotensin converting enzyme inhibitors or angiotensin receptor blockers (ARB), are not frequently used to treat postmenopausal hypertension 4. Perhaps one reason for this is that one study showed that monotherapy with an ARB only controlled blood pressure to normal levels in 30 % of the patients treated 9.
We have previously characterized the aging female spontaneously hypertensive rat (SHR) as a suitable model to study postmenopausal hypertension 10. In this experimental animal model, the estrous cycle stops at 12 mos of age and at 16 mos of age, BP is significantly higher than in young cycling female SHR. We showed previously that a portion of the hypertension in PMR is mediated by activation of the endothelin system, since the endothelin type A receptor blocker ABT627 decreases BP 11. However, the use of ABT627 only reduced BP about 20 mm Hg and BP remained significantly higher in the aged female SHR than in young female rats.
Young male SHRs have higher BP than aged-matched females. We found previously that in young SHRs the sex difference in BP is abolished by angiotensin converting enzyme inhibition 12. Furthermore, we showed recently that angiotensin type 1 receptor blockade with losartan in 16 mos old male SHR normalized BP 13, suggesting that the RAS via the AT1R is the dominant mechanisms that mediates hypertension in the aging male SHR. However, the role that the AT1R plays in mediating postmenopausal hypertension is unclear.
Thus the present study was performed to analyze the role of the RAS in hypertension in old and young female SHRs. We tested the hypothesis that as in male SHRs, activation of the RAS is the sole mediator for hypertension in young female SHRs, whereas in PMR, the RAS is only one of the mechanisms responsible for the hypertension. If this hypothesis were true, it would explain in part why BP is difficult to control in postmenopausal women. To address this hypothesis, components of the systemic and intrarenal RAS were measured in aging female and young female SHR, and the rats were given losartan, an angiotensin receptor blocker (ARB) , for 25 days and BP was measured in conscious freely moving rats by radio telemetry.
METHODS
Rats
Female SHRs, 8 mos of age, were obtained from Taconic Farms (Germantown, NY) and were aged in the Laboratory Animal Facility of the University of Mississippi Medical Center, until they were 16 mos of age (PMR). Young female SHR (YF), aged 4 mos, were obtained from the same vendor. All rats were maintained on standard rat chow (Teklad, Harlan SD, Indianapolis, IN) and tap water in an environment with 12h/12h-light/dark cycles. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center, and studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals, National Institutes of Health.
Depressor response to losartan
In order to determine whether the RAS plays a role in postmenopausal hypertension, the ARB, losartan was used. Young females and PMR (n=7 per group) were given losartan (40 mg/kg/d; a generous gift from Merck Laboratories) in the drinking water for 25 days. We showed previously that at this dose the hypertensinogenic effect of angiotensin II is completely blocked in conscious old female SHR 13. Controls received tap water and water consumption was monitored daily to verify doses were similar between old and young female animals.
Measurement of mean arterial pressure in conscious rats by radiotelemetry
Radiotelemetry BP was monitored, as we have previously reported 14, throughout baseline and losartan treatment. At the end of the experimental period, blood was withdrawn from the abdominal aorta to measure plasma renin activity (PRA) and angiotensinogen, as we previously described 13.
Study of components of the intrarenal RAS
PMR, aged 16 mos, and young females SHR, aged 4 mos (n=8 each group), were anesthetized with isoflurane anesthesia, and their kidneys were perfused with 2% heparin in saline, removed and separated into cortex and medulla and snap frozen in liquid nitrogen.
Measurement of intra-renal angiotensinogen, renin, angiotensin converting Enzyme, AT1R and AT2R mRNA expression with real time RT-PCR
mRNA was isolated from cortex and medulla of kidneys and real time RT-PCR was performed, as we previously reported 13. Elongation factor I (EF-I) primers were used as controls. Results are expressed as arbitrary units and standardized against EF-1 mRNA expression.
Statistics
Data are presented as mean ± SEM. Time series blood pressure studies were analyzed by repeated measures two-way analyses of variance (ANOVA) followed by Student-Newman-Keuls post hoc comparisons. All other comparisons between PMR and young female SHRs were performed by Student’s t-test. A value of p<0.05 was considered statistically significant. Statistical analyses were performed with SigmaPlot v11 (Systat Software, Inc., San Jose, CA).
