Abstract
Rationale: Pulmonary arterial hypertension (PAH) is a progressive condition with a poor prognosis. Platelet-derived growth factor receptor (PDGFR) signaling plays an important role in its pathobiology.
Objectives: To assess safety, tolerability, and efficacy of the PDGFR inhibitor imatinib in patients with PAH.
Methods: Patients with PAH in functional classes II–IV were enrolled in a 24-week randomized, double-blind, placebo-controlled pilot study. Patients received imatinib (an inhibitor of PDGFR activity) 200 mg orally once daily (or placebo), which was increased to 400 mg if the initial dose was well tolerated. The primary endpoints were safety and change from baseline in the 6-minute-walk distance (6MWD). Secondary endpoints included hemodynamics and functional classification.
Measurements and Main Results: Fifty-nine patients enrolled (imatinib [n = 28]; placebo [n = 31]); 42 completed the study. Dropouts were equally matched between the two groups. In the intention-to-treat (ITT) population there was no significant change in the 6MWD (mean ± SD) in the imatinib versus placebo group (+22 ± 63 versus −1.0 ± 53 m). There was a significant decrease in pulmonary vascular resistance (imatinib −300 ± 347 versus placebo –78 ± 269 dynes · s · cm−5, P < 0.01) and increase in cardiac output (imatinib +0.6 ± 1.2 versus placebo −0.1 ± 0.9 L/min, P = 0.02). Serious adverse events occurred in 11 imatinib recipients (39%) and 7 placebo recipients (23%). Three deaths occurred in each group. Post hoc subgroup analyses suggest that patients with greater hemodynamic impairment may respond better than patients with less impairment.
Conclusions: These data from a Phase II study are consistent with imatinib being well tolerated in patients with PAH, and provide proof of concept for further studies evaluating its safety, tolerability, and efficacy in PAH.
Clinical trial registered with www.clinicaltrials.gov (NCT00477269).
Keywords: pulmonary hypertension, imatinib, exercise, hemodynamics
AT A GLANCE COMMENTARY.
Scientific Knowledge on This Subject
Despite currently available treatments, pulmonary arterial hypertension (PAH) remains a progressive and frequently fatal condition. Platelet-derived growth factor (PDGF) and its receptor (PDGFR) have been implicated in the pathobiology of pulmonary hypertension in patients with PAH, and are potential new targets for the treatment of this disease. Imatinib, a tyrosine kinase inhibitor of PDGFR α and β kinases, Abl, DDR, and c-KIT may therefore prove efficacious in the treatment of PAH. This is a double-blind, placebo-controlled trial to assess the safety, tolerability, and efficacy of imatinib in patients with PAH on stable PAH medications.
What This Study Adds to the Field
The primary end points of this study were safety, tolerability, and change from baseline in 6-minute walking distance. Imatinib was well tolerated. Compared with placebo, there was no significant change in 6-minute walking distance; however, there was a significant decrease in pulmonary vascular resistance and an increase in cardiac output with imatinib. Post hoc analyses showed that patients with greater hemodynamic impairment (i.e. with pulmonary vascular resistance ≥1,000 dynes) may respond better than patients with less impairment. The results of this study suggest that imatinib is well tolerated in patients with PAH and may be efficacious as add-on therapy in PAH.
Pulmonary arterial hypertension (PAH) (defined as a mean pulmonary artery pressure [PAPm] of ≥25 mm Hg at rest and mean pulmonary capillary wedge pressure ≤15 mm Hg) leads to progressive increases in pulmonary vascular resistance (PVR), right ventricular failure, and death if untreated (1). Estimated 1- and 3-year survival rates in idiopathic PAH without targeted therapy are 68% and 48%, respectively (2).
