Abstract
Rationale: Prostacyclin analogs, used to treat idiopathic pulmonary arterial hypertension (IPAH), are assumed to work through prostacyclin (IP) receptors linked to cyclic AMP (cAMP) generation, although the potential to signal through peroxisome proliferator-activated receptor-γ (PPARγ) exists.
Objectives: IP receptor and PPARγ expression may be depressed in IPAH. We wished to determine if pathways remain functional and if analogs continue to inhibit smooth muscle proliferation.
Methods: We used Western blotting to determine IP receptor expression in peripheral pulmonary arterial smooth muscle cells (PASMCs) from normal and IPAH lungs and immunohistochemistry to evaluate IP receptor and PPARγ expression in distal arteries.
Measurements and Main Results: Cell proliferation and cAMP assays assessed analog responses in human and mouse PASMCs and HEK-293 cells. Proliferative rates of IPAH cells were greater than normal human PASMCs. IP receptor protein levels were lower in PASMCs from patients with IPAH, but treprostinil reduced replication and treprostinil-induced cAMP elevation appeared normal. Responses to prostacyclin analogs were largely dependent on the IP receptor and cAMP in normal PASMCs, although in IP−/− receptor cells analogs inhibited growth in a cAMP-independent, PPARγ-dependent manner. In IPAH cells, antiproliferative responses to analogs were insensitive to IP receptor or adenylyl cyclase antagonists but were potentiated by a PPARγ agonist and inhibited (∼ 60%) by the PPARγ antagonist GW9662. This coincided with increased PPARγ expression in the medial layer of acinar arteries.
Conclusions: The antiproliferative effects of prostacyclin analogs are preserved in IPAH despite IP receptor down-regulation and abnormal coupling. PPARγ may represent a previously unrecognized pathway by which these agents inhibit smooth muscle proliferation.
Keywords: prostacyclin analogues, human pulmonary smooth muscle cell proliferation, IP receptor, cyclic AMP, proliferator-activated receptor-gamma
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Parental prostacyclin therapy remains the gold standard for the treatment of pulmonary arterial hypertension (PAH), though decreased expression of the IP receptor, the classical biological target for prostacyclin, may occur in PAH. Whether this impacts on the ability of these agents to work in PAH is an important clinical question.
What This Study Adds to the Field
Down-regulation of the IP receptor, related to chronic treatment with prostacyclin, occurred in PAH but did not affect the ability of these agents to inhibit pulmonary smooth muscle cell proliferation from these patients. The mechanism differed from normal cells because neither the IP receptor nor cyclic AMP–mediated effects but were in part mediated by the peroxisome proliferator–activated receptor-gamma. Thus, peroxisome proliferator–activated receptor-gamma may represent a therapeutic target in PAH.
Idiopathic pulmonary arterial hypertension (IPAH) is a progressive, incurable disease leading to right heart failure and death. Untreated, median survival from diagnosis is 2.6 years in adults (1) and 10 months in children (2). Therapeutic interventions include prostacyclin, endothelin antagonists, and phosphodiesterase type 5 inhibitors, all of which improve hemodynamics, exercise tolerance, and clinical status (3). An impact on survival is assumed, although this has only been demonstrated for prostacyclin (3). Other prostacyclin analogs, such as iloprost and treprostinil, have been developed to offer greater plasma stability and alterative routes of administration. Prostacyclin and its analogs are thought to slow pulmonary vascular disease progression by reversing the abnormal remodeling process (4). Eventually, however, medications are no longer effective, and lung transplantation is required (5). Thus, the clinician cannot be certain that the patient continues to derive benefit from the drugs when they are clearly deteriorating.
In previous studies, replication of normal pulmonary artery smooth muscle cells (PASMCs) was reduced by prostacyclin analogs in a largely cyclic AMP (cAMP)-dependent manner (6, 7). However, the extent to which the prostacyclin (IP) receptor mediates the effects of prostacyclin analogs is not clear. Not only can these agents activate other prostanoid receptors (8), but they can also signal through nuclear peroxisome proliferator-activated receptors (PPARs) (9), a family of transcription factors regulating diverse biological processes such as cell growth, apoptosis, inflammation, and insulin sensitivity (10). Activation of PPARs can occur via direct ligand binding or as a consequence of receptor activation (11).
Whether prostacyclin analogs suppress replication of PASMCs from patients with IPAH and act through the same transduction pathway as normal PASMCs is unknown. We hypothesized that PASMCs from patients with IPAH would fail to respond normally because IP receptor density had decreased (12), because the receptor had become dysfunctional, or both. Reduced expression of PPARγ, reported in adult IPAH lungs (13), might also reduce prostacyclin analog signaling through this pathway. Therefore, we examined expression and function of IP receptors and PPARγ in PASMCs grown from the lungs of patients with IPAH and compared findings in PASMCs derived from normal lungs and from mice with and without the IP receptor. Despite reduced IP receptor levels in end-stage IPAH, the antiproliferative effects of prostacyclin analogs in PASMCs were preserved but did not involve the IP receptor. In normal cells, IP receptor–dependent and IP receptor–independent mechanisms prevailed. Furthermore, PPARγ was strongly expressed in the pulmonary arterial media in IPAH and assumed a greater role in mediating the antigrowth effects of treprostinil in PASMCs isolated from these patients compared with PASMCs from normal subjects.
METHODS
Expanded methods can be found in the online supplement.
Patient Characteristics
Lung tissue was taken after patient or relative consent and with Ethics Committee approval from Great Ormond Street (ICH and GOSH REC 05/Q0508/45), Papworth Hospital (REC H00/531/T) and Brompton and Harefield Trust (NHLI REC 01-210) through Dr. Wharton (Imperial College, London, UK). Samples were obtained from patients with IPAH who were undergoing transplant after failed treatment (six children, four adults) or who had not undergone therapy (eight children). Treated children received epoprostenol for 1.3 to 4 years; adult patients were on varying prostacyclin therapy for an average of 1.2 years. One adult had a mutation (N903S) in the bone morphogenetic protein receptor type II (BMPRII) (14). All patients had advanced pulmonary vascular disease (Heath and Edwards Grade 4–5). For controls, tissue was obtained from normal children (n = 5) and from adults undergoing transplant or lung resection for suspected malignancy (n = 6).
PASMCs and HEK-293 Cells
Peripheral PASMCs were isolated from patients with IPAH (n = 7) and from control adults (n = 6). Control intrapulmonary PASMCs derived from a 2.5-month-old child were obtained from Lonza Group Ltd. (Basel, Switzerland). PASMCs used in the study stained for α-smooth muscle actin and smooth muscle myosin heavy chain (see figure E2E in the online supplement). HEK-293 cells stably expressing the human IP receptor (HEK-293-IP) or empty vector (control) have previously been generated (11). Distal PASMCs from homozygous IP receptor–deficient (n = 6) and wild-type (n = 7) mice were isolated and cultured as described in the online supplement.
