Abstract
This study for the first time investigated resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder (BD) and compared them with findings in healthy controls and unipolar major depressive disorder (MDD) patient groups. Resting state correlations of low frequency BOLD fluctuations (LFBF) in echoplanar functional magnetic resonance (fMRI) data were acquired from a priori defined regions of interests (ROIs) in the pregenual anterior cingulate cortex (pgACC), dorsomedial thalamus (DMTHAL), pallidostriatum (PST) and amygdala (AMYG), to investigate corticolimbic functional connectivity in unmedicated BD patients in comparison to healthy subjects and MDD patients. Data were acquired from 11 unmedicated BD patients [six manic (BDM) and five depressed (BDD)], and compared with data available from 15 unmedicated MDD and 15 healthy subjects. BD patients had significantly decreased pgACC connectivity to the left and right DMTHAL, similar to findings seen in MDD. Additionally, BD patients had decreased pgACC connectivity with the left and right AMYG as well as the left PST. An exploratory analysis revealed that both BDD and BDM patients had decreased connectivity between the pgACC and DMTHAL. The results of the study indicate a common finding of decreased corticolimbic functional connectivity in different types of mood disorders.
Keywords: fMRI, Resting state connectivity, Bipolar disorder, Depression, Brain imaging
1. Introduction
Converging findings from animal and human studies point to the anterior cingulate–pallidostriatal–thalamic– amygdala circuit as a putative corticolimbic mood-regulating circuit (MRC) that may be dysfunctional in mood disorders (Drevets, 1998;Anand and Charney, 2000; Mayberg, 2003). Brain-imaging techniques such as functional magnetic resonance imaging (fMRI) and positron emission tomography have shown increased activation in major depressive disorder (MDD) of mood-generating limbic areas such as the amygdala (AMYG) (Ketter et al., 2001; Sheline et al., 2001; Drevets et al., 2002; Siegle et al., 2002; Anand et al 2005a), ventral striatum (VST), and dorsomedial thalamus (DMTHAL) (Drevets, 1998; Taber et al., 2004). Other areas of the brain that are also implicated are the insula, hippocampus and parahippocampal areas (Mayberg et al., 1999; Phillips et al., 2003; Anand et al., 2005a). Conversely, decreased activation of certain cortical areas have been reported in MDD — in particular, the pregenual and ventral subdivisions of the anterior cingulate cortex (pgACC and vACC) (Drevets et al., 1997; Mayberg et al., 1999), the anteromedial prefrontal cortex, the orbitofrontal cortex (OFC), and the dorsolateral prefrontal cortex (DLPFC) (Mayberg et al., 1999; Ketter et al., 2001).
Compared with the literature on MDD, considerably fewer studies have investigated regional brain activation in bipolar disorder (BD). In studies in which phase of illness has been characterized, most have been conducted in BD depression (BDD), which has also has been reported to be associated with increased limbic activation and decreased activation of cortical regions such as the DLPFC and ACC (Buchsbaum et al., 1986; Baxter et al., 1989; Drevets et al., 1997; Yurgelun-Todd et al., 2000; Ketter et al., 2001; Blumberg et al., 2003; Phillips et al., 2003; Chang et al., 2004). In mania (BDM), increased metabolism of the ventral and dorsal ACC, the striatum (Drevets et al., 1997; Blumberg et al., 2000), and the amygdala (Altshuler et al., 2005) [another study reported decreased amygdala activation (Lennox et al., 2004)], and decreased activity of the OFC (Blumberg et al., 1999), have been reported. Strakowski et al. (2004) have reported an abnormality in the anterior limbic network in BD in response to cognitive stimuli. Compared with healthy subjects, unmedicated euthymic BP patients showed increased activation in the limbic and paralimbic areas (parahippocampus, amygdala and insula) as well as ventral prefrontal regions when performing attentional tasks (Strakowski et al., 2004). Therefore, in BD, abnormalities within the prefrontal cortex, subcortical structures such as the striatum and thalamus, and medial temporal structures such as the amygdala and the parahippocampus, are likely to be present (Strakowski et al., 2005; Adler et al., 2006).
Methodological issues such as medication status and inadequate identification of the phase of illness may have contributed to discrepant results in some of the above studies. Unmedicated BD subjects are difficult to recruit for studies. Furthermore, the discrepant findings of changes in local activation also suggest that the abnormality may lie at a circuit level in terms of the corticolimbic connectivity rather than in localized brain regions.