Current drug therapy recommendations for PAH vary depending on the patient's functional class (FC) (World Health Organization's [WHO] Modification for Pulmonary Hypertension of the New York Heart Association Functional Class) (3). The oral phosphodiesterase type 5 (PDE5) inhibitors sildenafil and tadalafil (4), oral endothelin receptor antagonists (ERAs) bosentan, ambrisentan, and sitaxsentan (not in the United States), and prostacyclin analogs epoprostenol (intravenous), iloprost (inhaled), and treprostinil (inhaled, subcutaneous, or intravenous) are approved for patients in FC II–IV. Patients in FC III or IV who fail to improve or deteriorate with monotherapy can be treated with combination therapy; atrial septostomy; or transplantation (lung or heart-lung). However, to date, only lung transplantation offers a cure for PAH. PAH remains a progressive and frequently fatal condition (5). Two recent meta-analyses highlighted the beneficial effects of prostacyclin analogs, ERAs, and PDE5 inhibitors on exercise capacity and other clinical end points in patients with PAH, whereas only the most recent report by Galiè and coworkers (4) provided evidence of improved survival with the aforementioned treatments (6, 7).
Pathologic changes in the pulmonary arteries of patients with PAH include the formation of plexiform lesions and smooth muscle and fibroblast proliferation leading to vascular obstruction (8). Platelet-derived growth factor (PDGF) is a vascular smooth muscle cell mitogen activating signal transduction pathways associated with smooth muscle hyperplasia in pulmonary hypertension (9, 10). PDGF and its receptor (PDGFR) have been implicated in the pathobiology of pulmonary hypertension in animal studies and in patients with PAH (11–13), thereby offering a potential new target for treatment. Recent data suggest that c-Kit may also be involved in the pathobiology of PAH (14).
Imatinib, a tyrosine kinase inhibitor that inhibits PDGFR α and β kinases, Abl, DDR, and c-KIT (15, 16), may therefore prove efficacious in the treatment of PAH. Several case reports have provided promising results, warranting further study of imatinib in PAH (17–19).
We compared the effects of imatinib with placebo in a randomized, double-blind, placebo-controlled pilot study in patients with PAH who had not adequately improved with prostacyclin analogs, ERAs, PDE5 inhibitors, or combinations of these therapies (i.e., remained in WHO FC II–IV). Some of the results from this study have been reported previously in the form of abstracts (20–22).
METHODS
Study Objectives and Design
The primary objectives were to assess the safety and tolerability of imatinib compared with placebo in patients with PAH and to evaluate its efficacy using the 6-minute-walk test (6MW test). Secondary objectives included changes in hemodynamic variables and FC.
Patients (≥ 18 yr) in FC II–IV with idiopathic or heritable PAH, or PAH associated with connective tissue disease or congenital heart disease (WHO group I), and PVR greater than 300 dynes · s · cm−5 were eligible. Patients were on stable PAH medications for more than 3 months before enrolment, but remained symptomatic in the opinion of the investigator and in FC II–IV (e.g., patients who were able to carry out activities of daily living but had poor prognosis by other assessments, such as hemodynamics, right ventricular function by echo, presence of pericardial effusion, high B-type natriuretic peptide, high uric acid, or low plasma sodium). Women of child-bearing potential were required to use double-barrier contraception.
Patients with other causes of PAH were excluded. Patients were not allowed to use nonspecific PDE inhibitors, chronic inhaled nitric oxide therapy, or catecholamines during the study. Additional exclusion criteria included participation in another clinical trial within 3 months; donation or loss of blood (>400 ml) within 8 weeks; and a history of another significant illness within 4 weeks. Patients were also excluded if they had preexisting lung disease; coagulation disorders; thrombocytopenia; major bleeding or intracranial hemorrhage; history of latent bleeding risk; elevated liver transaminases (>4 times upper limit of normal), elevated bilirubin (>2 times upper limit of normal); elevated serum creatinine (>200 μmol/l); history of elevated intracranial pressure; pregnancy; breast feeding; sickle cell anemia; history of clinically significant drug allergy or atopic allergy; history of immunodeficiency or hepatitis B or C; or history of drug or alcohol abuse. Patients were excluded if they had known hypersensitivity to the study drug, any condition that could alter the study drug pharmacokinetics or put them at risk, if their underlying disease was likely to result in failure to survive the study, or if they were unable to perform the 6MW test because of a condition other than PAH. Eligible patients were enrolled at seven centers in Germany, the United Kingdom, Austria, and the United States and randomized 1:1 to treatment with either imatinib or placebo.
The study was designed, implemented, and reported in accordance with the International Conference on Harmonization Harmonized Tripartite Guidelines for Good Clinical Practice and all applicable local regulations (including European Directive 2001/83/EC and US Code of Federal Regulations Title 21) and with the ethical principles laid down in the Declaration of Helsinki. This study was approved by institutional review boards at all centers and all patients signed informed consent before enrolment. All deaths and safety data were reviewed throughout the study by an external data safety monitoring board.