Cell Proliferation Assays
For determination of cell number in HEK-293 cells and PASMCs, cells were seeded onto 6-well plates at a density of 0.5 to 2 × 104 cells/ml, grown for 24 hours, and starved in low serum for 48 hours. Cells were then incubated in medium containing 10% FBS with and without the relevant test agent and counted at 24-hour intervals using an automated cell counter (Sysmex F-520P; Malvern Instruments Ltd., Worcestershire, UK). FBS-induced growth was assessed at a single point in cells incubated for 48 (HEK-293) or 96 hours (PASMCs). Analog doses were chosen to give a maximal rise in cyclic AMP (0.1–1 μM) (7) and substantial (∼40–50%) inhibition of cell growth. [3H]Thymidine incorporation into distal PASMCs from wild-type and IP receptor–deficient mice was performed as previously described (15). Assays were undertaken in the presence of 0.1% FBS, 10% FBS, or platelet-derived growth factor (PDGF)-BB (10 ng/ml).
IP Receptor Antibody Production
A peptide corresponding to the C terminus of the human IP receptor (RRDPRAPSAVGKE) was synthesized and conjugated to hemocyanin before injection into rabbits using standard protocols (Eurogentec, Seraing, Belgium). Bleeds were assayed for activity using a standard ELISA assay, and those showing reactivity were affinity purified.
Immunohistochemistry and Immunofluorescence
Blocks of lung tissue containing preacinar and intraacinar arteries were obtained from normal children and from nontreated and treated children with IPAH. Blood vessels were immunostained as previously described (16) using cell-specific markers (Figure E2C and E2D) and antibodies to PPARγ (Cell Signaling Technology, Danvers MA) and the IP receptor. Slides were examined using a Leica DM LB microscope (Leica Microsystems, Wetzlar, Germany), and images were acquired and analyzed in a blinded fashion (details provided in the online supplement). For immunofluoresence, monolayers of cultured cells were fixed and permeabilized and then stained for the IP receptor and nuclei (TO-PRO-3; Invitrogen, Paisley, UK). Images were viewed and analyzed using a laser-scanning confocal microscope.
Western Blotting
IP receptor protein expression was determined using conventional techniques. Blots were processed and developed using the ECL Plus chemiluminescent immunoblot detection system and hyperfilm (Amersham Biosciences, Little Chalfont, UK).
Intracellular cAMP Measurement
cAMP was measured according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI, R&D Systems Europe Ltd, Abingdon, UK, or NEN Life Science Products, Boston, MA). Additional information is provided in the online supplement.
PPARγ Reporter Gene Assay
The luciferase reporter pGAL5TKpGL3 was cotransfected into HEK-293-IP cells with pMLuc2 (Renilla control vector) and GAL4-hPPARγ-pcDNA3 (vector containing the human PPARγ ligand-binding domain) as described (11). Data are shown as luciferase activity normalized to Renilla.
Statistical Analysis
Experiments were repeated at least three times, and results are expressed as mean ± SEM of n observations or as box-whisker plots (Figure 2C). A Student's t test or one-way ANOVA with correction for multiple comparisons was used. A P value < 0.05 was considered significant.
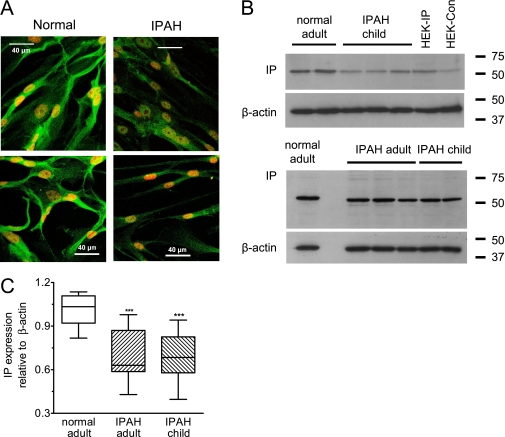
Figure 2.
(A) Subconfluent pulmonary arterial smooth muscle cells (PASMCs) from different isolates stained with the IP receptor antibody using FITC-conjugated Alexa fluor 488 (green stain) as the secondary. TO-PRO-3 (red) was used to stain nuclei, and all images were taken under identical conditions. (B) Western blotting showing IP receptor expression in crude homogenates from PASMCs and HEK-293 cells expressing the IP receptor (HEK-IP) or empty vector (HEK-Con). For PASMC and HEK-293 lysates, 10 and 20 μg of protein, respectively, was loaded to match β-actin levels. (C) Box-whisker-plot of IP receptor expression in PASMCs shown relative to β-actin and normalized with respect to control. Bands were measured by densitometry using NIH Image software. Samples for each isolate (12 in total) were run on three or four separate occasions with blots always containing normal and disease samples. The passage number used was P4-P7 for controls, P3-P6 for adult idiopathic pulmonary arterial hypertension, and P3-P4 for child idiopathic pulmonary arterial hypertension. ***P < 0.001.
RESULTS
Comparative Proliferation Rates of Human and HEK-293 Cell Lines
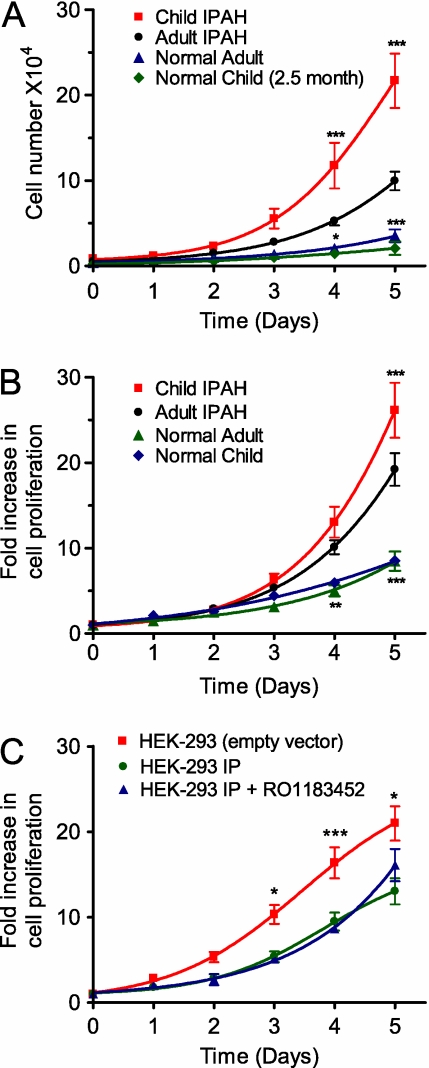
Isolates of PASMCs derived from lungs of adults and children with IPAH proliferated significantly (P < 0.001; n = 9–12) more than normal adult PASMCs cultured under the same conditions (Figures 1A and 1B). However, the growth of pediatric IPAH cells was approximately double that of adult IPAH cells over a 5-day period (Figure 1A), although this difference was largely masked if growth was normalized to cell number at t = 0 (Figure 1B). This striking difference between hypertensive and normal PAMSCs was similar to that observed between HEK-293-IP and control HEK-293 cells (Figure 1C), where stably expressing the IP receptor significantly (P < 0.001; n = 6) slowed HEK-293 cell growth. Differences in replication rates are unlikely to relate to basal IP receptor activity because pretreatment with the IP receptor antagonist RO1183452 (17) failed to increase the growth of HEK-293-IP cells (Figure 1C).