Recently, there has been considerable interest generated from the discovery of spontaneous low frequency (<0.08 Hz) blood oxygen level-dependent (BOLD) fluctuations (LFBF) in resting state in echoplanar imaging (EPI) data (Raichle et al., 2001). It has been recognized that these LFBFs are not caused by instrumentation or physiological effects (such as cardiac and respiratory cycles) originating outside the brain (Biswal et al., 1995). It has also been shown that these resting state signal changes reflect alterations in blood flow and oxygenation that may be coupled to neuronal activity and that LFBFs correlate between brain areas of plausible functional connectivity (Biswal et al., 1995; Lowe et al., 2000; Cordes et al., 2001; Peltier and Noll, 2002; Hampson et al., 2002; Salvador et al., 2005). Published studies of connectivity abnormalities using the LFBF correlation method have been reported in neuropsychiatric conditions such as attention deficit hyperactivity disorder (ADHD) (Castellanos et al., 2008), schizophrenia (Liang et al., 2006; Garrity et al., 2007; Zhou et al., 2007), Alzheimer’s disease (Greicius et al., 2004), substance abuse (Li et al., 2000), multiple sclerosis (Lowe et al., 2002), and autism (Cherkassky et al., 2006).
We have previously reported the results of our study in which corticolimbic connectivity was measured using the resting state LFBF correlation method in unmedicated MDD patients and healthy subjects (Anand et al., 2005a). The results of this study indicated that resting state functional connectivity between the pgACC and the limbic regions – amygdala (AMYG), pallidostriatum (PST) and dorsomedial thalamus (DMTHAL) – is decreased in MDD (Anand et al., 2005a,b). In this study, we report, for the first time, corticolimbic connectivity abnormalities in unmedicated BD patients in both the manic and depressed phase of the illness and a comparison with corticolimbic connectivity abnormalities previously reported in MDD patients and healthy controls. Our unitary hypothesis was that mood dyregulation arises from decreased corticolimbic connectivity, and hence in BD (whether in the manic or the depressed phase) decreased connectivity will be seen similar to that seen in MDD.
2. Methods
2.1. Subjects
Medication-free unipolar depressed (MDD), bipolar depressed (BDD) and bipolar manic (BDM) outpatients were recruited from the outpatient clinic at University Hospital, Indiana University School of Medicine, and by advertisement, from the community. Closely matched healthy subjects were recruited through advertisements. All subjects took part in the study after signing an informed consent form approved by the Investigational Review Board (IRB) at Indiana University School of Medicine. Both patients and healthy control subjects were paid $50 for screening and $50 for each MRI scan. Inclusion criteria for MDD patients were as follows: age 18–60 years and ability to give voluntary informed consent; satisfy Diagnostic and Statistical Manual fourth edition (DSM-IV) criteria for Major Depressive Episode; have a 25-item Hamilton Depression Rating Scale (HDRS) (Thase et al., 1991) score > 18; satisfy criteria to undergo an MRI scan based on an MRI screening questionnaire; and be able to be managed as outpatients. The inclusion criterion for BD patients was that they satisfy DSM-IV criteria for Bipolar Disorder either in the hypomanic or manic (Young Mania rating Scale (Young et al., 1978) (YMRS) > 10) or depressed episode (HDRS > 18). Other inclusion criteria were the same as those for MDD patients. Exclusion criteria for patients were as follows: meeting DSM-IV criteria for schizophrenia, schizoaffective disorder, or an anxiety disorder as a primary diagnosis; use of psychotropic agents in the past 2 weeks; use of fluoxetine in the past 4 weeks; being acutely suicidal or homicidal or requiring inpatient treatment; meeting DSM-IV criteria for substance dependence within the past year, except caffeine or nicotine; positive urinary toxicology screening at baseline; use of alcohol in the past week; serious medical or neurological illness; current pregnancy or breast-feeding; metallic implants or other contraindications to MRI. Inclusion criteria for healthy subjects were as follows: ages 18–60 years and ability to give voluntary informed consent; no history of psychiatric illness or substance abuse or dependence; no significant family history of psychiatric or neurological illness; not currently taking any prescription or centrally acting medications; no use of alcohol in the past week; and no serious medical or neurological illness. Exclusion criteria for healthy subjects were as follows: under 18 years of age; pregnant or breast-feeding; metallic implants or other contraindication to MRI.
2.2. Behavioral ratings
Subjects were rated on the 25-item HDRS and the YMRS at the time of the baseline scan.