Interventions
Treatment with imatinib (or placebo) was initiated at a dose of 200 mg orally once daily for the first 2 weeks of treatment. If treatment was well tolerated, the dose was increased to 400 mg/day. If the 400-mg dose was not well tolerated, down-titration to 200 mg was permitted. Patients and investigators were blind to the treatment allocation. The blinding could be broken in an emergency.
Efficacy Assessments
The primary efficacy outcome was the between-group difference in the 6MW distance (6MWD) (23) at baseline and at 6 months. Complete hemodynamic parameters were assessed with standard techniques. FC was classified according to the WHO modification of the New York Heart Association criteria for pulmonary hypertension.
Safety Assessments
Blood cell counts, hepatic and renal function parameters, and echocardiography were monitored during the study. Patients were also interviewed by way of regular telephone calls between scheduled study visits.
Statistical Analysis
The planned sample size of 60 subjects was selected to address both safety and the primary efficacy outcome (6MWD). For the primary efficacy outcome it was estimated that the study had 80% power to detect a 55-m increase in the 6MWD with 95% confidence (two-sided P < 0.05), based on a SD of 75 m.
Analyses were conducted within the intention-to-treat population, which consisted of all patients who received at least one dose of study medication. Dropouts were excluded from the analysis. The primary efficacy analysis (6MWD) was performed using analysis of covariance with baseline value as a covariate. Analysis of covariance was also used to assess between-group differences in hemodynamics and blood gases. Missing data were not imputed; only subjects with assessment both at baseline and posttreatment were included in the analysis of covariance. FC was compared using Fisher test.
To generate new hypotheses and to identify patient subgroups that may respond better than other subgroups to imatinib, additional post hoc, nonprescribed subgroup analyses were conducted in patients with PVR values of greater than or equal to 1,000 versus less than 1,000 dynes · s · cm−5 (median of the data) at baseline.
RESULTS
Disposition and Baseline Characteristics
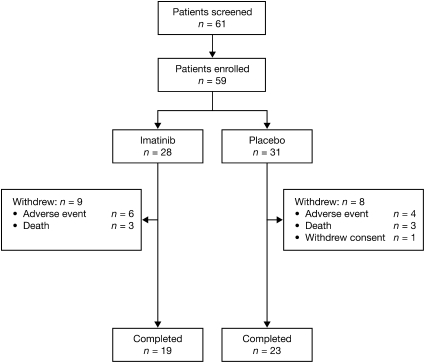
Fifty-nine patients (40 female, 19 male) enrolled and 42 (71.2%) completed the 6-month study (Figure 1). The reasons for withdrawals in the treatment group included death (n = 3); worsening of PAH (n = 2); worsening of general condition (n = 1); respiratory infection (n = 1); right ventricular failure (n = 1); and other adverse events (AEs) (n = 1, loss of appetite and fatigue). The reasons for withdrawals in the placebo group included death (n = 3); worsening of PAH (n = 2); worsening of general condition (n = 1); subject withdrew consent (n = 1); and other AEs (n = 1, back pain, acid reflux, dizziness, heart flutters, chest wall pain). Baseline characteristics were similar between the two treatment groups (Table 1). Overall, patients had a mean age of 44.3 years, mean weight of 68.7 kg, and mean body mass index of 24.6 kg/m2. Fifty-five of the 59 patients were white and 78% had idiopathic PAH (Table 1). Eighteen patients (32%) were WHO FC II; 36 (63%) were WHO FC III; and 3 (5%) were WHO FC IV. There was no significant difference in WHO FC between imatinib and placebo groups (P = 0.24). At baseline, 79% of the imatinib- and 81% of the placebo-group patients were receiving combination targeted therapy (Table 1).
Figure 1.
Patient disposition in the intention-to-treat population.
TABLE 1.