Figure 1.
Cell proliferation rates in pulmonary arterial smooth muscle cells (A and B) derived from normal and idiopathic pulmonary arterial hypertension (IPAH) patients and (C) HEK-293 cells expressing the IP receptor (HEK-293-IP) or the pcDNA3.1Zeo vector alone. Starved cells were grown in media containing 10% FBS. Results are presented as cell number (A) or expressed as mean fold increase in proliferation (B and C). HEK-293-IP cells were also treated with the IP receptor antagonist (1 μM; RO1183452), present throughout the 5-day period (C). Data are expressed as mean ± SEM, with growth assays repeated two to four times for each isolate. *P < 0.05, **P < 0.01, and ***P < 0.001 with respect to adult IPAH or HEK-293-IP.
IP Receptor Expression in Human PASMCs
Given that IP receptor loss accelerates cell growth, we examined if expression might be down-regulated in PASMCs derived from patients with IPAH. We used a C-terminal antibody that preferentially stains HEK-293 cells stably expressing the IP receptor but not control cells (Figure E1A). In normal PASMCs cells, IP receptor staining was localized to the membrane and within the cytosol, whereas in IPAH cells, staining was weaker and localized more in the cytosol (Figure 2A). Western blotting confirmed the existence of a major immunoreactive band with an apparent molecular weight of approximately 53 kD in all human PASMC samples tested (Figure 2B). A band of similar weight was observed in HEK-293-IP cells but was barely detectable in control HEK-293 cells. Analysis of bands with normalization to β-actin staining showed that in IPAH cells from adults and children, band intensity was approximately 65% of that observed in normal human PASMC lysates (P < 0.01) (Figure 2C). Likewise, the IP receptor mRNA band intensity was consistently weaker in IPAH cells (Figure E1C).
IP Receptor and PPARγ Expression in Pulmonary Arteries from Normal Subjects and Patients with IPAH
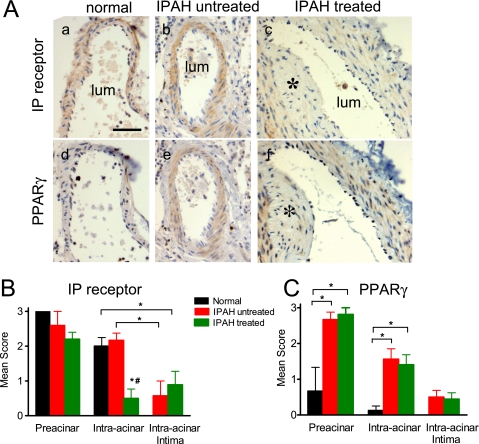
Immunohistochemical staining was performed in small pulmonary arteries from normal children and compared with those from untreated and treated children with IPAH (Figure 3). IP receptor expression was strong in the smooth muscle layer of normal children (Figure 3Aa) and well preserved in untreated IPAH samples (Figures 3Ab and Figure 3B). No significant staining was observed with the secondary antibody alone (Figure E2B). In treated IPAH samples, IP receptor expression was weaker (P < 0.05) in PASMCs of intraacinar arteries compared with untreated samples, although no difference was observed in preacinar arteries. In intimal proliferative cells, IP receptor staining was weak regardless of treatment (Figures 3Ab and 3Ac and Figure 3B). With respect to PPARγ, staining was weak in smooth muscle layer of normal peripheral arteries (Figure 3Ad) but consistently present in this layer in all IPAH samples (Figures 3Ae and 3Af). Analysis showed expression was significantly increased in pre- and intraacinar arteries (P < 0.05; n = 5–8) (Figure 3C). In regions of intimal proliferation, staining was weak or undetectable (Figure 3Af, asterisk).
Figure 3.
(A) Immunohistochemical staining for the IP receptor (a, b, c) and peroxisome proliferator-activated receptor-gamma (PPARγ) (d, e, f) in serial sections of pulmonary arteries from a normal and from an untreated or treated child with idiopathic pulmonary arterial hypertension. Asterisk depicts regions of intimal proliferation and the scale bar represents 50 μm for all panels. Average IP receptor (B) and PPARγ (C) expression in medial preacinar and intraacinar arteries and in intimal proliferative cells. Staining was scored blinded in one to three sections per sample where 0, 1, 2, 3 are equivalent to no, weak, intermediate, or strong staining. *P < 0.05 compared with normal and #P < 0.05 compared with untreated intraacinar.