RESULTS
Antihypertensive response to ARB in PMR and young female SHR
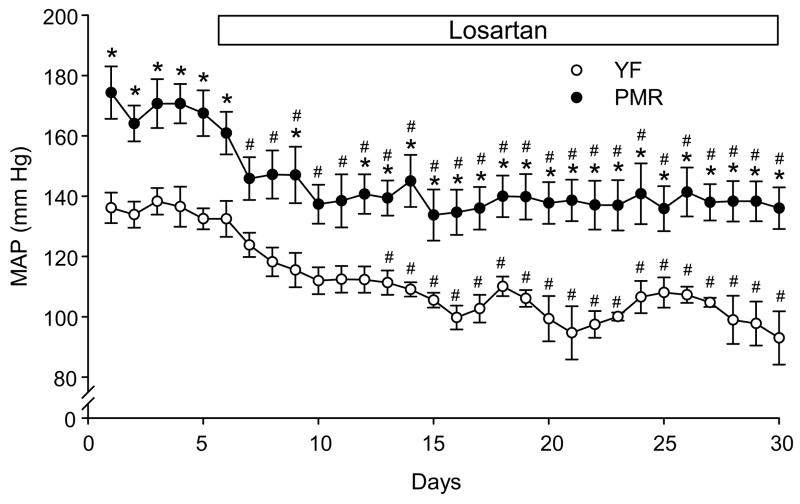
As shown in Figure 1, BP was measured in conscious rats instrumented with radiotelemetry probes. MAP was recorded 24 h per day. MAP was measured during 5 days of baseline and then losartan, was given in their drinking water for 25 days. MAP was significantly higher in PMR than young females (170 ±2 vs 135±1 mm Hg, p<0.05) during the baseline period. Losartan treatment significantly decreased MAP after one day of losartan administration in PMR. After one week MAP was significantly reduced to normotensive levels in young females treated with losartan compared to baseline period, but not in treated PMR. The reduction in MAP with losartan was similar in young females and PMR (PMR: 31±3 vs 33±5 mm Hg, p=0.73). In addition, at the end of the experimental protocol MAP was still significantly elevated above normotensive levels in PMR but was normalized in young females SHR (7 day average: 138±1 vs. 102±2 mmHg; p<0.05).
Figure 1. A: Effect of chronic AT1 receptor blocker, losartan, on mean arterial pressure in PMR and young female SHR.
Losartan treatment significantly decreases mean arterial pressure (MAP) after one day of treatment in PMR, but after 7 days in YF-SHR. At the end of the experimental protocol BP was normalized in young female SHR, but remained significantly higher in PMR. Data are expressed as mean ± SEM. *, P<0.05 compared with young female SHR; #, p<0.05 compared with day 5 (last day of baseline) of the same experimental group.
Characterization of systemic RAS components
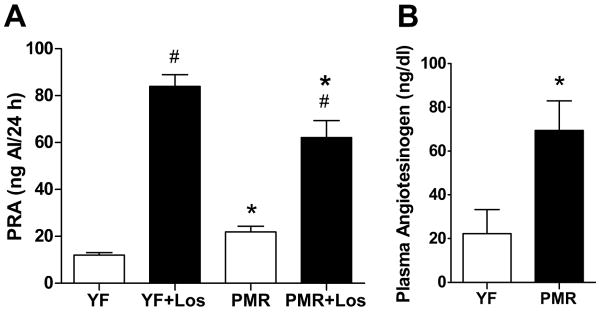
As shown in Figure 2A, PRA was significantly higher in PMR compared with their young female counterparts, and as expected, losartan treatment significantly increased PRA in both PMR and young female SHR. PRA was increased more with losartan in young females than PMR, however. Plasma angiotensinogen levels were approximately 3 fold higher in PMR than in young females (Figure 2B).
Figure 2. A) Plasma renin activity in losartan-treated and untreated PMR and young female SHR.
PMR has significantly higher levels of plasma renin activity than young females SHR. Losartan increased PRA to similar levels in both young and old female SHR. Data are expressed as mean ± SEM. *, P<0.05 compared with young females SHR; #, p<0.05, compared with untreated SHR. B) Plasma Angiotensinogen level in PMR and young female SHR. PMR had higher levels of angiotensinogen than young females. Data are expressed as mean ± SEM. *, P<0.05 compared with young females.