2.3. MRI data
Scans were performed in either the morning or the early afternoon.
2.3.1. Image acquisition
Imaging data were acquired using a General Electric (Waukesha, WI) 1.5 T MRI scanner. Subjects were placed in a birdcage head coil and individually fitted to a bite bar partially composed of dental impression compound attached to the coil to reduce head motion. For the resting state connectivity scan, the subjects were asked to keep their eyes closed, stay awake, and not think of anything in particular. The MRI sequence included a T1-weighted whole brain image using a Spoiled Gradient-Echo Recalled sequence (SPGR) sequence to provide real 1 × 1 × 1 mm3 spatial resolution. Next, a T1-weighted axial image, to identify slices for the various regions of interest (ROIs), was acquired with the following sequence: TR/TE 500/12 ms; 16 slices; Thickness/Gap 7.0/2.0 mm; matrix 256 × 128; FOV 24 × 24 cm; 1 NEX. The short TR limits the number of slices that can be acquired; therefore four noncontiguous axial slices were acquired that covered the areas of interest at the level of the pgACC, DMTHAL/PST and AMYG identified by a trained radiology staff member (YW) during the scan. Other scans for local brain activation in response to emotional stimuli and connectivity scans during steady state exposure to neutral, positive and negative pictures were also acquired during the fMRI session as previously described (Anand et al., 2005a); however, this report is mainly focused on the results of resting state connectivity. During the resting state, awake with eyes closed, subjects were asked to stay awake and think of nothing in particular. After the scan, the patients were interviewed, and it was assessed whether they complied with the instructions or were awake throughout the scan. Subjects who were judged not have complied with the instructions were excluded from the study.
2.4. Image analysis
The raw imaging data were Hamming-filtered to improve signal-to-noise ratio with minimal reduction in spatial resolution (Lowe and Sorenson, 1997). Motion was measured, but motion correction was not performed on the data as it can lead to increase in spatial correlation in LFBF data (Lowe et al., 1998). Moreover, with a limited number of slices motion correction is not reliable with the usual registration routines.
2.4.1. Selection of regions of interest (ROIs)
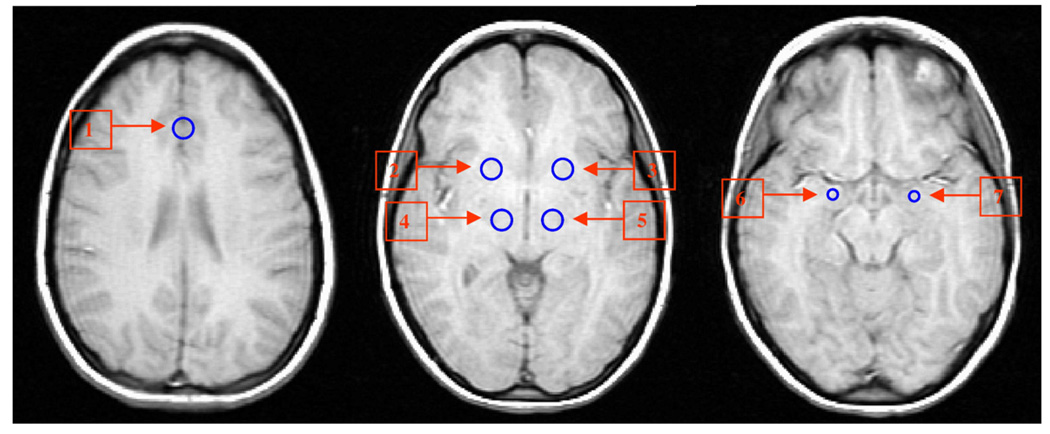
ROIs were placed by a trained radiology staff member (YW) corresponding to the a priori defined areas of the MRC (Fig. 1). The pregenual ACC (the area just anterior to the genu of the corpus callosum) (subregion of Brodmann area 24) was chosen as the reference ROI as a number of neurological studies have indicated that this area, as well as the more inferior subgenual ACC, is involved in the regulation of emotions (Damasio, 1997; Critchley, 2004), and activity in these areas has been shown to accompany reward-based emotional/motivational processing (Critchley, 2004). Another more caudal area of the subgenual ACC (Area 25) has also been described in imaging studies to be involved in emotion regulation (Mayberg et al., 2005). We chose to study the pgACC because the signal there was less likely to be corrupted by susceptibility artifacts than the more ventral subgenual ACC or Area 25. The pallidostriatal ROI was defined as reported by Burruss (2000) and partially covered putamen and lateral palladium. The DMTHAL ROI was centered in the medial dorsal posterior part of the thalamus. The AMYG ROI was centered on the AMYG based on anatomical landmarks for that region. The “draw dataset” function in Analysis of Functional Neuroimages (AFNI) software was used to define ROIs as fixed size circles with a radius of 6 mm for the ACC, DMTHAL and PST and 4 mm for the AMYG (Fig. 1). The radiologist was not aware of the group status while placing ROIs. As non-contiguous EPI slices were selected, corresponding to the axial high-resolution T1 images that covered ROIs, the distance between the four EPI slices varied according to individual anatomy and position. ROI time series were averaged using the AFNI function “3dmaskave.” This function produces the time-wise arithmetic average for each voxel in an ROI mask. The result is an average time series for each ROI.