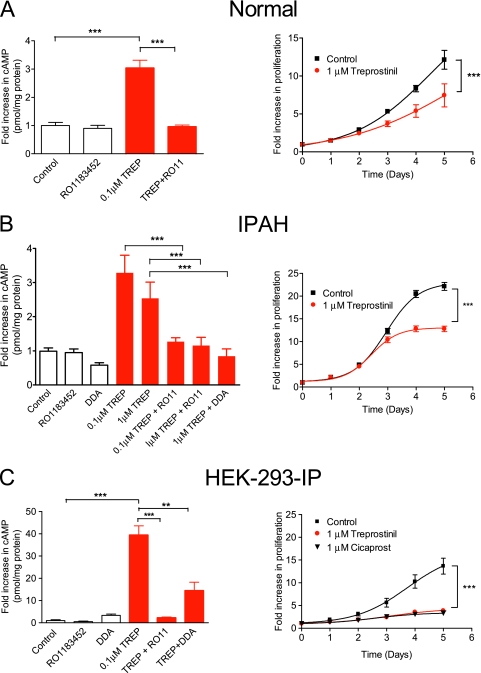
Treprostinil Effects on cAMP and Cell Growth
To test receptor functionality, the effects of treprostinil (provided by United Therapeutics, Silver Spring, MD) on cAMP levels were assessed at the peak time (30 min) of elevation in normal human and IPAH PASMCs. Despite differences in IP receptor expression, treprostinil (100 nM) elevated cAMP on average by 3-fold in normal and IPAH cells. cAMP elevation was abolished by pretreatment with IP receptor antagonist RO1183452 (1 μM) and also in HEK-293-IP cells, where increases were previously substantial (40-fold) (Figure 4C). The fact that RO1183452 has no significant affinity at other prostanoid receptors (17) suggests that the IP receptor is the major source of cAMP in PASMCs. Next, we assessed time-dependent effects of treprostinil on cell proliferation. In normal and IPAH cell isolates, treprostinil inhibited proliferation. Thus, at Days 4 and 5, proliferation was approximately 40% lower than in FBS alone (P < 0.001) (Figures 4A and 4B). The antiproliferative effects of treprostinil (and those of another prostacyclin analog, cicaprost) on HEK-293-IP cells were greater (∼72% inhibition at Day 5) than observed in human PASMCs (Figure 4C).
Figure 4.
Effect of treprostinil (TREP) on cAMP generation and proliferation in distal pulmonary arterial smooth muscle cells derived from (A) normal subjects and (B) patients with idiopathic pulmonary arterial hypertension and in (C) HEK-293-IP cells. For cAMP measurements, TREP (0.1 or 1 μM) was applied for 30 minutes with or without the IP receptor antagonist (RO1183452 or RO11; 1 μM) or 2′5′dideoxyadenosine (DDA; 100 μM). Inhibitors were given 1 hour before the addition of treprostinil. Results expressed as fold increase above basal in pmol of cAMP per mg of total protein. Growth-arrested cells were stimulated with 10% FBS ± treprostinil or cicaprost and counted at various time points. All data are expressed as mean ± SEM (n = 9–15). **P < 0.01; ***P < 0.001.
Role of the IP Receptor in Mediating Antigrowth Effects
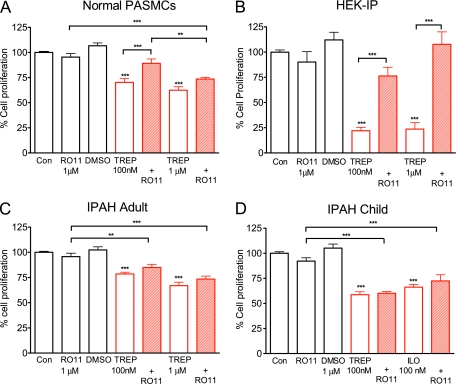
Whether prostacyclin analogs mediate their antigrowth effects in the lung solely through the IP receptor remains an important clinical question. Therefore, we investigated the effect of RO1183452 on analog inhibition of cell growth at 4 days in human PASMCs. In normal PASMCs, RO1183452 significantly (P < 0.001; n = 12) reversed the antiproliferative effects of 100 nM treprostinil (Figure 5A) and iloprost (Schering AG, Berlin, Germany). (Figure E3), although, with respect to treprostinil, this was incomplete when compared with the solvent control DMSO (P < 0.01). At higher treprostinil doses (1 μM), there was only a trend toward reversal with RO1183452 (Figure 5A), which contrasted with HEK-293-IP cells, where responses were fully inhibited by the IP receptor antagonist at that dose (Figure 5B). The antiproliferative effects of treprostinil and iloprost were essentially insensitive to RO1183452 in IPAH cells from children and adults (Figures 5C and 5D).
Figure 5.
Growth-arrested distal human pulmonary arterial smooth muscle cells (PASMCs) (A, C, and D) or HEK-293-IP cells (B) were incubated in media containing 10% FBS and left untreated (Con) or treated with the IP receptor antagonist (RO1183452), DMSO (0.1%), the IP receptor agonist (TREP, treprostinil or ILO, iloprost), or a combination. Data, expressed as % cell proliferation relative to growth response mediated by 10% FBS alone, are shown as mean ± SEM (n = 9). **P < 0.01 and ***P < 0.001 compared with control or as shown.
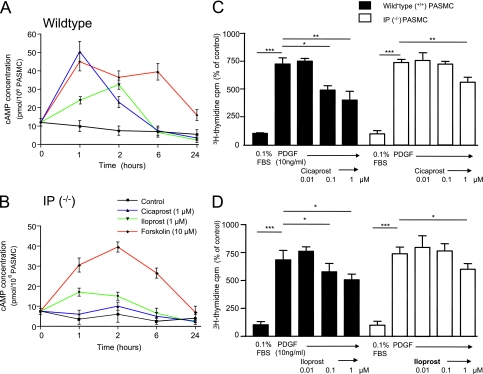
To confirm that PGI2 analogs could inhibit cell growth independently of the IP receptor, primary cell lines of distal PASMCs were generated from wild-type and IP receptor (IP−/−)–deficient mice. In wild-type cells, cicaprost (Schering AG) elevated cAMP by 4-fold (Figure 6A) but had no effect in IP−/− cells (Figure 6B). Iloprost elevated cAMP in mutant cells but by a substantially lesser amount. This contrasted with forskolin, whose effects on cAMP levels were similar in both cell types. In 3H-thymidine incorporation studies, PDGF (10 ng/ml) induced a significant increase in DNA synthesis compared with control wells. In wild-type distal PASMCs, cicaprost and iloprost elicited concentration-dependent attenuation of PDGF-induced 3H-thymidine incorporation (Figures 6C and 6D). In mutant cells, these agents only inhibited DNA synthesis at the highest agonist concentration (1 μM), consistent with IP-receptor dependent and independent mechanisms of growth inhibition.
Figure 6.
Time-dependent effects of cicaprost (1 μM), iloprost (1 μM), and forskolin (10 μM) on intracellular cAMP concentration in (A) wild-type and (B) IP−/− cultured distal pulmonary arterial smooth muscle cells (PASMCs) expressed as pmol of cAMP per 106 cells. (C) 3H-thymidine incorporation in IP receptor wild-type (+/+) mice. (D) 3H-thymidine incorporation in IP receptor deficient (−/−) mice. All data are expressed as mean ± SEM (n = 3). *P < 0.05, **P < 0.0.01, and ***P < 0.001.