Characterization of intrarenal RAS components
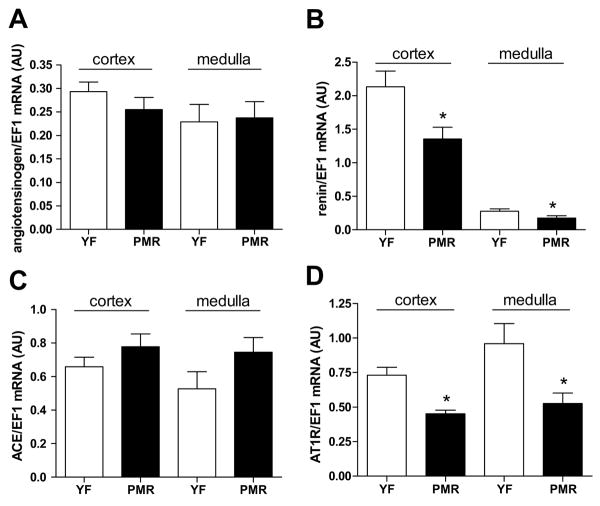
Expression of mRNA for components of the RAS in kidneys harvested from old female SHR, aged 16 mos, and young female SHR, age 4 mos, were measured by real time RT-PCR. There was no significant difference in the intrarenal levels of angiotensinogen or ACE mRNA in cortex or medulla between PMR and young females (Figure 3A, C). Intrarenal renin mRNA was significantly reduced in both cortex and medulla of PMR compared to young females (Figure 3B). In addition, intrarenal mRNA expression of AT1R (Figure 3D) was significantly down-regulated in PMR compared to young females SHR. Intrarenal mRNA expression of AT2R was not different between the groups (data not shown).
Figure 3. mRNA levels of kidney RAS components from PMR and young females by real time RT-PCR.
A) Angiotensinogen mRNA level was the same in cortex and medulla in PMR and young females; B) Renin mRNA expression was significantly down-regulated in PMR compared to young rats; C) ACE mRNA level was the same in cortex and medulla in PMR and young females D) AT1R mRNA expression was significantly down-regulated in PMR compared to young rats. Data are expressed as mean ± SEM. *, P<0.05 compared with young females.
DISCUSSION
The main findings of this paper are: 1) the RAS through AT-1R is one mechanism involved in the pathophysiology of postmenopausal hypertension in PMR; 2) activation of the RAS through AT-1R is likely the most important regulator of BP in young female SHR; 3) plasma renin activity and plasma angiotensinogen levels are significantly higher in PMR; 4) intrarenal mRNA expression of renin and AT1R are significantly down-regulated in PMR; and 5) ARB treatment with losartan failed to normalize MAP in PMR, suggesting that other factors may contribute to the higher MAP observed in PMR compared with young females SHR.
The WHI report stated that although 64% of the hypertensive women in the study were treated with antihypertensive medication, BP was only controlled in about a third of them 4. Similarly, the NHANES III (1988 to 1991) data indicate that while 81% of hypertensive women, aged 18 to 74 years, were aware of their condition, and 65% were undergoing treatment, only 38% had their hypertension under control 1. These data are surprising since women typically see their physicians more frequently than age-matched men and they are often more compliant with medications than are men. These data strongly indicate then that the mechanism(s) responsible for postmenopausal hypertension is (are) poorly understood.
Our present study supports the notion that postmenopausal hypertension is mediated in part by activation of the RAS, since the ARB, losartan, significantly decreased MAP in PMR. However, since the magnitude of the depressor response with losartan was similar in both young females and PMR suggests that the RAS is not more activated in PMR than young females. Unlike in young females though, MAP was not normalized in PMR with losartan. In young SHRs, MAP is higher in males than in females and inhibition of the RAS with enalapril, an angiotensin converting enzyme inhibitor, eliminates this sex difference 12. In other studies, we found that treatment of old male SHR and PMR with an ARB, normalized BP in old male SHR but not old females 13. Based on these data we concluded that hypertension in both young and old male SHR is predominantly mediated by the RAS. In contrast, taking these previous data with our current findings, our data suggest that another mechanism(s) besides the RAS is (are) involved in postmenopausal hypertension in PMR.