Fig. 1.
Region of interest (ROI) placement for sampling of low frequency BOLD fluctuations (LFBF) for corticolimbic connectivity analysis. 1. Pregenual anterior cingulate cortex (pgACC); 2, 3: pallidostriatum (PST); 4, 5: dorsomedial thalamus (DMTHAL); 6, 7: amygdala (AMYG).
2.4.2. LFBF correlation analysis
The analysis was done as previously described (Anand et al., 2005a). Briefly, the first 50 scans were discarded to allow MR signal to reach steady state and the next 512 time points were included in the analysis. Averaged data from all the voxels within each ROI (as defined above) were detrended for global signal drifts using previously described methods (Lowe and Russell, 1999) and then passed through a finite-impulse response (FIR) filter to remove all frequencies above 0.08 Hz. This procedure removes the oxygenation fluctuations from physiological processes such as direct sampling of respiratory and cardiac-related oxygenation fluctuations (Lowe et al., 1998; Cordes et al., 2001). Next, the Pearson correlation coefficient (cc) was calculated between the averaged LFBF time series of pgACC as the reference region with the averaged time series of each of the limbic ROIs across all time points (512 time points) (Lowe et al., 1998). The correlation coefficient was then transformed to a t statistic (Lowe et al., 1998; Anand et al., 2005a) to enable comparison between groups. This t-score was used as the measure of corticolimbic functional connectivity.
2.5. Statistical analysis
One-way analysis of variance (ANOVA) was performed to evaluate the effect of diagnostic groups on pgACC connectivity with the DMTHAL, PST and AMYG on each side. For significant ANOVA results, post hoc analysis was performed using Fisher's protected least square difference (Fisher's PLSD).
3. Results
Twelve unmedicated bipolar subjects in either the manic (BDM) or the depressed (BDD) phase completed the study. One BDD subject’s imaging data were discarded because of technical difficulties during the acquisition of the scan. The results of the remaining 11 BD subjects (6 BDM, 5 BDD) were then compared with those of 15 unipolar depressed (MDD) subjects and 15 healthy subjects who we had previously studied using the same paradigm (Anand et al., 2005a). One BDM patient, after inclusion in the study, reported taking a small dose of gabapentin for chronic leg pain. The dose of gabapentin was very small, and it was not being taken for mood stabilization. Exclusion of this patient’s data did not change the results of the study. Therefore, it was decided not to exclude this patient. Table 1 presents the demographics and illness characteristics of each group. The BD group was slightly older (age: 33±12 years) than the MDD group (29±9 years), but the difference was not significant. A bite bar customized for each individual was used to minimize the effect of motion. Mean displacement over surface (Jiang et al., 1995), calculated to assess for motion effects, was 0.33±0.19 mm, 0.20±0.06 mm and 0.18±0.10 mm, respectively, in the BD, MDD and healthy subjects groups.
Table 1.
