Abstract
Rationale: Cigarette smoke (CS) is the leading cause of chronic obstructive pulmonary disease, accounting for more than 90% of cases. The prevalence of chronic obstructive pulmonary disease is much higher in the elderly, suggesting an age dependency. A prominent defense against the oxidant burden caused by CS is the glutathione (GSH) adaptive response in the lung epithelial lining fluid (ELF) and tissue. However, as one ages the ability to maintain GSH levels declines.
Objectives: Examine the effect of aging on the GSH adaptive response to CS and resulting lung sensitization to inflammation and oxidation.
Methods: Both young (2 mo old) and aged (8, 13, 19, and 26 mo old) mice were used to study the effects of age on the GSH adaptive response after an acute exposure to CS.
Measurements and Main Results: Young mice had a robust sixfold increase in ELF GSH after a single exposure to CS. The GSH response to CS decreased as a function of age and diminishes in the older mice to only a twofold increase over air controls. As a consequence, levels of CS-induced tumor necrosis factor-α and nitric oxide synthase, markers of inflammation, and 8-hydroxy-2-deoxyguanosine, a marker of DNA oxidation, were elevated in the aged mice compared with the young mice. Additionally, depletion of ELF GSH with buthionine sulfoximine in young mice recapitulated changes in ELF tumor necrosis factor-α as seen in old mice.
Conclusions: These data suggest that the age-related maladaptive response to CS sensitizes the lung to both inflammation and oxidation potentially contributing to the development of CS-induced chronic obstructive pulmonary disease.
Keywords: smoking, antioxidants, inflammation, oxidation
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
The effects of cigarette smoke in causing lung diseases including chronic obstructive pulmonary disease and emphysema are well known, yet do not manifest until later in life, which suggests an inherent adaptive mechanism of the lung. Although cigarette smoke is known to induce antioxidant systems including glutathione, the same antioxidant systems have been shown to decrease with age.
What This Study Adds to the Field
This study shows that age decreases glutathione and the glutathione adaptive response to cigarette smoke resulting in an exacerbation of inflammation and oxidation in the lung. Our results also show that a previously overlooked age factor may have a larger role in contributing to the sensitivity to cigarette smoke.
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the United States, accounting for more than 110,000 deaths per year (1). COPD encompasses both chronic bronchitis and emphysema and is characterized by airway narrowing, air trapping, alveolar destruction, and excessive airway inflammation and oxidative stress. Cigarette smoke (CS) is the most common cause of COPD but many of the other contributing factors leading to the pathogenesis of the disease are poorly understood. The prevalence of COPD is reported to be nearly three times higher in individuals over the age of 60 and it has been hypothesized that COPD is actually an accelerated aging phenotype caused primarily by the inhaled oxidants in CS (2, 3). Many of the morphologic changes seen with COPD are also present in the aging lung, independent of smoking (4). There is ample evidence of an age-dependant increase in the prevalence of COPD and there is also supporting evidence that age contributes to the pathogenesis of the disease (5, 6).
In both aging alone and COPD there are marked increases in both inflammation and oxidative stress (7, 8). Increases in proinflammatory cytokines including IL-6, INF-γ, and tumor necrosis factor (TNF)-α in the airways of older humans and rodents have been previously observed (9). In addition, increases in the oxidation products 4-hydroxynonenal, protein carbonyls, and 8-hydroxy-2-deoxyguanosine (8OHdG) have been associated with both aging and COPD (10–12). It is well known that cellular antioxidants can prevent many of the oxidation end products but recent research suggests that they may also play a role in modulating inflammation (13–15).
Glutathione (GSH) is a major low-molecular-weight antioxidant thiol and is of particular importance in the lung because it is concentrated in the epithelial lining fluid (ELF) between 10 and 100 times more than in the plasma (16). Healthy smokers have been shown to have about two to three times more GSH in the airways than nonsmokers; conversely, these GSH levels decline in both aging and COPD (17). GSH has also been shown to be important in modulating cytokine release from cells in response to proinflammatory stimuli (18). GSH levels can influence both inflammation and oxidative stress, two major contributing factors to COPD. The lung's ability to produce a GSH adaptive response to environmental oxidants, like CS, may be a key factor in minimizing inflammation and oxidative damage to the lung. Yet, during the aging process normal levels of GSH are not maintained, potentially contributing to the pathogenesis of COPD.
Currently, it is unclear whether aging affects the lung's ability to increase GSH in response to an oxidative insult, such as CS. Therefore in the present study young mice (2 mo old) and aged mice (8, 13, 19, and 26 mo old) were exposed to either air or acute CS and the GSH adaptive response in the airways and tissue was examined. In addition, the ability of aged mice to induce common antioxidant enzymes in response to CS and the resulting inflammation and oxidative stress was assessed. These studies demonstrate a profound age-associated disruption in the lung GSH adaptive response to CS, which may be a contributing factor in chronic CS-induced lung disease. Some of the results of these studies have previously been presented as an abstract (19).
METHODS
Animals and CS Exposure
Both male and female C57B/6 mice aged 2, 8, 13, 19, or 26 months were used; there were no differences in GSH between males and females with either air or CS exposure (data not shown). Young mice were obtained from Jackson's laboratory and aged mice were either obtained from the National Jewish Health animal colony or the National Institute on Aging colony, and there was no difference in basal GSH levels between the two sources of mice (data not shown). The mice were exposed to smoke from Kentucky reference cigarette 3R4F (University of Kentucky) for 5 hours per day for 1 or 5 days using a Teague TE-10 smoking system (Teague Enterprises, Woodland, CA) (20). The average particulate matter was 100 mg/m3 and carbon monoxide levels were less than 300 ppm. Mice were anesthetized and sacrificed 16 hours after the CS exposure by cardiac exsanguination and heparinized blood was collected. Bronchoalveolar lavage (BAL) was performed using two 750-μl rinses of cold isotonic potassium phosphate buffer. The two rinses were pooled and the resulting bronchoalveolar lavage fluid (BALF) was stored on ice until analysis. BAL cells were separated by centrifugation and the resulting cell-free BALF was aliquoted for GSH analysis. Cytospin (Shandon, Pittsburgh, PA) slides were prepared from the resulting BAL cell pellet. Lung tissue was harvested after vascular perfusion, snap frozen in liquid nitrogen, and stored at −80°C until analyzed. All animal experiments were approved by the National Jewish Health animal care and use committee.