Role of cAMP
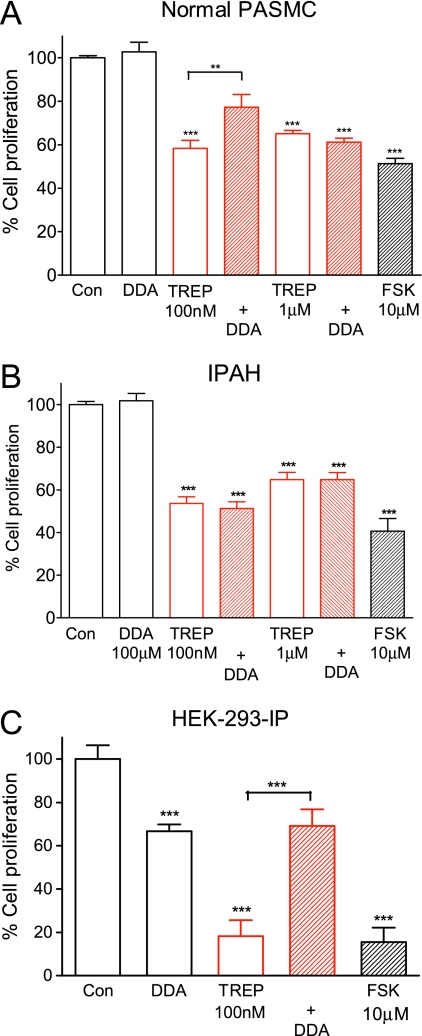
We reported previously that the adenylyl cyclase antagonist 2'5′ dideoxyadenosine (DDA) was an effective inhibitor of the growth suppression induced by treprostinil and iloprost in proximal PASMCs (7). Therefore, we sought to investigate the role of cAMP in distal PASMCs. Treatment with 100 μM DDA alone had no significant effect on cell proliferation in human PASMCs (n = 9; P = 0.37) (Figures 7A and 7B), although it did inhibit proliferation in HEK-293-IP cells (33.4 ± 3.2% inhibition compared with untreated controls; n = 8; P < 0.001) (Figure 7C). Responses to 100 nM treprostinil were significantly (n = 9; P < 0.01) attenuated approximately 60% by DDA in normal PASMCs, but no effect of DDA was observed in IPAH cells from adults and children. By contrast, reversal of the antiproliferative response was complete in HEK-293-IP cells compared with serum-induced growth in the presence of DDA. It was incomplete (75%) with respect to serum alone (Figure 7C), perhaps reflecting some residual cAMP elevation with treprostinil in the presence of DDA. At the higher treprostinil concentration (1 μM), DDA had no effect on normal or IPAH cells despite it fully inhibiting cAMP generation (Figure 4B). These results suggest a largely cAMP-independent mechanism of growth inhibition for prostacyclin analogs in IPAH cells despite the ability of the adenylyl cyclase activator forskolin to inhibit proliferation in these cells (Figure 7B).
Figure 7.
Role of cAMP in mediating treprostinil (TREP) effects. Growth-arrested (A and B) distal pulmonary arterial smooth muscle cells (PASMCs) or (C) HEK-293-IP cells were stimulated with 10% FBS ± TREP (100 nM or 1 μM) in combination with 2′5′dideoxyadenosine (DDA; 100 μM). Cells were pretreated with 2′5′dideoxyadenosine 1 hour before stimulation with TREP. Data, expressed as % cell proliferation relative to the proliferative response mediated by 10% FBS alone, are shown as mean ± SEM (n = 9). ***P < 0.001.
Role of PPARγ
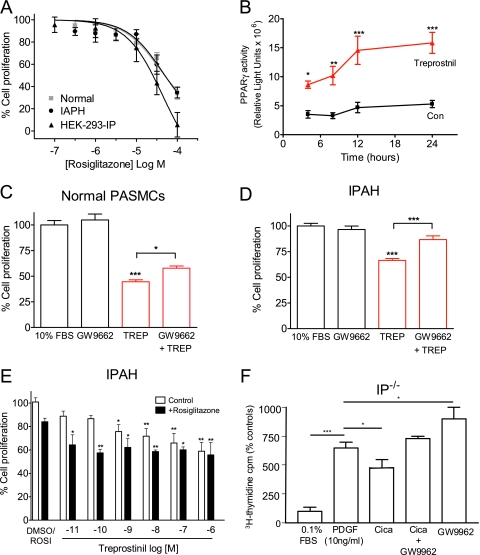
Given the evidence that loss of PPARγ might cause or contribute to pulmonary arterial hypertension (PAH) and vascular remodeling (13, 18), we first sought to confirm that the PPARγ pathway was functional in our cells. We used the selective PPARγ agonist rosiglitazone, which inhibited serum-induced growth with a similar potency in normal, IPAH, and HEK-293-IP cells (EC50 35, 43, and 45 μM, respectively) (Figure 8A). To confirm specificity of PPARγ modulators and to provide further evidence for PPARγ activation, we transfected HEK-293-IP cells with a PPARγ reporter construct. This was activated 6-fold by rosiglitazone (1 μM), an effect abolished by pretreatment with the PPARγ antagonist GW9662 at 100 nM or 1 μM, with partial inhibition at 10 nM (Figure E4). Likewise, treprostinil (1 μM) significantly (2- to 3-fold) increased PPARγ activity, with luciferase activity detected as early as 4 hours, peaking at approximately 12 hours, and remaining stable thereafter (Figure 8B). Over the same time period, there was no significant change in basal activity (P = 0.18; n = 4).
Figure 8.
Effect of peroxisome proliferator-activated receptor-gamma (PPARγ) modulators on cell growth. (A) Concentration-response curve to the PPARγ agonist rosiglitazone (ROSI) in human pulmonary arterial smooth muscle cells (PASMCs) and HEK-293-IP cells. Growth-arrested cells were stimulated with 10% FBS with and without agonist. Cells were counted, and results are expressed as % cell proliferation relative to FBS alone. (B) Time-course of treprostinil effects on the luciferase activity. HEK-293-IP were transfected with GAL5TKpGL3 (reporter construct), the control renilla vector pMLuc2, and GAL4-hPPARγ-pcDNA3 (containing PPARγ fusion protein). Results are expressed as luciferase light units normalized to renilla activity. Effect of the PPARγ antagonist GW9662 (1 μM) on the antiproliferative effects of treprostinil (TREP; 100 nM) in (C) normal and in (D) IPAH PASMCs. (E) Concentration-response to TREP in the absence and presence of rosiglitazone (300 nM) in PASMCs from patients with idiopathic pulmonary arterial hypertension (IPAH) compared with DMSO (0.05%) or ROSI alone. (F) Effect of cicaprost (1 μM) and GW9662 (0.1 μM) on 3H-thymidine incorporation in cultured distal PASMC from IP receptor–deficient mice. In C and F, cells were pretreated with GW9662 for 1 hour before addition of the agonist. Data are expressed as mean ± SEM (n = 9–15 for A, D, and E; n = 3–5 for C and F). *P < 0.05, **P < 0.01, and ***P < 0.001 with respect to appropriate control or as indicated.