Demographic and clinical characteristics of subjects
| Bipolar (BP) (N=11) | Bipolar manic (BPM) (N=6) |
Bipolar depressed (BPD) (N=5) |
Unipolar depressed (MDD) (N=15) |
Healthy controls (N=15) |
|
|---|---|---|---|---|---|
| Age | 33±12 | 34±14 | 32±9 | 29±9 | 28±7 |
| Gender | 7 female 4 male | 4 female, 2 male | 3 female, 2 male | 11 female, 4 male | 11 female, 4 male |
| Ethnicity | 9 Caucasian, 2 African American | 5 Caucasian, 1 African American | 4 Caucasian, 1 African American | 14 Caucasian, 1 African American | 14 Caucasian, 1 African American |
| YMRS on day of scan | 13±8 (range: 1–25) | 18±4 (range: 15–25) | 6±7 (1–19) | NA | 0 |
| 25-item HDRS score on day of scan | 18±13 (3–34) | 8±6 (3–16) | 29±6 (21–34) | 31±8 (19–46) | 0 |
| Number of previous mood episodes | 18±5 | 18±6 | 18±4 | 3±2 | NA |
| Type | Bipolar I: 10; Bipolar II: 1 | Bipolar I: 5; Bipolar II: 1 | All Bipolar I | NA | NA |
| Duration of illness (years) | 14±10 | 14±11 years | 14±10 years | 6±7 years | NA |
| Drug free period (weeks) | Treatment Naïve: 1 Rest: 95±218 (range 2–676 weeks) | Treatment Naïve: 1 patient, 1 taking gabapentin | 21±21 (range 2–52 weeks) | Treatment Naïve: 8 patients | NA |
| Rest of the patients: 189±324 (range 24–676) | Rest of the patients: 24±33 weeks (Range 2–24 weeks) |
HDRS: Hamilton Depression Rating Scale. YMRS: Young Mania Rating Scale. Unless otherwise indicated, data are expressed as mean±S.D.
3.1. Resting state connectivity in bipolar disorder compared with unipolar depression and healthy subjects
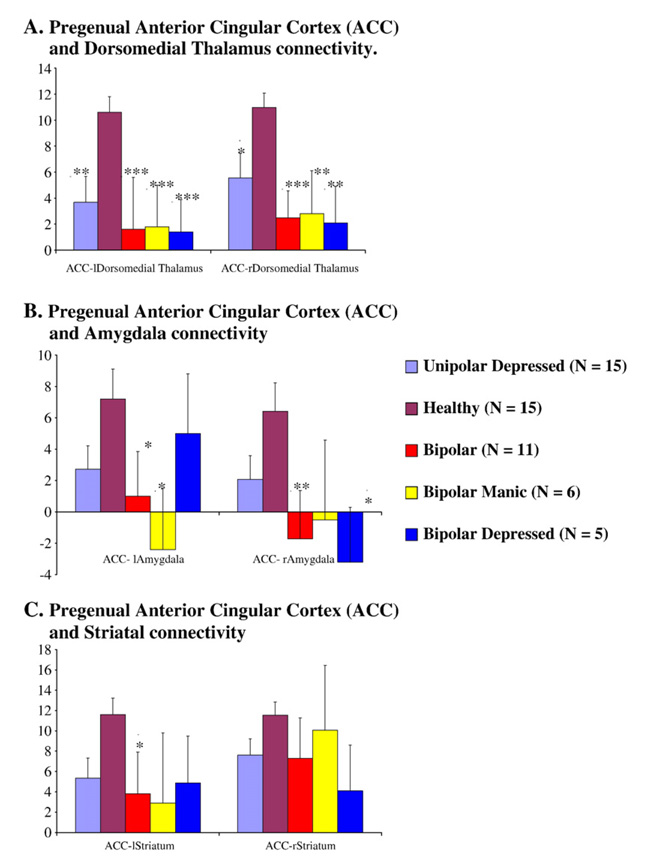
Both the unipolar depressed group and the bipolar group had decreased connectivity, compared with healthy subjects, between the pgACC and the AMYG, THAL and PST on each side (Fig. 2). The ANOVA for differences between the three groups was significant for the right AMYG (F=3.74, df=2, P<0.04) and for the left DMTHAL (F=7.27, df=2, P<0.003) and the right DMTHAL (F=6.270, df=2, P<0.005), and there was a trend for significance for the left AMYG and the left PST. Post hoc tests of significance revealed that the difference was significant between BD as a group and healthy subjects for the pgACC and for the left (P<0.05) and right AMYG (P<0.01) as well as the left PST (P<0.04) connectivity. Both the BD and the MDD groups had decreased connectivity between the pgACC and the left DMTHAL (BD: P<0.005; MDD: P<0.01) and right DMTHAL (BD: P<0.005; MDD: P<0.05) as well as the left PST (BD: P<0.04; MDD: P<0.07). No significant differences were seen between BD and MDD subjects.
Fig. 2.