Treatment with l-Buthionine-Sulfoximine
Mice were administered l-buthionine-sulfoximine (BSO) (Sigma, St. Louis, MO) in the drinking water at a final concentration of 20 mM for a total of 11 days. Based on previous reports, the depletion of GSH by 20 mM BSO has been shown to stabilize within 7 to 10 days and be associated with lowering GSH in the lavage fluid to roughly half of the control levels (21). Additionally, BSO has been shown to be stable in drinking water for at least 14 days without significant loss (22).
Measurement of GSH
Both total (GSH) and oxidized (GSSG) GSH were measured spectrophotometrically in the BALF, plasma, and lung tissues as previously described (23, 24). GSH was measured by adding the standard or sample to 100 μl of a 1:1 mixture of 3 units/ml GSH reductase (GR) with 0.67 mg/ml 5,5′-Dithiobis (2-nitrobenzoic acid). The reaction was initiated by the addition of 20 μl of 0.67 mg/ml NADP reduced and the increase in absorbance at 450 nm was monitored. For GSSG measurements the samples were incubated with 4-vinyl pyridine (Sigma Aldrich, St. Louis, MO) for 1 hour to conjugate any reduced GSH before analysis, GSSG samples were analyzed the same way as GSH samples. Values measured in BALF are normalized to urea and GSH values in tissues are normalized to protein content. The limits of detection were 0.2 μM for both GSH and GSSG.
Determination of ELF Dilution Factor Using Urea
Urea is freely permeable between the plasma and ELF making it a commonly used marker for the dilution of the ELF from the BALF (25). Urea was measured in both the BALF and plasma using a blood-urea-nitrogen reagent kit (Teco Diagnostics, Anaheim, CA). The blood-urea-nitrogen reagent was added to standards or samples and monitored at 340 nm for 10 minutes using a SpectraMax 340PC microplate reader (Molecular Devices, Downington, PA). The plasma urea values are divided by the BALF urea values to give the ELF dilution factor, which is then multiplied by the BALF GSH values to obtain the normalized ELF GSH levels. There was no statistical difference in dilution values between groups.
Western Blotting for Protein Expression
Frozen lung tissue (∼25 mg) was homogenized in phosphate-buffered saline (PBS) containing protease inhibitors (Roche, Tucson, AZ) and large debris was removed by centrifugation. A sample containing 35 μg total protein was run on 7.5% acrylamide gel and transferred to a polyvinylidene fluoride membrane. Membranes were blocked with 5% bovine serum albumin and probed with primary antibodies for γ-glutamylcysteine ligase (GCL) at 1:2,500, GR at 1:1,000, or glyceraldehydes phosphate dehydrogenase 1:5,000 (Abcam, Cambridge, MA) for 2.5 hours at room temperature. The blots were then washed and probed with the secondary peroxidase conjugated goat antimouse antibody (Abcam) at a dilution of 1:45,000 for 30 minutes at room temperature. Proteins were visualized using ECL plus (GE Healthcare, Pittsburgh, PA) and band density was quantified using NIH ImageJ software.
Measurement of Inflammation in the BALF
TNF-α was used as a marker of inflammation in the BALF. TNF-α was quantified using a mouse TNF-α ELISA kit (BD Biosciences, Rockville, MD) based on known standard amounts of TNF-α, with the lowest standard at 7 pg/ml. The capture antibody was diluted 1:250 in coating buffer (200 mM sodium phosphate, pH 6.5) and coated on 96-well plates overnight at 4°C. The plate was then washed using wash buffer (PBS containing 0.05% Tween20) and blocked using assay diluent buffer (PBS containing 10% fetal bovine serum, pH 7.0) for 1 hour at room temperature. After washing, standard or sample was added in duplicate and incubated at room temperature for 2 hours. The plate was then washed before incubation with the detection antibody and streptavidin conjugated horseradish peroxidase diluted 1:250 for 1 hour at room temperature. A substrate solution consisting of tetramethylbenzidine and H2O2 mixed 1:1 (BD Biosciences) was added for 30 minutes, after which the tetramethylbenzidine stop solution (BioFX Laboratories, Owings Mills, MD) was added. The plate was read at 450 nm with correction at 570 nm using a SpectraMax 340PC microplate reader (Molecular Devices).
Analysis of DNA Oxidation in the Lung
To examine the effect of both age and smoke on oxidation in the lung, 8OHdG was measured. DNA from 25 mg lung tissue was extracted using DNeasy tissue kit (Qiagen, Valencia, CA). DNA concentration and purity was measured using a Nanodrop 1000 spectrophotometer (Thermo Fisher, Waltham, MA). The sample DNA was then subjected to enzymatic digested and analyzed for 8OHdG and 2-deoxyguanosine, respectively, by HPLC as previously described (23). Concentrations were determined based on known standards and expressed as a ratio of 8OHdG/105 2-deoxyguanosine.
Immunocytochemistry of Activated BAL Macrophages
Cytospin slides were air dried then methanol fixed and stored until stained. The slides were blocked with 5% bovine serum albumin for 1 hour and then again with a biotin/avidin blocking solution (Vector Laboratories, Burlingame, CA) for 15 minutes each. The cells were double stained with the macrophage marker F4/80 (Invitrogen, Carlsbad, CA) conjugated to biotin diluted at 1:100 in PBS and nitric oxide synthase (NOS2) (BD Biosciences) diluted to 1:100 in PBS incubated for 2.5 hours each. Goat biotin conjugated secondary (Vector Laboratories) was used at a dilution of 1:250 for NOS2 and incubated for 30 minutes. Fluorescein or AMCA conjugated avidin (Vector Laboratories) was used to detect bound antibodies with a Leica DM4000B fluorescent microscope fitted with a SPOT RTke digital camera (Diagnostic Instruments, Sterling Heights, MI) at ×20 magnification. Control slides using nonspecific IgG or lacking a primary antibody were used to assess background fluorescence. Three fields per slide were captured and the NOS2 fluorescence was quantified using Image J (National Institutes of Health) and normalized to the number of cells per field. The three fields were averaged per animal, with an n = 4–6 animals per group.