Next we determined whether PPARγ could contribute to prostacyclin analog effects in smooth muscle. Pretreatment of normal human PASMCs with 1 μM GW9662 caused a small (∼ 24%) but significant (P < 0.05) reversal of the antiproliferative effects of 100 nM treprostinil (Figure 8C). By contrast, the antagonist reversed the antiproliferative effects of treprostinil by approximately 60% (n = 15; P < 0.001) in IPAH cells, although it had no effect on growth by itself (Figure 8D). Moreover, in IPAH cells, rosiglitazone (300 nM) potentiated the antiproliferative effects of treprostinil such that it could significantly (P < 0.01) inhibit growth below 1 nM. No additive effects were seen at higher treprostinil (> 10 nM) concentrations, suggesting that both agents activate a common pathway. Finally, we found that GW9662 (100 nM) could reverse the effects of cicaprost on PDGF-induced 3H-thymidine incorporation in IP−/− cells, suggesting that the prostacyclin analog can affect PPARγ independently of the IP receptor and cAMP. However, GW9662 augmented PDGF-induced 3H-thymidine incorporation when applied by itself, indicative of increased basal PPARγ activity in IP−/− cells, although we cannot exclude non–PPARγ-mediated effects.
DISCUSSION
From a clinical perspective, we addressed the question of whether patients deteriorating with advanced pulmonary disease continue to derive benefit from a therapy that might down-regulate the target (IP receptor) it is seeking to activate. Specifically, we wished to establish whether prostacyclin analogs would suppress the replication of PASMCs isolated from patients with IPAH and to determine whether this involved the IP receptor. We found that the ability of treprostinil and iloprost to increase cAMP and suppress growth of PASMCs was not impaired in end-stage disease despite a reduction in IP receptor expression, which was more marked in pulmonary vessels from prostacyclin-treated than from untreated patients with IPAH. The striking finding in this study was that neither the IP receptor nor cAMP appeared to mediate the antiproliferative effects of prostacyclin analogs in IPAH cells as they did to a large extent in normal PASMCs. Experiments in IP−/− cells confirmed that non-IP receptor mechanisms prevailed at higher (1 μM) analog concentrations. Significant enhancement of PPARγ expression was found in the medial layer of arteries from patients with IPAH, and the PPARγ antagonist GW9662 substantially inhibited the antiproliferative effect of treprostinil in IPAH cells but had less effect in normal PASMCs. Moreover, the PPARγ agonist rosiglitazone potentiated the antiproliferative effects of treprostinil in a manner that suggests they share a common pathway. Thus, we show for the first time that PPARγ may represent an important therapeutic target by which prostacyclin analogs work in IPAH.
Role of the IP Receptor in IPAH
We found that IP receptor mRNA and protein levels were reduced in PASMCs from patients with IPAH, consistent with recent studies showing IP receptor loss in whole lungs from patients with IPAH and in rats after monocrotaline treatment (12). Furthermore, intimal proliferating cells of distal vessels stained weakly for the IP receptor, which agrees with reports of decreased expression of prostacyclin synthase in small pulmonary arterial vessels in IPAH and its absence in concentric plexiform lesions (19). Several reasons suggest that reduced IP receptor expression might be detrimental in IPAH. IP receptor–null mice develop more severe PAH and pulmonary arterial medial thickening in response to chronic hypoxia than wild-type mice (20). They are also more susceptible to injury-induced vascular proliferation and thrombosis and have elevated plasma levels of thromboxane A2 (21), all common features of IPAH (22–24). In this study, we found that reduced IP receptor expression was associated with a doubling of the growth rate in IPAH compared with normal PASMCs. Conversely, HEK-293 cells stably expressing the IP receptor grew at half the rate of those without the receptor. Thus, IP receptor deficiency could contribute to enhanced growth in IPAH by allowing growth factor signaling, which is elevated in patients IPAH (25), to go unopposed. However, IP receptor deficiency alone is unlikely to explain the more aggressive proliferative rate of IPAH cells from children compared with adults because both groups had similar levels of IP receptor expression. Moreover, IP−/− receptor mice do not develop spontaneous pulmonary hypertension, suggesting that the IP receptor is a modulator rather than an instigator of the disease process (20).
IP receptor desensitization could be a problem to the clinician using long-term prostacyclin to treat IPAH. IP receptor staining was less in intraacinar arteries from treated compared with untreated patients with IPAH. The former had been on prostacyclin therapy, some as long as 4 years. Iloprost is known to induce rapid time- and concentration-dependent phosphorylation and internalization of the IP receptor (26). Such an effect could explain the more diffuse and largely cytosolic staining we observed in PASMCs of treated patients with IPAH. The mechanism of receptor desensitization can be overcome with EP1 receptor blockade in rabbit lungs (27). That iloprost (and to a lesser extent prostacyclin) can bind to contractile EP1 and EP3 receptors with a similar potency to IP receptors (8, 9) suggests that tachyphylaxis to these agents may be accentuated in IPAH. Alternatively, loss of the IP receptor may drive prostacyclin analogs to signal through other Gs-coupled prostanoid receptors, including EP4 for iloprost (12) and EP2 for treprostinil (28). Thus, the possibility that these receptors contribute analog effects in IPAH cells cannot be excluded. Consistent with this notion, we found that iloprost increased cAMP in IP receptor–deficient PASMCs. Whether the combination of reduced receptor expression and increased desensitization explains the customary need to escalate the dose of prostacyclin and its analogs as PAH progresses is unclear because the IP receptor did not seem to mediate the antiproliferative effects of treprostinil or iloprost in PASMCs from patients with IPAH.