Cortiolimbic connectivity in major depression (N=15), healthy controls (N=15), bipolar disorder (N=11), bipolar mania (N=6), and bipolar depression (N=5). Post hoc t-tests results for significant differences with each of the mood disorder groups and healthy subjects as described in the text are denoted by *P<0.05, **P<0.01 and ***P<0.005. A. Pregenual anterior cingular cortex (ACC) and dorsomedial thalamus connectivity. B. Pregenual anterior cingular cortex (ACC) and amygdala connectivity. C. Pregenual anterior cingular cortex (ACC) and striatal connectivity.
3.2. Resting state connectivity in bipolar depression and bipolar mania compared to healthy subjects
An exploratory analysis, keeping in mind the small number of subjects in each BD subgroup, was conducted. Both BDD and BDM patients exhibited decreased corticolimbic connectivity compared with healthy subjects. One-way ANOVA for differences between the groups was significant for the left DMTHAL (F=5, df=3, P<0.005) and for the right DMTHAL (F=4, df=3, P<0.005), and there was a trend for significance for the right and left AMYG (P<0.07). A post hoc t-test revealed a significant decrease for left DMTHAL connectivity between BDD and healthy controls (P<0.005) and BDM and healthy controls (P<0.005), for the right DMTHAL between BDD and healthy controls (P<0.01) and BDM and healthy controls (P<0.01). Additionally, significant differences were found for pgACC connectivity to the right AMYG between BDD patients and healthy controls (P<0.05), and for the left AMYG for BDM patients and healthy controls (P<0.05).
4. Discussion
The findings of this study indicated decreased corticolimbic connectivity in BD patients compared with healthy subjects, similar to results previously reported for MDD; however, the abnormalities seemed to be more severe in the BD group. This is not a surprising finding as BD is a more severe illness of mood regulation than MDD. The BD subgroup also had a longer duration of illness and had had more mood episodes than the MDD group, and it was slightly older than the MDD and healthy control groups. The greater severity of mood disorder in the BD group could also explain the greater decrease in connectivity in this group. Motion was slightly greater in the bipolar group, but when used as a covariate in the analysis, it did not change the findings of the study.
An exploratory analysis was done (keeping in mind the small number of patients in each subgroup of BD) and showed that the BDD and the BDM subgroups had similar decreases in corticolimbic connectivity compared with healthy subjects. Some differences were noted, e.g. the decreased pgACC-left AMYG connectivity only in BDM and not in BDD, and the decreased pgACC-right AMYG connectivity in BDD, which will need to investigated in future studies with a larger number of subjects.
The decreased corticolimbic LFBF correlations results indicate possible decreased phase coherence between LFBF sampled in the ACC and the limbic regions in BD and MDD patients. Phase synchrony has been related to the integrity of the circuits between two brain regions (Spencer et al., 2004). Single neuron studies with intraneuronal electrodes and, to some extent, electroencephalograhic studies have shown that if two brain regions are locked in phase with each other, their functioning is closely connected (Varela et al., 2001). Hence, decreased phase coherence could be associated with a decreased regulatory effect of the ACC over the limbic areas leading to mood dysregulation in bipolar and unipolar depression as well as mania.
The decreased corticolimbic connectivity seen in mood disorders across diagnosis and phase of illness suggests that the decreased connectivity may be a trait abnormality. However, in a previous study (Anand et al., 2005b), we found that antidepressant treatment leads to an increase in corticolimbic connectivity in MDD patients, and therefore the connectivity abnormality may be state-dependent. To investigate whether the decreased connectivity is state- or trait-dependent, these findings will need to be investigated in BD before and after treatment and also in unmedicated euthymic BD and MDD patients.
The ROIs were placed within the corticolimbic system based on a priori identified and agreed upon anatomical landmarks as discussed in Section 2.4. The EPI slices that were selected on matching high-resolution T1 images were not contiguous but included only slices chosen by the radiologist to cover the ROIs. Therefore, the location of the EPI slices, the distance between the four EPI slices, and the placement of the ROIs did vary slightly from subject to subject due to differences in subjects’ anatomy and head position. To place ROIs in exactly the same location for all subjects, it would have been necessary to normalize the data into a standardized space and therefore have considerably larger slice coverage. This was not possible without a very significant increase of the TR. As discussed in Section 2, the data were acquired with a short TR to avoid aliasing effects of fluctuations in the BOLD signals due to cardiac and respiratory cycles. In future studies, to acquire data from the whole brain, methods such as recording of cardiac and respiratory cycles along with fMRI acquisition with subsequent retrospective correction for effects of these physiological variables on the BOLD signal could be used (Glover et al., 2000).