Statistical Analysis
All data are represented as the mean ± SEM and includes an n = 4–6. Significance was set at a P less than 0.05. Correlations were performed using a Spearman test and other data sets used an unpaired t test or a two-way analysis of variance with a Bonferroni posttest using Prism 5 software (GraphPad, La Jolla, CA).
RESULTS
Age-Dependant Decrease in Basal ELF GSH Levels
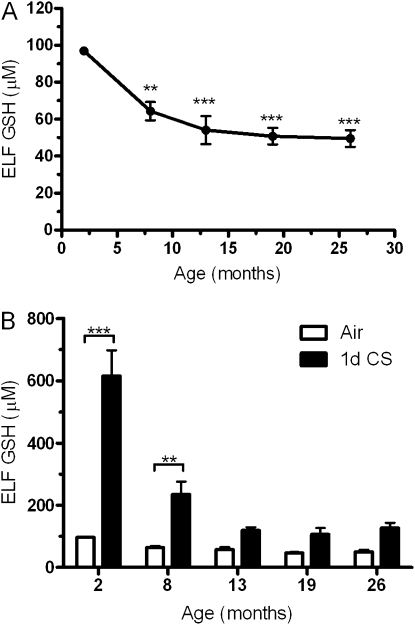
BAL fluid from mice aged 2, 8, 13, 19, and 26 months were collected and analyzed for GSH to assess whether there are changes in the ELF GSH content with age alone (Figure 1A). The basal level of GSH declines to roughly 65% of the young mice beginning at 8 months, whereas by 13 months and out to 26 months, the age-related decline becomes much more gradual remaining at roughly 50% of the young controls.
Figure 1.
Age decreases both basal and the adaptive epithelial lining fluid (ELF) glutathione (GSH) levels. (A) Basal levels of GSH in the ELF were measured in mice 2, 8, 13, 19, or 26 months of age. All age groups have significantly reduced ELF GSH compared with the 2-month-old mice. (B) Both young and aged mice were exposed to air (open bars) or cigarette smoke (closed bars) for 1 day and the GSH adaptive response was examined. Data represented as mean ± SEM with **P < 0.01, and ***P < 0.001; n = 4–6 compared with control.
The GSH Adaptive Response Is Diminished in Aged Mice
An acute 1-day CS exposure was performed because the initial adaptive response is first established at this point, which is then simply maintained over chronic exposures. Because lower levels of GSH found in aged mice may make them more susceptible to inhaled oxidants, we sought to investigate the ability to establish an ELF GSH adaptive response to acute CS. Both young and aged mice were exposed to CS for 1 day and BAL fluid was collected 16 hours after the exposure. Young mice had a typical robust GSH adaptive response to the CS with levels reaching roughly six times the air controls, whereas the aged mice had a significantly lower adaptive response (Figure 1B). Beginning at 8 months of age the CS-induced adaptive response is diminished with GSH levels only reaching roughly 230 μM compared with 600 μM in the young mice. At 13 months and out to 26 months of age the CS-induced adaptive response is significantly defective with ELF GSH levels in all three ages reaching only 120 μM. There was a significant interaction (P < 0.001) between both age and CS. Because of both the depletion of the GSH pool and the dilution of the ELF by BAL, the ELF GSSG levels were not detectable in the aged mice.
Lung Tissue GSH Is Decreased with Age
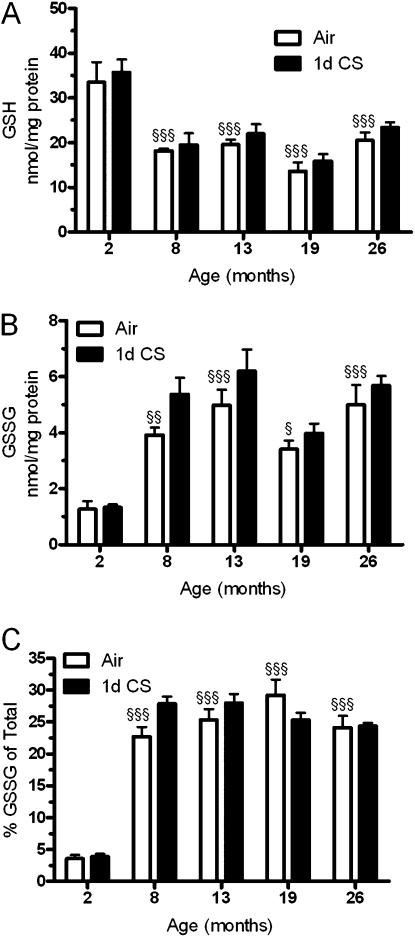
Many of the components of the ELF originate from the various cell types that reside within the lung, and therefore we investigated changes in lung tissue GSH levels. Similar to observed changes in the ELF there was an overall decrease in lung GSH in all of the aged groups (Figure 2A), which was not affected by acute CS exposure. We also examined the changes in the amount of GSSG (Figure 2B) within the tissue and found that although the GSH is down there are age-related increases in GSSG in the older mice. Although CS only had a slight effect on GSSG levels there were small increases in the 8- and 13-month-old mice. The amount of GSSG relative to total GSH is kept fairly low; therefore, comparing the amount of GSSG relative to GSH can be a predictor of oxidative stress. When the percentage of GSSG was determined (Figure 2C) it was not surprising that the young mice had a very low percentage with GSSG being only 3% of total GSH, whereas all of the aged mice had nearly 10 times that amount, with GSSG comprising more than 25% of total GSH.
Figure 2.
Age is associated with increased oxidative stress in the lung. Lung homogenate levels of (A) glutathione (GSH) and (B) oxidized glutathione (GSSG) were measured in both young and aged mice exposed to either air (open bars) or cigarette smoke (closed bars) for 1 day. (C) The percentage of glutathione in the oxidized state was calculated and found to be increased at all ages compared with the young mice. Data represented as mean ± SEM with *P < 0.05, **P < 0.01 compared between air and CS; §P < 0.05, §§P < 0.01, §§§P < 0.001 compared with air-exposed young mice; n = 4–6.