Antiproliferative Mechanism of Prostacyclin Analogs
Our results imply a substantial role for IP receptor–induced cAMP mediating growth inhibition in normal human PASMCs and HEK-293-IP cells. Not only did the IP receptor antagonist and adenylyl cyclase inhibitor substantially inhibit antiproliferative responses to low (≤ 100 nM) concentrations of prostacyclin analogs, but gene deletion of the IP receptor in mouse PASMCs did also. These results confirm earlier observations of cAMP involvement in analog inhibition of mitogenic responses to PDGF and serum in human PASMCs (6, 7). However, the antiproliferative effects of prostacyclin analogs were still observed in IP−/− cells, and neither the IP receptor antagonist nor the adenylate cyclase inhibitor 2′5′dideoxyadenosine significantly reversed the effects to higher (1 μM) analog concentrations in normal human PASMCs, strongly suggesting that additional mechanisms are involved. Indeed, the small but significant reversal of the antiproliferative effect with the PPARγ antagonist GW9662 in normal human and IP−/− mouse PASMCs suggests the involvement of PPARγ. Likewise, treprostinil activated a luciferase reporter construct encoding the human PPARγ ligand binding domain in HEK-293-IP cells. We and others have shown that cicaprost and iloprost can also activate PPARγ, inhibiting serum-induced cell growth (11) or lung tumorigenesis (29). The mechanism of PPARγ activation is not well understood. It does not appear to require cAMP but may involve IP receptor–dependent and IP receptor–independent pathways (11, 29). Our results in IP−/− mouse PASMCs favor the latter, although we cannot rule out the possibility in these experiments that GW9662 is acting in a non–PPARγ-dependent manner because it enhanced PDGF-induced growth on its own. However, knockdown of PPARγ in endothelial cells enhances lung PDGF receptor protein levels (30), and PPARγ can oppose PDGF signaling in human PASMCs (18), thus providing a plausible explanation for the specificity of the observed effects of GW9662.
Role of PPARγ in IPAH
An important finding was the striking increase of PPARγ expression in the medial layer of diseased pulmonary arteries and the enhanced effects of the PPARγ antagonist on cell growth. This suggests a greater role for PPARγ in the regulation of PASMC growth in IPAH. As in the present study, Voelkel and colleagues found little PPARγ staining in the medial layer of normal lungs or in the proliferating cells of plexiform lesions, but they did not examine PPARγ expression in the pulmonary smooth muscle of patients with IPAH (13). The consequence of elevated PPARγ expression within the medial layer and its impact on disease progression warrants further investigation to determine whether increased expression translates into enhanced PPARγ activity or responsiveness to endogenous ligand activators, such as the prostaglandin metabolite 5-deoxy-delta-12,14-prostaglandin J2 (10). The overlapping concentration–response curves to the antiproliferative effects of the PPARγ agonist rosiglitazone we observed in PASMCs from normal and IPAH cells suggests not. PPARγ activity may remain low because of PDGF-mediated phosphorylation and inactivation of PPARγ or loss of signaling through BMRPII (31), a receptor pathway that inhibits PASMC proliferation by counteracting the effects of PDGF on PPARγ (18). However, PASMCs derived from a patient with a loss-of-function mutation in BMPRII (14) can be rescued by PPARγ agonists, suggesting that PPARγ remains a viable target when combatting abnormal proliferation in IPAH regardless of the BMPRII status of the patient (18). The enhanced antiproliferative effects of treprostinil in the subnanomolar range when applied in combination with rosiglitazone may argue for sensitization of the PPARγ pathway. The effects of rosiglitazone were not additive at higher treprostinil concentrations, suggesting that both agents activate a common pathway that eventually saturates. The mechanism of this potentiation is unknown. Treprostinil and rosiglitazone can interact with the ligand-binding domain of PPARγ (10, 11), so that one agent could enhance the binding of the other ligand activator, thus allowing PPARγ to activate at lower agonist concentrations. PPARγ also contains many phosphorylation sites (32), so that prostacyclin analogs may modulate PPARγ agonist activity in that way. It remains to be determined whether a combination therapy involving a prostacyclin and PPARγ agonist would translate into patient benefit with increased survival. Targeted deletion of PPARγ in smooth muscle causes pulmonary hypertension and muscularization of distal pulmonary arteries (18), demonstrating the importance of PPARγ in regulating lung function. The idea that PPARγ might be a clinically relevant target in pulmonary vascular disease is supported by recent work showing that rosiglitazone can reverse mucularization of distal pulmonary arteries in male apoE−/− mice with severe PAH (33).
In summary, we have identified a novel mechanism by which prostacyclin analogs inhibit PASMC growth in patients with IPAH. The mechanism does not require the classical pathway of IP receptor activation and cAMP elevation but involves PPARγ, itself a major regulator of vascular remodeling and inflammation in the lung. Clarification of these interrelated signaling pathways will be crucial in identifying novel targets to improve the treatment of patients with PAH. The combination of prostacyclin analogs and PPARγ agonists appears likely to be a helpful antiproliferative therapy to target abnormal vascular remodeling.
Supplementary Material
Acknowledgments
The authors thank Thomas Briston for technical help.
Supported by a British Heart Foundation Ph.D. studentship (FS/02/060) and a Medical Research Senior Fellowship grant (G117/440) (L.H.C.) and by an MRC Clinical Training Fellowship (P.G.P.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201001-0011OC on July 9, 2010
Author Disclosure: E.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.G.P. is an employee of Pfizer Ltd. and holds $10,001 to $50,000 in stock ownership or options from Pfizer. J.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.W.M. received more than $100,001 from Novartis plc in industry-sponsored grants. S.G.H. received up to $1,000 from Pfizer and up to $1,000 from GlaxoSmithKline in consultancy fees, up to $1,000 from Pfizer, up to $1,000 from Actelion, up to $1,000 from GlaxoSmithKline, and up to $1,000 from Bayer in advisory board fees and has received $50,001 to $100,001 from Actelion in industry-sponsored grants as support for part time research fellow. L.H.C. received $1,001 to $5,000 from CoNCERT Pharmaceuticals, Inc. and $10,001 to $50,000 from Cytokinetics, USA in consultancy fees, $1,001 to $5,000 from United Therapeutics in advisory board fees, $1,001 to $5,000 from United Therapeutics in lecture fees for pulmonary conferences, $50,001 to $100,001 from Pfizer for an educational grant, $10,001 to $50,000 from United Therapeutics for an educational grant, and more than $100,001 from the British Heart Foundation in sponsored grants.