The correlation of LFBF between two areas is a measure of functional connectivity, i.e. that the two are in synchrony (Friston et al., 1993). However, this could also occur due to the influence of a third factor that may be simultaneously affecting both the areas. In the future, to measure the direct effect of one area over another, i.e. to measure effective connectivity, techniques such as structural equation modeling (SEM) (Seminowicz et al., 2004) or newer techniques such as dynamic causal modeling (DCM) (Friston et al., 2003) could be used. An investigation of structural connectivity using diffusion tensor imaging (DTI) could also shed light on the relationship between functional and structural connectivity.
The analysis performed here is a straightforward hypothesis-driven analysis based on an a priori expectation of involved regions of the brain. The a priori defined ROI approach has the advantage of reducing the magnitude of correction needed for a large number of voxels; one can correct only for a small number of ROIs, thereby considerably increasing statistical power (Poldrack, 2007). The same analysis was performed on controls and patients, and statistically meaningful conclusions were drawn. Connectivities with other regions were not investigated, and no conclusions were drawn regarding regions that were not examined.
Out of the three a priori identified limbic structures whose connectivity with the pgACC was investigated in this study, the connectivity of the pgACC-DMTHAL was present in all mood disorders (Fig. 2). This is not surprising as the thalamus is an integral part of the cingulate–pallidostriatal–thalamic–amygdala mood-regulating circuit (Taber et al., 2004). The DMTHAL has major connections with the ACC, the ventral PST, and the AMYG, and therefore it is central to the circuit (Taber et al., 2004). Decreases in pgACC connectivity were also seen for the AMYG. The AMYG is located in the more ventral part of the brain, and the BOLD signal from the AMYG has a lower signal-to-noise ratio due to susceptibility artifacts. Therefore, the variance for the data was greater in this region. In future studies, more sophisticated techniques using advanced hardware and techniques such as z-shimming to reduce susceptibility artifacts could be used to image the ventral areas of the brain (Glover, 1999).
Another limitation of this study was that we did not measure differences in gray matter density within ROIs between groups due to the small number of subjects studied. It is possible that the ROIs may have contained more or less gray matter in the different groups, leading to partial-volume effects that could have affected the results. In future studies, measurement of gray matter density using techniques such as voxel-based morphometry (Ashburner and Friston, 2000) could be used to investigate differences in gray matter density between groups.
The duration of medication-free period for psychiatric studies is always a compromise between what is ideal and what is clinically feasible. We chose a minimum period of 2 weeks for patients to be off medication (except for fluoxetine, for which we required a 4-week drug-free period) and inclusion criteria for no substance dependence in the past year and a negative urine drug screen at the time of screening for the study. However, long-term effects of psychotropic agents may still be present. Future studies will need to be conducted to address this issue with a larger number of subjects with longer medication-free and substance-free periods before the study.
The findings of this study are consistent with a common abnormality of corticolimbic functional connectivity in bipolar disorder and depression, and they need to be confirmed with a larger number of patients and with more sophisticated techniques to measure functional connectivity within the brain.
Footnotes
The results of this study have been previously presented at the Society of Biological Psychiatry meeting in San Diego (2007).
References
- Adler CM, DelBello MP, Strakowski SM. Brain network dysfunction in bipolar disorder. CNS Spectrums. 2006;11:312–320. doi: 10.1017/s1092852900020800. [DOI] [PubMed] [Google Scholar]
- Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A, Bartzokis G, Mintz J, Mazziotta J, Cohen MS. Increased amygdala activation during mania: a functional magnetic resonance imaging study. American Journal of Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- Anand A, Charney DS. Abnormalities in catecholamines and pathophysiology of bipolar disorder. In: Soares JC, Gershon S, editors. Bipolar Disorder: Basic Mechanisms and Therapeutic Implications. New York: Marcel Dekker; 2000. pp. 59–94. [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Kalnin A, Mathews VP, Lowe MJ. Activity and connectivity of mood regulating circuit in depression: a functional magnetic resonance study. Biological Psychiatry. 2005a;15:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu JW, Gao SJ, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Antidepressant effect on connectivity of the mood-regulating circuit: an fMRI study. Neuropsychopharmacology. 2005b;30:1334–1344. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry — the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Archives of General Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Stern E, Ricketts S, Martinez D, de Asis J, White T, Epstein J, Isenberg N, McBride PA, Kemperman I, Emmerich S, Dhawan V, Eidelberg D, Kocsis JH, Silbersweig DA. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. American Journal of Psychiatry. 1999;156:1986–1988. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Stern E, Martinez D, Ricketts S, de Asis J, White T, Epstein J, McBride PA, Eidelberg D, Kocsis JH, Silbersweig DA. Increased anterior cingulate and caudate activity in bipolar mania. Biological Psychiatry. 2000;48:1045–1052. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, Fulbright RK, Gore JC, Charney DS, Krystal JH, Peterson BS. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. American Journal of Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Wu J, DeLisi LE, Holcomb H, Kessler R, Johnson J, King AC, Hazlett E, Langston K, Post RM. Frontal cortex and basal ganglia metabolic rates assessed by positron emission tomography with [18F]2-deoxyglucose in affective illness. Journal of Affective Disorders. 1986;10:137–152. doi: 10.1016/0165-0327(86)90036-4. [DOI] [PubMed] [Google Scholar]