Systemic GSH Declines with Age
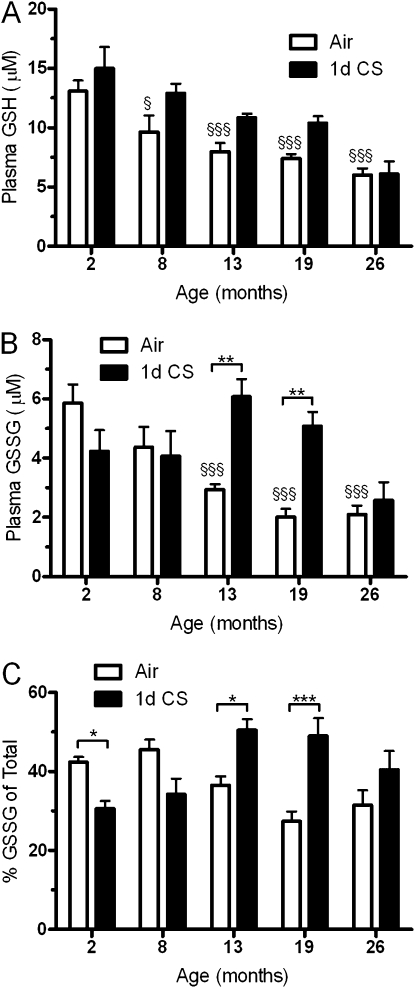
Because the lung may be partially dependant on GSH used from other sources, the plasma GSH and GSSG were analyzed. Interestingly, there is a decline in both GSH (Figure 3A) and GSSG (Figure 3B) with age, whereas CS had modest effects on the GSH in the 8-, 13-, and 19-month-old mice and on the GSSG levels in only the 13- and 19-month-old mice. There was no significant interaction between age and CS with GSH (P = 0.3904), whereas there was a significant interaction for GSSG (P = 0.0002). To examine the redox balance between GSH and GSSG, the percentage of GSH was calculated (Figure 3C). Although the percentage is unchanged with age alone, there was a significant decrease in the percentage of GSSG with CS exposure in the 2-month-old mice in contrast to significant increases in the 13- and 19-month-old mice. There were small although not significant changes in these parameters with both the 8- and 26-month-old mice.
Figure 3.
Aged mice have higher systemic oxidative stress. Blood was collected and plasma levels of (A) glutathione (GSH) and (B) oxidized glutathione (GSSG) were examined in both young and aged mice exposed to air (open bars) or cigarette smoke (closed bars) for 1 day. (C) The percentage of glutathione as its disulfide was calculated and found to be higher with cigarette smoke only in the 13- to 26-month-old mice. Data represented as mean ± SEM with *P < 0.05, **P < 0.01, ***P < 0.001 compared between air and CS; §P < 0.05, §§§P < 0.001 compared with air-exposed young mice; n = 4–6.
Both GCL and GR Expression Are Not Induced with CS in Aged Mice
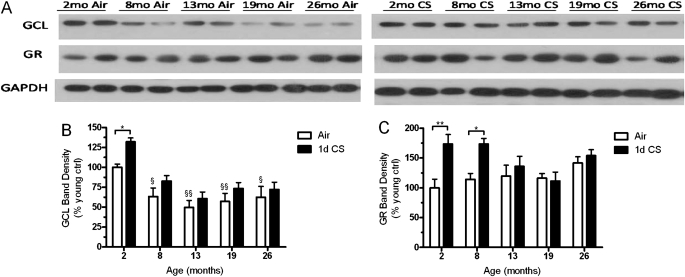
There is ample literature showing that during the aging process the expression levels of critical enzymes in the GSH pathway are decreased in the liver (26). GCL and GR, involved in the synthesis and recycling GSSG, may be differentially modulated in the lung with age and CS exposure. In the lung, GCL expression is clearly increased in response to CS in the 2-month-old mice (Figure 4B), whereas not only is there an age-related decrease in GCL but there is no increase in expression levels with CS exposure in the aged mice. Additionally, GR expression (Figure 4C) is increased with CS in both the 2- and 8-month-old mice, whereas there was no increase in GR expression in the 13-, 19-, or 26-month-old mice. Although there was no significant change in GR expression with age there was a significant interaction between age and CS (P = 0.0479).
Figure 4.
The ability to synthesize and maintain reduced glutathione decreases with age. (A) Representative Western blot probed for γ-glutamylcysteine ligase (GCL), GSH reductase (GR), and glyceraldehyde phosphate dehydrogenase in lung homogenate. Protein expression levels of both (B) GCL, the rate-limiting enzyme in glutathione synthesis, and (C) GR were quantified. GCL declines with age and is induced with cigarette smoke (CS) in young mice but not aged mice. GR expression is unchanged with age but is only induced by CS in the 2- and 8-month-old mice. Data represented as mean ± SEM with *P < 0.05, **P < 0.01 compared between air and CS; §P < 0.05, §§P < 0.01 compared with air-exposed young mice; n = 4–6.
Increases in Inflammation and Oxidation Occur with CS in Aged Animals
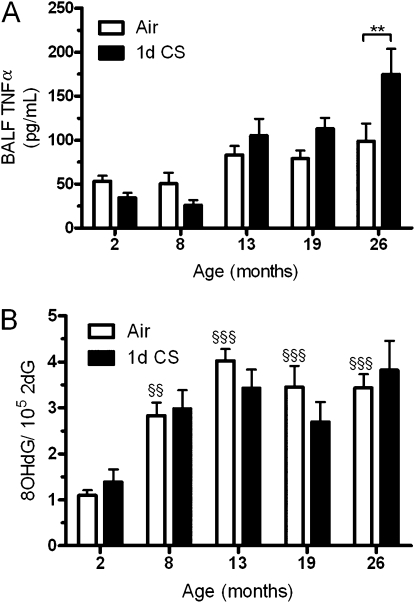
GSH is important for maintaining a reducing environment even in the presence of such oxidants as CS. Because the aged mice clearly have a diminished capacity to maintain elevated levels of GSH, we sought to determine whether they in turn have elevated levels of inflammation and oxidation. TNF-α was used as a marker of inflammation in the airways and the DNA oxidation product 8OHdG was quantified in the lung tissue. There was a clear increase in both TNF-α (Figure 5A) and 8OHdG (Figure 5B) with age, whereas the acute 1-day CS exposure had no significant effect on 8OHdG or TNF-α except in the 26-month-old mice.