References
- 1.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 1991;115:343–349. [DOI] [PubMed] [Google Scholar]
- 2.Sandoval J, Bauerle O, Gomez A, Palomar A, Martinez Guerra ML, Furuya ME. Primary pulmonary hypertension in children: clinical characterization and survival. J Am Coll Cardiol 1995;25:466–474. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes CJ, Davidson A, Gibbs JS, Wharton J, Wilkins MR. Therapeutic targets in pulmonary arterial hypertension. Pharmacol Ther 2009;121:69–88. [DOI] [PubMed] [Google Scholar]
- 4.Schermuly RT, Yilmaz H, Ghofrani HA, Woyda K, Pullamsetti S, Schulz A, Gessler T, Dumitrascu R, Weissmann N, Grimminger F, et al. Inhaled iloprost reverses vascular remodeling in chronic experimental pulmonary hypertension. Am J Respir Crit Care Med 2005;172:358–363. [DOI] [PubMed] [Google Scholar]
- 5.Haworth SG. Pulmonary hypertension in the young. Heart 2002;88:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wharton J, Davie N, Upton PD, Yacoub MH, Polak JM, Morrell NW. Prostacyclin analogues differentially inhibit growth of distal and proximal human pulmonary artery smooth muscle cells. Circulation 2000;102:3130–3136. [DOI] [PubMed] [Google Scholar]
- 7.Clapp LH, Finney PA, Turcato S, Tran S, Rubin LJ, Tinker A. Differential effects of stable prostacyclin analogues on smooth muscle proliferation and cyclic AMP generation in human pulmonary artery. Am J Respir Cell Mol Biol 2002;26:194–201. [DOI] [PubMed] [Google Scholar]
- 8.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structure, properties, and functions. Physiol Rev 1999;79:1193–1226. [DOI] [PubMed] [Google Scholar]
- 9.Wise H. Multiple signalling options for prostacyclin. Acta Pharmacol Sin 2003;24:625–630. [PubMed] [Google Scholar]
- 10.Ward JE, Tan X. Peroxisome proliferator activated receptor ligands as regulators of airway inflammation and remodelling in chronic lung disease. PPAR Res 2007;2007:14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falcetti E, Flavell DM, Staels B, Tinker A, Haworth SG, Clapp LH. IP receptor-dependent activation of PPARγ by stable prostacyclin analogues. Biochem Biophys Res Commun 2007;360:821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai YJ, Pullamsetti SS, Dony E, Weissmann N, Butrous G, Banat GA, Ghofrani HA, Seeger W, Grimminger F, Schermuly RT. Role of the prostanoid EP4 receptor in iloprost-mediated vasodilatation in pulmonary hypertension. Am J Respir Crit Care Med 2008;178:188–196. [DOI] [PubMed] [Google Scholar]
- 13.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARγ) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res 2003;92:1162–1169. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H, Trembath RC, Morrell NW. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res 2005;96:1053–1063. [DOI] [PubMed] [Google Scholar]
- 15.Morrell NW, Upton PD, Kotecha S, Huntley A, Yacoub MH, Polak JM, Wharton J. Angiotensin II activates MAPK and stimulates growth of human pulmonary artery smooth muscle via AT1 receptors. Am J Physiol 1999;277:L440–L448. [DOI] [PubMed] [Google Scholar]
- 16.Hall SM, Hislop AA, Wu Z, Haworth SG. Remodelling of the pulmonary arteries during recovery from pulmonary hypertension induced by neonatal hypoxia. J Pathol 2004;203:575–583. [DOI] [PubMed] [Google Scholar]
- 17.Bley KR, Bhattacharya A, Daniels DV, Gever J, Jahangir A, O'yang C, Smith S, Srinivasan D, Ford AP, Jett MF. RO1138452 and RO3244794: characterization of structurally distinct, potent and selective IP (prostacyclin) receptor antagonists. Br J Pharmacol 2006;147:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, et al. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 2008;118:1846–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch D, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 1999;159:1925–1932. [DOI] [PubMed] [Google Scholar]
- 20.Hoshikawa Y, Voelkel NF, Gesell TL, Moore MD, Morris KG, Alger LA, Narumiya S, Geraci MW. Prostacyclin receptor-dependent modulation of pulmonary vascular remodeling. Am J Respir Crit Care Med 2001;164:314–318. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, Lawson JA, FitzGerald GA. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science 2002;296:539–541. [DOI] [PubMed] [Google Scholar]
- 22.Olschewski H, Rose F, Schermuly R, Ghofrani HA, Enke B, Olschewski A, Seeger W. Prostacyclin and its analogues in the treatment of pulmonary hypertension. Pharmacol Ther 2004;102:139–153. [DOI] [PubMed] [Google Scholar]
- 23.Adatia I, Barrow SE, Stratton PD, Miall-Allen VM, Ritter JM, Haworth SG. Thromboxane A2 and prostacyclin biosynthesis in children and adolescents with pulmonary vascular disease. Circulation 1993;88:2117–2122. [DOI] [PubMed] [Google Scholar]
- 24.Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 1992;327:70–75. [DOI] [PubMed] [Google Scholar]
- 25.Perros F, Montani D, Dorfmuller P, Durand-Gasselin I, Tcherakian C, Le PJ, Mazmanian M, Fadel E, Mussot S, Mercier O, et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2008;178:81–88. [DOI] [PubMed] [Google Scholar]
- 26.Smyth EM, Li WH, FitzGerald GA. Phosphorylation of the prostacyclin receptor during homologous desensitization: a critical role for protein kinase c. J Biol Chem 1998;273:23258–23266. [DOI] [PubMed] [Google Scholar]
- 27.Schermuly RT, Pullamsetti SS, Breitenbach SC, Weissmann N, Ghofrani HA, Grimminger F, Nilius SM, Schror K, Kirchrath JM, Seeger W, et al. Iloprost-induced desensitization of the prostacyclin receptor in isolated rabbit lungs. Respir Res 2007;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aronoff DM, Peres CM, Serezani CH, Ballinger MN, Carstens JK, Coleman N, Moore BB, Peebles RS, Faccioli LH, Peters-Golden M. Synthetic prostacyclin analogs differentially regulate macrophage function via distinct analog-receptor binding specificities. J Immunol 2007;178:1628–1634. [DOI] [PubMed] [Google Scholar]
- 29.Nemenoff R, Meyer AM, Hudish TM, Mozer AB, Snee A, Narumiya S, Stearman RS, Winn RA, Weiser-Evans M, Geraci MW, et al. Prostacyclin prevents murine lung cancer independent of the membrane receptor by activation of peroxisomal proliferator–activated receptor γ. Cancer Prev Res (Phila Pa) 2008;1:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator-activated receptor-gamma in mice causes PDGF receptor-beta-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol 2009;297:L1082–L1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewachter L, Adnot S, Guignabert C, Tu L, Marcos E, Fadel E, Humbert M, Dartevelle P, Simonneau G, Naeije R, et al. Bone morphogenetic protein signalling in heritable versus idiopathic pulmonary hypertension. Eur Respir J 2009;34:1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelman L, Michalik L, Desvergne B, Wahli W. Kinase signaling cascades that modulate peroxisome proliferator-activated receptors. Curr Opin Cell Biol 2005;17:216–222. [DOI] [PubMed] [Google Scholar]
- 33.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation 2007;115:1275–1284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.