The RAS is the most important regulator of BP and fluid homeostasis 6. PRA is higher in postmenopausal women than in premenopausal ones 7. Recently it was shown that monotherapy with candesartan, an ARB, only controlled BP in 36% of women after 1 mos of treatment. These data are also consistent with our present findings 9. PRA was also higher in our PMR model, and why this is the case for both women and PMR is not clear. We hypothesize that an increase in androgens could increase angiotensinogen, the substrate for renin and thus increase PRA. We could also speculate that sympathetic activity may be increased with aging in PMR and women which could increase renin levels. Sympathetic activity has been shown to play a role in mediating the hypertension in SHR 15. Since our data also show that AT1R mRNA expression is down-regulated in PMR compared to young females, it is possible that intrarenal Ang II levels may be increased since downregulation of the AT1R is known to occur when Ang II levels are increased and since Ang II has been shown to destabilize AT1R mRNA16. However, the fact that ACE expression was not different between PMR and young female controls does not support an increase in Ang II although the levels of ACE are not rate limiting in the synthesis of Ang II. Future studies will be necessary to determine if Ang II levels are indeed increased in kidneys of PMR.
Many investigators have found either by direct measurement of expression or as suggested by binding studies and response to antagonists that AT1 receptor expression increases with aging in humans and most rat models 17-21. Most studies were performed in male animals or the human studies did not segregate data in women from data in men. However, Hinojosa Laborde and colleagues did find increased AT1R binding (Bmax) in aging female Dahl salt sensitive rats 18. This is in contrast to our present findings that AT1R mRNA expression was downregulated in PMR. The reasons for the inconsistencies between these studies are not readily apparent. Blood pressure in DS females is responsive to the presence of estrogens, i.e. ovariectomized rats exhibit increased blood pressure 22. Estrogens have been shown to downregulate AT1R expression 23,24. Therefore, with aging and reduction in estrogens, AT1R expression may increase in DS rats. Estrogens do not play a role in mediating hypertension in female SHR since ovariectomy has no effect on their blood pressure 10, thus they may not exhibit increases in AT1R expression that usually accompanies estrogen depletion. One caveat for our present study is that AT1 receptor mRNA expression may not adequately represent the protein expression or receptor binding capacities.
What other mechanisms may contribute then to hypertension in postmenopausal women and rats? Previously we showed that endothelin receptor (ETA) blockers decrease BP in PMR but have no effect on BP in young female SHR 11. Despite the effect of endothelin receptor antagonists on MAP in PMR, the BP in PMR was still approximately 170 mm Hg. These data support a role for endothelin in mediating a portion of the postmenopausal hypertension in PMR. Taken with the observation that angiotensin II can stimulate synthesis of pre-proendothelin 25, our data suggest that both the RAS and endothelin may be involved in postmenopausal hypertension in our PMR and may play a role in postmenopausal hypertension in women as well. As mentioned previously, androgens have been shown to increase angiotensinogen in male rats26, and PMR exhibit a 4 fold increase in plasma testosterone compared to young female SHR 10. Thus it is possible that the increase in testosterone may contribute to the increased angiotensinogen we found in PMR compared to young females. It is also possible that reductions in the vasodilator arm of the RAS, the Ang 1-7 pathway, could play a role in the hypertension in PMR although we did not find differences in intrarenal mRNA expression of AT2R. Future studies will be necessary to thoroughly investigate this alternative.
In summary, we found that the ARB, losartan, reduces MAP in both PMR and young females, but normalized BP in young females and not in PMR. We also found that AT1R and intrarenal renin mRNA expression is reduced in PMR despite higher PRA. Plasma angiotensinogen levels were also higher in PMR. Taken together, these data suggest that increased angiotensinogen may lead to increased Ang II despite lower intrarenal renin expression and thus plays a role in hypertension in PMR. However, since losartan did not normalize MAP in PMR as it did in young females, it is likely that other factors also contribute to hypertension in PMR and perhaps in postmenopausal women as well.