Figure 5.
Measures of inflammation and oxidation with 1-day cigarette smoke (CS) exposure. (A) bronchoalveolar lavage fluid (BALF) TNF-α, a marker of inflammation, was measured after 1 day of CS exposure by ELISA. (B) To measure DNA oxidation, 8-hydroxy-2-deoxyguanosine (8OHdG) was quantified by HPLC after 1 day of CS exposure. Age alone increases both TNF-α and 8-hydroxy-2-deoxyguanosine in air controls (open bars), whereas 1 day of CS exposure (closed bars) has little effect. Data represented as mean ± SEM with, **P < 0.01, compared between air and CS; §§P < 0.01, §§§P < 0.001 compared with air-exposed young mice; n = 4–6.
The short CS exposure time frame may not allow for both TNF-α and 8OHdG to accumulate in the most susceptible 13-, 19-, and 26-month-old mice; therefore, a longer 5-day CS exposure was done. The young mice had a typical robust CS-induced GSH response with levels being increased threefold, whereas the aged mice showed a deficient GSH response with levels only increasing to near young control levels (Figure 6A). The GSH response in the young mice with 5-day CS exposure is lower than at 1 day because of an initial exaggerated GSH response, which then maintains at a new elevated steady state over the repeated exposures. When the BALF TNF-α levels (Figure 6B) were quantified there was both an age and CS effect and a significant interaction (P = 0.0171) with the largest differences between air and CS in the 13- and 26-month-old mice. Additionally, there was significant negative correlation between TNF-α release and ELF GSH levels in the air (r = −0.4299; P = 0.0259), 1-day (r = −0.4296; P = 0.0181), and 5-day (r = −0.5008; P = 0.0088) CS exposure groups (see Figure E1 in the online supplement). The oxidation product 8OHdG (Figure 6C) was even more pronounced with the longer CS exposure with significant effects from age and CS and a significant interaction (P = 0.0272). The longer CS exposure in the aged mice resulted in roughly 2.5–3 times more lung 8OHdG than seen in the young mice.
Figure 6.
Inflammation and oxidation are increased in the aged mice after 5 days of cigarette smoke (CS) exposure. (A) Epithelial lining fluid (ELF) glutathione was measured after exposure to air (open bars) or CS (solid bars) for 5 days in the 2-, 13-, 19-, and 26-month-old mice. Both (B) bronchoalveolar lavage fluid (BALF) TNF-α and (C) 8-hydroxy-2-deoxyguanosine (8OHdG) were more elevated in the aged mice than in the young mice with CS exposure. Data represented as mean ± SEM with *P < 0.05, **P < 0.01, ***P < 0.001 compared between air and CS; n = 4–6.
Smoke Exposure Increases the Number of Activated Macrophages in Aged Mice
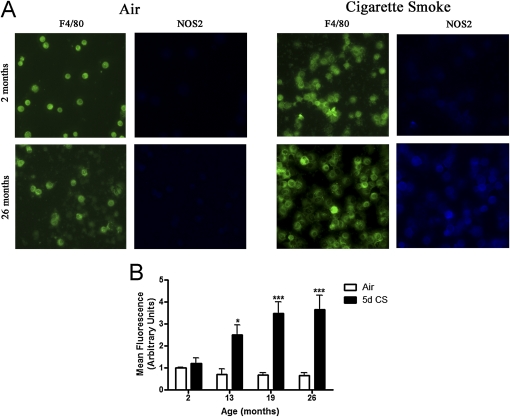
Once activated, macrophages can release a number of proinflammatory factors including TNF-α. Inducible NOS2 is a marker of activated macrophages and is also a major source of nitric oxide. The BAL cells from mice exposed to air or 5-day CS were analyzed for NOS2 expression by immunocytochemistry (Figure 7A) and the mean fluorescence was quantified (Figure 7B). The young mice exposed to CS did not show an increase in NOS2 expression, whereas the 13-, 19-, and 26-month-old mice showed more than threefold increase in NOS2 expression.
Figure 7.
Increased cigarette smoke (CS)–induced activation of bronchoalveolar lavage (BAL) macrophages in aged mice. The macrophage surface marker F4/80 was used to identify BAL macrophages and nitric oxide synthase was used as a marker of macrophage activation. (A) Representative images of F4/80 positive BAL macrophages from 2- and 26-month-old mice exposed to air or CS for 5 days. (B) Fluorescent quantification of nitric oxide synthase expression in 2-, 13-, 19-, and 26-month-old mice exposed to air or 5-day CS. Data represented as mean ± SEM with *P < 0.05, **P < 0.01, ***P < 0.001 compared between air and CS; n = 4–6.
BSO Administration Results in Depletion of ELF GSH and Increased BALF TNF-α Levels
Because a number of pathways are dysregulated during the aging process, young mice were treated with BSO to inhibit GCL activity, thus depleting the levels of GSH to a similar degree as seen in the aged mice. Mice were administered BSO chronically in the drinking water over the course of 11 days, which resulted in the depletion of ELF GSH from 114 to 50 μM (Figure 8A). Consequently, the BALF TNF-α levels rose from 25 to 120 pg/mL (Figure 8B). These data show a strong link between ELF GSH and BALF TNF-α levels that is independent of any other nonspecific age-related effects.
Figure 8.
Depletion of epithelial lining fluid (ELF) glutathione with l-buthionine-sulfoximine (BSO) treatment leads to increased bronchoalveolar lavage fluid (BALF) TNF-α levels. Young mice were administered l-buthionine-sulfoximine (20 mM) in the drinking water for 11 days, which resulted in the depletion of the (A) epithelial lining fluid glutathione and increase in (B) bronchoalveolar lavage fluid TNF-α levels compared with controls. Data represented as mean ± SEM with ***P < 0.001 significantly different than control; n = 5.