- Burruss JW. Functional neuroanatomy of the frontal lobe circuits. Radiology. 2000;214:227–230. doi: 10.1148/radiology.214.1.r00ja43227. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJS, Rotrosen J, Adler LA, Milham MP. Cingulate–precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal–subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Archives of General Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR: American Journal of Neuroradiology. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. The human cortex responds to an interoceptive challenge. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6333–6334. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Towards a neuropathology of emotion and mood. Nature. 1997;386:769–770. doi: 10.1038/386769a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annual Review of Medicine. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacology, Biochemistry & Behavior. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. Journal of Cerebral Blood Flow & Metabolism. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. American Journal of Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [Erratum appears in American Journal of Psychiatry 164(7), 1123, 2007] [DOI] [PubMed] [Google Scholar]
- Glover GH. 3D z-shim method for reduction of susceptibility effects in BOLD fMRI. Magnetic Resonance in Medicine. 1999;42:290–299. doi: 10.1002/(sici)1522-2594(199908)42:2<290::aid-mrm11>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101 doi: 10.1073/pnas.0308627101. 4637-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Human Brain Mapping. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A, Kennedy DN, Baker JR, Weisskoff RM, Tootell RBH, Woods RP, Benson RR, Kwong KK, Brady TJ, Rosen BR, Belliveau JW. Motion detection and correction in functional MR imaging. Human Brain Mapping. 1995;3:224–235. [Google Scholar]
- Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, Benson BE, Willis MW, Danielson A, Frye MA, Herscovitch P, Post RM. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biological Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- Lennox BR, Jacob R, Calder AJ, Lupson V, Bullmore ET. Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychological Medicine. 2004;34:795–802. doi: 10.1017/s0033291704002557. [DOI] [PubMed] [Google Scholar]
- Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, Salmeron BJ, Stein EA. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magnetic Resonance in Medicine. 2000;43:45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Dzemidzic M, Lurito JT, Mathews VP, Phillips MD. Correlations in low-frequency BOLD fluctuations reflect cortico-cortical connections. Neuroimage. 2000;12:582–587. doi: 10.1006/nimg.2000.0654. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, Mathews VP. Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology. 2002;224:184–192. doi: 10.1148/radiol.2241011005. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Russell DP. Treatment of baseline drifts in fMRI time series analysis. Journal of Computer Assisted Tomography. 1999;23:463–473. doi: 10.1097/00004728-199905000-00025. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Sorenson JA. Spatially filtering functional magnetic resonance imaging data. Magnetic Resonance in Medicine. 1997;37:723–729. doi: 10.1002/mrm.1910370514. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic–cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic–cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Peltier SJ, Noll DC. T(2)(*) dependence of low frequency functional connectivity. Neuroimage. 2002;16:985–992. doi: 10.1006/nimg.2002.1141. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Regions of interest analysis for fMRI. SCAN. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman MR, Pickard JD, Menon DK, Bullmore ET. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereberal Cortex. 2005;15(9):1332–1342. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. Limbic–frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP. A preliminary FMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29:1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Molecular Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Taber KH, Wen C, Khan A, Hurley RA. The limbic thalamus. Journal of Neuropsychiatry & Clinical Neurosciences. 2004;16:127–132. doi: 10.1176/jnp.16.2.127. [DOI] [PubMed] [Google Scholar]
- Thase ME, Carpenter L, Kupfer DJ, Frank E. Clinical significance of reversed vegetative subtypes of recurrent major depression. Psychopharmacology Bulletin. 1991;27:17–22. [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nature Reviews Neuroscience. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Young RC, Schreiber MT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disorders. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Liu H, Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neuroscience Letters. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]