PERSPECTIVES
Postmenopausal hypertension is a complex disease with multiple contributing mechanisms involved. The Joint National Committee (JNC) VI and JNCVII Guidelines recommend the use of diuretics and β-blockers in uncomplicated hypertensive patients. Postmenopausal hypertension is often associated with more cardiovascular risk factors than found in essential hypertensive patients. At the present many postmenopausal hypertensive women are treated with calcium channel blockers, diuretics, or a combination of the two 4. Treatment with calcium channel blockers is associated with increased morbidity and mortality in some cohorts 27,28 and also may increase the transmission of the systemic pressure to the glomeruli, exacerbating renal injury 29, and thus further complicate hypertension. In addition, diuretics can worsen metabolic alterations often found in postmenopausal hypertension. Unfortunately, ACE inhibitors and ARBs are rarely used to treated postmenopausal hypertension. Our previous data with endothelin receptor antagonists and our current data with ARBs reinforce the notion that postmenopausal hypertension is a complex disease that needs to be treated with blockers specific for the systems that mediate the hypertension rather than non-specific treatment with calcium channel blockers and diuretics that may cause more harm than good. Our data also reinforce the fact that additional studies are necessary in postmenopausal women to determine the exact mechanisms by which their blood pressure is elevated.
Acknowledgments
SOURCES OF FUNDING These studies were supported by National Institutes of Health National Heart, Lung and Blood Institute HL66072, HL51971 and HL69194 to JFR and American Heart Association Scientist Development Grant 0830239N to LLY and Scientist Development Grant 0803416N to RI.
Footnotes
Disclosures: Jane F. Reckelhoff: NIH for grant support
Licy L. Yanes: American Heart Association for grant support
Radu Iliescu: American Heart Association for grant support
Damian G. Romero: none
Huimin Zhang: None
Deborah Davis:None
References
- 1.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the us adult population. Results from the third national health and nutrition examination survey, 1988-1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 2.Wiinberg N, Hoegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal danish subjects, related to age and gender. Am J Hypertens. 1995;8:978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB. The Framingham study: Historical insight on the impact of cardiovascular risk factors in men versus women. J Gend Specif Med. 2002;5:27–37. [PubMed] [Google Scholar]
- 4.Wassertheil-Smoller S, Anderson G, Psaty BM, Black HR, Manson J, Wong N, Francis J, Grimm R, Kotchen T, Langer R, Lasser N. Hypertension and its treatment in postmenopausal women: Baseline data from the women’s health initiative. Hypertension. 2000;36:780–789. doi: 10.1161/01.hyp.36.5.780. [DOI] [PubMed] [Google Scholar]
- 5.Ong KL, Tso AW, Lam KS, Cheung BM. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension. 2008;51:1142–1148. doi: 10.1161/HYPERTENSIONAHA.107.105205. [DOI] [PubMed] [Google Scholar]
- 6.Hall JE, Brands MW, Henegar JR. Angiotensin ii and long-term arterial pressure regulation: The overriding dominance of the kidney. J Am Soc Nephrol. 1999;10(Suppl 12):S258–265. [PubMed] [Google Scholar]
- 7.James GD, Sealey JE, Muller F, Alderman M, Madhavan S, Laragh JH. Renin relationship to sex, race and age in a normotensive population. J Hypertens Suppl. 1986;4:S387–389. [PubMed] [Google Scholar]
- 8.Schunkert H, Danser AH, Hense HW, Derkx FH, Kurzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation. 1997;95:39–45. doi: 10.1161/01.cir.95.1.39. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Vega F, Abellan J, Vegazo O, De Vinuesa SG, Rodriguez JC, Maceira B, de Castro SS, Nicolas RR, Luno J. Angiotensin ii type 1 receptor blockade to control blood pressure in postmenopausal women: Influence of hormone replacement therapy. Kidney Int Suppl. 2002:S36–41. doi: 10.1046/j.1523-1755.62.s82.8.x. [DOI] [PubMed] [Google Scholar]
- 10.Fortepiani LA, Zhang H, Racusen L, Roberts LJ, 2nd, Reckelhoff JF. Characterization of an animal model of postmenopausal hypertension in spontaneously hypertensive rats. Hypertension. 2003;41:640–645. doi: 10.1161/01.HYP.0000046924.94886.EF. [DOI] [PubMed] [Google Scholar]