DISCUSSION
GSH is an important thiol antioxidant that is up-regulated in chronic smokers but decreased in the airways of patients with COPD (17). The ability to establish and maintain a GSH adaptive response to CS may be critical in preventing the development of COPD. Although COPD is a disease closely associated with CS, it typically only occurs in older individuals, suggesting that aging factors influence the development of the disease (3, 27). However, the ability to induce a GSH adaptive response to CS and whether it is dysfunctional in an aged lung has not been investigated.
The ELF contains a number of protective macromolecules to detoxify inhaled oxidants (16, 28). ELF GSH is of particular interest because it is maintained at high concentrations compared with plasma. Although others have shown that basal GSH levels in the BALF decline with age, these measures are largely dependent on dilution and recovery volumes and can be quite variable based on technique (29, 30). Here we have shown that dilution-corrected ELF levels of basal GSH do decline in accordance with the previous published BALF levels. The ELF GSH is an important defense against inhaled oxidants, but in aged airways the decline in GSH may suggest an increased susceptibility to inhaled xenobiotics, potentially increasing one's risk for respiratory diseases.
Additionally, to the best of our knowledge we have shown for the first time that the ability to establish an ELF GSH adaptive response to CS is impaired in aged mice. Although others have investigated the role of age on GSH metabolism in response to CS, these studies are largely based on tissue levels of GSH (31). In addition to the age-related changes in the tissue that we and others (26, 32) have demonstrated, there are important changes that occur in the extracellular space, particularly in the ELF, that contributes to the sensitivity of the aged airways to CS. Our data would suggest that as the lung ages the ability to increase ELF GSH in response to inhaled oxidants decreases and may contribute to the large proportion of individuals over 60 years of age with COPD (2). These findings not only would have consequences for older individuals who smoke but for any repeatedly inhaled noxious compounds.
Secondarily, we have demonstrated a defective GSH adaptive response in wild-type aged mice, not an accelerated aging strain. These strains, which are a common alternative, are meant to develop senile changes in major organs within a shorter lifespan, which could present more of an artificial model system (3). In addition, several senescence accelerated strains have been shown to have higher levels of GSSG in the BALF and tissue compared with aged-matched C57B/6 mice (33). These studies indicate an imbalance in the GSH redox couple even at young ages, potentially making them an inadequate model to investigate GSH changes with CS. One could also speculate that it may ultimately be the changes in the redox couple that contributes to the accelerated aging phenotype in these mice.
A large shift toward an oxidizing environment in a young animal might signify a severe disease, whereas with aging this shift seems to be a natural process. We found that the percentage of GSSG was greatly elevated in the aged mice in accordance with the higher state of oxidative stress that occurs with aging. Clinically, this is supported by the numerous metabolic and oxidative stress-related diseases that tend to arise with age (34–36). Contributing to the shift in redox status, the expression of common antioxidant defenses including Nrf2, GCL, and GR has been shown to decrease in the liver with age (26, 37).
Although age may decrease many of the antioxidant defenses, alternatively CS has been shown to induce expression of these very same defenses (38, 39). In accordance with previous reports in the liver (26), we found the expression of GCL in the lung was decreased with age, corresponding with the decline in GSH levels seen in the lung tissue. Although CS did induce expression of GCL in the young mice, all the older mice failed to show any significant increases in GCL, confirming that the aged mice are unable to increase antioxidant pathways comparable with a young mouse during stress. This inability to significantly increase GSH synthesis in the lung may increase the dependence on systemic GSH, ultimately burdening other organs, such as the liver, which can be a systemic source of GSH (40, 41). This also signifies that with aging comes an increase in susceptibility to oxidants, such as CS, as demonstrated by the higher levels of both inflammation and oxidation in aged mice exposed to CS.
Chronic inflammation is a hallmark of both aging and COPD, with higher levels of proinflammatory cytokines, including IL-6, INF-γ, and TNF-α, independently associated with both (9, 42). TNF-α is arguably one of the more important inflammatory cyotkines involved in smoke-induced lung damage because TNF-α has been shown to drive roughly 70% of smoke-induced emphysema in mice (43). In agreement with previous published research we observed increases in both TNF-α and 8OHdG with age (9, 44). Unexpectedly, we found little change in inflammation and DNA oxidation after an acute 1-day CS exposure. We theorized that this may be caused by the short time frame and thus longer exposures are necessary for both markers to accumulate. When we exposed mice repeatedly over 5 days we did see the expected increase in both TNF-α and 8OHdG with CS, suggesting that the aged mice do have increased sensitivity over repeated exposures toward CS-induced lung inflammation and oxidation because of the defective GSH adaptive response. The main cell type found in the BAL is the macrophage, which when activated can release proinflammatory factors, such as TNF-α, and a host of different reactive oxygen and nitrogen species. Corroborating the TNF-α result, there was an increase in activated macrophages, as measured by NOS2 expression, in the aged mice suggesting that the GSH adaptive response influences the degree of macrophage activation and corresponding TNF-α release. These findings suggest a mismatch between GSH, which declines with age and inflammatory responses that are exaggerated in the older animals and may be part of the unregulated inflammation that is associated with many age-related disorders.
Depletion of GSH in alveolar type II cells has previously been shown to exacerbate the cytokine release in response to proinflammatory stimuli, whereas supplementation with glutamine to enhance GSH levels has been shown to attenuate cytokine release (18, 45). In the present study we have shown that in vivo, depletion of ELF GSH by BSO results in the increase of BALF TNF-α independent of the effects of aging. This link between GSH levels and the proinflammatory cytokine response suggests the higher levels of TNF-α in the aged animals may be caused in part by the decreased GSH, highlighted by the significant negative correlation seen between BALF TNF-α and ELF GSH. Accordingly, the slight decreases observed in TNF-α with 1-day CS in the 2- and 8-month-old mice correspond to a larger adaptive response compared with the old mice suggesting elevated GSH can attenuate lung inflammation. Our observed increase in 8OHdG may be caused by the aged animals having a defective DNA repair system but recent literature would suggest the opposite. Mice that were 25 months old were reported to have the same expression and activity levels of nucleotide repair enzymes compared with 3-month-old mice (12), suggesting that the accumulation of 8OHdG observed in the aged mice is not caused by defective DNA repair but presumably by the lower antioxidant defenses.
Unwittingly, aging is also an unrecognized factor in many typical animal models of emphysema that are caused by prolonged CS exposures. Emphysema-like changes arises only after prolonged exposure to CS when mice are exposed to CS typically for 6–8 months resulting in a final age of roughly 8–10 months old (46, 47). Interestingly, this corresponds with the age when we observe a gradual loss of the GSH adaptive response to CS. The diminished GSH adaptive response seems to be caused by both a decrease in GSH synthesis and diminished systemic availability contributing to the differences between 8-month-old and young mice. Our data would suggest that many of the CS-induced emphysema studies in the literature are studies examining the combination of both aging and chronic CS.
The fact that these changes do not arise until the adaptive responses decline suggests that lung damage may only begin to occur when the adaptive response declines. Although CS is still the primary cause of COPD, it is a highly age-dependant disease with most cases arising in people over the age of 60 (2). Half of all people over the age of 65 have at least three chronic diseases, many of which may adversely affect the adaptive response (48). Our laboratory has previously demonstrated that infection with mycoplasma also adversely affects GSH adaptive responses to CS resulting in increased lung oxidation (20). This would suggest that the development of COPD may be influenced by a combination of two or more conditions that overwhelm the natural adaptive responses, which then manifests into disease symptoms. These types of events may also be important in triggering exacerbation of disease in patients with COPD.
To the best of our knowledge, we have shown for the first time that age adversely affects the lung GSH adaptive response to acute CS exposure in mice. We have also shown that this maladaptive response leads to increases in inflammation in the airways and increased DNA oxidation in the lung. We have also shown that although CS-induced emphysema studies are informative, there exists an underlying age factor that occurs within 8 months of age. Finally, COPD may be the manifestation of multiple syndromes and our data suggest that the combination of age and CS is enough to overwhelm the adaptive response to the point where inflammation and oxidation begin to accumulate, which could potentially contribute to the development of COPD in susceptible individuals.
Supplementary Material
Acknowledgments
The authors thank Stephanie Case and Maisha Minor for their help with the smoke exposure system.
Supported by National Institutes of Health grant R01 HL084469 (BJD).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201003-0442OC on July 9, 2010
Author Disclosure: N.S.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.W.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.J.D. was a consultant for Aeolus Pharmaceuticals (more than $100,001) and received lecture fees from Kyowa Hakko ($1,001–$5,000). He received grant support from Aeolus Pharmaceuticals and owns stocks or options of Aeolus Pharmaceuticals ($50,001–$100,000).
References
- 1.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev 2007;87:1047–1082. [DOI] [PubMed] [Google Scholar]
- 2.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB, et al. International variation in the prevalence of COPD (the bold study): a population-based prevalence study. Lancet 2007;370:741–750. [DOI] [PubMed] [Google Scholar]
- 3.Fukuchi Y. The aging lung and chronic obstructive pulmonary disease: similarity and difference. Proc Am Thorac Soc 2009;6:570–572. [DOI] [PubMed] [Google Scholar]
- 4.Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest 2005;128:1239–1244. [DOI] [PubMed] [Google Scholar]
- 5.MacNee W. Accelerated lung aging: a novel pathogenic mechanism of chronic obstructive pulmonary disease (COPD). Biochem Soc Trans 2009;37:819–823. [DOI] [PubMed] [Google Scholar]
- 6.Tuder RM. Aging and cigarette smoke: fueling the fire. Am J Respir Crit Care Med 2006;174:490–491. [DOI] [PubMed] [Google Scholar]
- 7.Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl 2001;34:50s–59s. [PubMed] [Google Scholar]
- 8.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 2006;28:219–242. [DOI] [PubMed] [Google Scholar]
- 9.Sharma G, Hanania NA, Shim YM. The aging immune system and its relationship to the development of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009;6:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merker K, Sitte N, Grune T. Hydrogen peroxide-mediated protein oxidation in young and old human MRC-5 fibroblasts. Arch Biochem Biophys 2000;375:50–54. [DOI] [PubMed] [Google Scholar]
- 11.Braga M, Sinha Hikim AP, Datta S, Ferrini MG, Brown D, Kovacheva EL, Gonzalez-Cadavid NF, Sinha-Hikim I. Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis 2008;13:822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikkelsen L, Bialkowski K, Risom L, Lohr M, Loft S, Moller P. Aging and defense against generation of 8-oxo-7,8-dihydro-2'-deoxyguanosine in DNA. Free Radic Biol Med 2009;47:608–615. [DOI] [PubMed] [Google Scholar]
- 13.Haddad JJ. Glutathione depletion is associated with augmenting a proinflammatory signal: evidence for an antioxidant/pro-oxidant mechanism regulating cytokines in the alveolar epithelium. Cytokines Cell Mol Ther 2000;6:177–187. [DOI] [PubMed] [Google Scholar]
- 14.Haddad JJ, Safieh-Garabedian B, Saade NE, Land SC. Thiol regulation of pro-inflammatory cytokines reveals a novel immunopharmacological potential of glutathione in the alveolar epithelium. J Pharmacol Exp Ther 2001;296:996–1005. [PubMed] [Google Scholar]
- 15.Haddad JJ, Safieh-Garabedian B, Saade NE, Lauterbach R. Inhibition of glutathione-related enzymes augments LPS-mediated cytokine biosynthesis: involvement of an ikappab/NF-kappab-sensitive pathway in the alveolar epithelium. Int Immunopharmacol 2002;2:1567–1583. [DOI] [PubMed] [Google Scholar]