- 11.Yanes LL, Romero DG, Cucchiarelli VE, Fortepiani LA, Gomez-Sanchez CE, Santacruz F, Reckelhoff JF. Role of endothelin in mediating postmenopausal hypertension in a rat model. Am J Physiol Regul Integr Comp Physiol. 2005;288:R229–233. doi: 10.1152/ajpregu.00697.2003. [DOI] [PubMed] [Google Scholar]
- 12.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: Role of the renin-angiotensin system. Hypertension. 2000;35:480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- 13.Yanes LL, Romero DG, Iles JW, Iliescu R, Gomez-Sanchez C, Reckelhoff JF. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R383–390. doi: 10.1152/ajpregu.00510.2005. [DOI] [PubMed] [Google Scholar]
- 14.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin ii when the endogenous renin-angiotensin system is blocked. Hypertension. 2008;51:1170–1176. doi: 10.1161/HYPERTENSIONAHA.107.106922. [DOI] [PubMed] [Google Scholar]
- 15.Iliescu R, Yanes LL, Bell W, Dwyer T, Baltatu OC, Reckelhoff JF. Role of the renal nerves in blood pressure in male and female SHR. Am J Physiol Regul Integr Comp Physiol. 2006;290:R341–344. doi: 10.1152/ajpregu.00035.2005. [DOI] [PubMed] [Google Scholar]
- 16.Elton TS, Martin MM. Angiotensin II type 1 receptor gene regulation: Transcriptional and posttranscriptional mechanisms. Hypertension. 2007;49:953–961. doi: 10.1161/HYPERTENSIONAHA.106.070565. [DOI] [PubMed] [Google Scholar]
- 17.Wray DW, Nishiyama SK, Harris RA, Richardson RS. Angiotensin II in the elderly: Impact of angiotensin II type 1 receptor sensitivity on peripheral hemodynamics. Hypertension. 2008;51:1611–1616. doi: 10.1161/HYPERTENSIONAHA.108.111294. [DOI] [PubMed] [Google Scholar]
- 18.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension. 2004;44:405–409. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 19.Cao XJ, Li YF. Alteration of messenger rna and protein levels of cardiac alpha(1)-adrenergic receptor and angiotensin ii receptor subtypes during aging in rats. Can J Cardiol. 2009;25:415–420. doi: 10.1016/s0828-282x(09)70509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilliam-Davis S, Payne VS, Kasper SO, Tommasi EN, Robbins ME, Diz DI. Long-term AT1 receptor blockade improves metabolic function and provides renoprotection in Fischer-344 rats. Am J Physiol Heart Circ Physiol. 2007;293:H1327–1333. doi: 10.1152/ajpheart.00457.2007. [DOI] [PubMed] [Google Scholar]
- 21.Jones ES, Black MJ, Widdop RE. Angiotensin AT2 receptor contributes to cardiovascular remodelling of aged rats during chronic AT1 receptor blockade. J Mol Cell Cardiol. 2004;37:1023–1030. doi: 10.1016/j.yjmcc.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of Dahl hypertension. Hypertension. 2000;35:484–489. doi: 10.1161/01.hyp.35.1.484. [DOI] [PubMed] [Google Scholar]
- 23.Rogers JL, Mitchell AR, Maric C, Sandberg K, Myers A, Mulroney SE. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R794–799. doi: 10.1152/ajpregu.00424.2006. [DOI] [PubMed] [Google Scholar]
- 24.Nickenig G, Baumer AT, Grohe C, Kahlert S, Strehlow K, Rosenkranz S, Stablein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Bohm M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation. 1998;97:2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 25.Alexander BT, Cockrell KL, Rinewalt AN, Herrington JN, Granger JP. Enhanced renal expression of preproendothelin mRNA during chronic angiotensin ii hypertension. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1388–1392. doi: 10.1152/ajpregu.2001.280.5.R1388. [DOI] [PubMed] [Google Scholar]
- 26.Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension. 1992;19:456–463. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- 27.Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92:1326–1331. doi: 10.1161/01.cir.92.5.1326. [DOI] [PubMed] [Google Scholar]
- 28.Psaty BM, Heckbert SR, Koepsell TD, Siscovick DS, Raghunathan TE, Weiss NS, Rosendaal FR, Lemaitre RN, Smith NL, Wahl PW, Wagner EH, Furberg CD. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274:620–625. [PubMed] [Google Scholar]
- 29.Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest. 1995;96:793–800. doi: 10.1172/JCI118125. [DOI] [PMC free article] [PubMed] [Google Scholar]