- 16.Cantin AM, Fells GA, Hubbard RC, Crystal RG. Antioxidant macromolecules in the epithelial lining fluid of the normal human lower respiratory tract. J Clin Invest 1990;86:962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drost EM, Skwarski KM, Sauleda J, Soler N, Roca J, Agusti A, MacNee W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 2005;60:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang F, Wang X, Wang W, Li N, Li J. Glutamine reduces TNF-alpha by enhancing glutathione synthesis in lipopolysaccharide-stimulated alveolar epithelial cells of rats. Inflammation 2008;31:344–350. [DOI] [PubMed] [Google Scholar]
- 19.Gould NS, Min E, Chu HW, Martin R, Day BJ. Lung glutathione adaptive responses decline with age [abstract]. Toxicologist 2010;114:A1524. [Google Scholar]
- 20.Kariya C, Chu HW, Huang J, Leitner H, Martin RJ, Day BJ. Mycoplasma pneumoniae infection and environmental tobacco smoke inhibit lung glutathione adaptive responses and increase oxidative stress. Infect Immun 2008;76:4455–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun JD, Ragsdale SS, Benson JM, Henderson RF. Effects of the long-term depletion of reduced glutathione in mice administered l-buthionine-s,r-sulfoximine. Fundam Appl Toxicol 1985;5:913–919. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe T, Sagisaka H, Arakawa S, Shibaya Y, Watanabe M, Igarashi I, Tanaka K, Totsuka S, Takasaki W, Manabe S. A novel model of continuous depletion of glutathione in mice treated with l-buthionine (s,r)-sulfoximine. J Toxicol Sci 2003;28:455–469. [DOI] [PubMed] [Google Scholar]
- 23.Gould NS, White CW, Day BJ. A role for mitochondrial oxidative stress in sulfur mustard analog 2-chloroethyl ethyl sulfide-induced lung cell injury and antioxidant protection. J Pharmacol Exp Ther 2009;328:732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 2006;1:3159–3165. [DOI] [PubMed] [Google Scholar]
- 25.Chinard FP. Quantitative assessment of epithelial lining fluid in the lung. Am J Physiol 1992;263:L617–L618. [DOI] [PubMed] [Google Scholar]
- 26.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci USA 2004;101:3381–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest 2009;135:173–180. [DOI] [PubMed] [Google Scholar]
- 28.Noel-Georis I, Bernard A, Falmagne P, Wattiez R. Database of bronchoalveolar lavage fluid proteins. J Chromatogr B Analyt Technol Biomed Life Sci 2002;771:221–236. [DOI] [PubMed] [Google Scholar]
- 29.Teramoto S, Fukuchi Y, Uejima Y, Teramoto K, Ito H, Orimo H. Age-related changes in the antioxidant screen of the distal lung in mice. Lung 1994;172:223–230. [DOI] [PubMed] [Google Scholar]
- 30.Cross CE, van der Vliet A, O'Neill CA, Louie S, Halliwell B. Oxidants, antioxidants, and respiratory tract lining fluids. Environ Health Perspect 1994;102:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teramoto S, Uejima Y, Teramoto K, Ouchi Y, Fukuchi Y. Effect of age on alteration of glutathione metabolism following chronic cigarette smoke inhalation in mice. Lung 1996;174:119–126. [DOI] [PubMed] [Google Scholar]
- 32.Shih PH, Yen GC. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology 2007;8:71–80. [DOI] [PubMed] [Google Scholar]
- 33.Teramoto S, Fukuchi Y, Uejima Y, Teramoto K, Orimo H. Biochemical characteristics of lungs in senescence-accelerated mouse (SAM). Eur Respir J 1995;8:450–456. [DOI] [PubMed] [Google Scholar]
- 34.Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol 2008;122:456–468, quiz 469–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benz CC, Yau C. Ageing, oxidative stress and cancer: paradigms in parallax. Nat Rev Cancer 2008;8:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell 2005;120:449–460. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Down-regulated NF-e2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2008;39:673–682. [DOI] [PubMed] [Google Scholar]
- 38.Lyakhovich VV, Vavilin VA, Zenkov NK, Menshchikova EB. Active defense under oxidative stress. The antioxidant responsive element. Biochemistry (Mosc) 2006;71:962–974. [DOI] [PubMed] [Google Scholar]
- 39.Rahman I, Smith CA, Lawson MF, Harrison DJ, MacNee W. Induction of gamma-glutamylcysteine synthetase by cigarette smoke is associated with AP-1 in human alveolar epithelial cells. FEBS Lett 1996;396:21–25. [DOI] [PubMed] [Google Scholar]
- 40.Garibotto G, Sofia A, Saffioti S, Russo R, Deferrari G, Rossi D, Verzola D, Gandolfo MT, Sala MR. Interorgan exchange of aminothiols in humans. Am J Physiol Endocrinol Metab 2003;284:E757–E763. [DOI] [PubMed] [Google Scholar]
- 41.Ookhtens M, Kaplowitz N. Role of the liver in interorgan homeostasis of glutathione and cyst(e)ine. Semin Liver Dis 1998;18:313–329. [DOI] [PubMed] [Google Scholar]
- 42.Szulakowski P, Crowther AJ, Jimenez LA, Donaldson K, Mayer R, Leonard TB, MacNee W, Drost EM. The effect of smoking on the transcriptional regulation of lung inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;174:41–50. [DOI] [PubMed] [Google Scholar]
- 43.Churg A, Wang RD, Tai H, Wang X, Xie C, Wright JL. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med 2004;170:492–498. [DOI] [PubMed] [Google Scholar]
- 44.Hashimoto K, Takasaki W, Yamoto T, Manabe S, Sato I, Tsuda S. Effect of glutathione (GSH) depletion on DNA damage and blood chemistry in aged and young rats. J Toxicol Sci 2008;33:421–429. [DOI] [PubMed] [Google Scholar]
- 45.McRitchie DI, Isowa N, Edelson JD, Xavier AM, Cai L, Man HY, Wang YT, Keshavjee SH, Slutsky AS, Liu M. Production of tumour necrosis factor alpha by primary cultured rat alveolar epithelial cells. Cytokine 2000;12:644–654. [DOI] [PubMed] [Google Scholar]
- 46.Rangasamy T, Misra V, Zhen L, Tankersley CG, Tuder RM, Biswal S. Cigarette smoke-induced emphysema in a/j mice is associated with pulmonary oxidative stress, apoptosis of lung cells, and global alterations in gene expression. Am J Physiol Lung Cell Mol Physiol 2009;296:L888–L900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adair-Kirk TL, Atkinson JJ, Griffin GL, Watson MA, Kelley DG, DeMello D, Senior RM, Betsuyaku T. Distal airways in mice exposed to cigarette smoke: Nrf2-regulated genes are increased in clara cells. Am J Respir Cell Mol Biol 2008;39:400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005;294:716–724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.