BASELINE CHARACTERISTICS OF THE INTENTION-TO-TREAT POPULATION
| Imatinib (n = 28) | Placebo (n = 31) | |
|---|---|---|
| Age, y, mean (SD) | 44.4 (15.3) | 44.2 (15.7) |
| Sex, male/female, n (%) | 10 (36)/8 (64) | 9 (29)/22 (71) |
| Ethnicity, n (%) | ||
| White | 26 (92) | 29 (94) |
| Asian | 0 | 1 (3) |
| Black | 1 (4) | 0 |
| Pacific Islander | 0 | 1 (3) |
| Hispanic | 1 (4) | 0 |
| Weight, kg, mean (SD) | 70 (15) | 67 (23) |
| Height, cm, mean (SD) | 169 (9) | 164 (9) |
| Diagnosis, n (%) | ||
| Idiopathic PAH | 21 (75) | 25 (81) |
| Heritable PAH | 2 (7) | 0 |
| PAH associated with systemic sclerosis | 1 (4) | 5 (16) |
| Other | 4 (14) | 1 (3) |
| WHO classification, n (%)* | ||
| Class II | 11 (41) | 7 (23) |
| Class III | 14 (52) | 22 (73) |
| Class IV | 2 (7) | 1 (3) |
| PAH specific treatments, n (%) | ||
| ERA alone | 2 (7) | 4 (13) |
| Sildenafil alone | 2 (7) | 0 (0) |
| Prostacylin analog† alone | 2 (7) | 1 (3) |
| ERA + prostacylin analog† | 1 (4) | 3 (10) |
| ERA + sildenafil | 12 (43) | 9 (29) |
| Sildenafil + prostacyclin analog† | 5 (18) | 3 (10) |
| ERA + sildenafil + prostacyclin | 4 (14) | 10 (32) |
| Calcium channel blocker for PAH | 0 | 1 (3) |
Definition of abbreviations: ERA = endothelin receptor antagonists (bosentan and ambrisentan); PAH = pulmonary arterial hypertension; WHO = World Health Organization.
WHO assessment was not available for one patient receiving imatinib.
Iloprost, epoprostenol, trepostinil, or beraprost.
Efficacy Outcomes
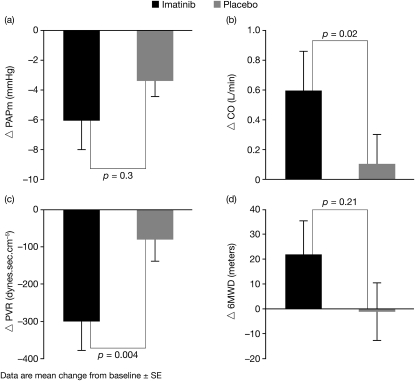
The mean (± SD) 6MWD did not change significantly in the imatinib group versus placebo (+22 ± 63 versus −1 ± 53 m; mean treatment difference 21.7 m; 95% confidence interval [CI], −13 to 56.5; P = 0.21) (Table 2, Figure 2). There was, however, a significant decrease in PVR (mean treatment difference −230.7 dynes; 95% CI, −383.7 to −77.8; P = 0.004) and increase in cardiac output (CO) (mean treatment difference 0.68 L/min; 95% CI, 0.10–1.26; P = 0.02) in imatinib recipients compared with placebo (Figure 2, Table 3). There was no significant difference in PAPm (Figure 2) or change in FC between imatinib and placebo-treated patients (data not shown).
TABLE 2.
SIX-MINUTE-WALK DISTANCE AND PULMONARY HEMODYNAMICS AT BASELINE AND END OF STUDY AFTER IMATINIB AND PLACEBO THERAPY IN PATIENTS WITH PULMONARY ARTERIAL HYPERTENSION
| Imatinib |
Placebo |
|||
|---|---|---|---|---|
| Patients (n) | Absolute Value | Patients (n) | Absolute Value | |
| 6MWD, m (SD) | ||||
| Baseline | 28 | 392 (89) | 29* | 369 (118) |
| Study end | 21 | 419 (85) | 22 | 399 (86) |
| PAPm, mm Hg (SD) | ||||
| Baseline | 27 | 62 (16) | 28 | 59 (12) |
| Study end | 20 | 53 (11) | 22 | 56 (12) |
| CO, L/min (SD) | ||||
| Baseline | 27 | 4.2 (1.3) | 28 | 4.1 (1.4) |
| Study end | 20 | 4.9 (1.2) | 22 | 4.4 (1.6) |
| PVR, dynes · s · cm−5(SD) | ||||
| Baseline | 27 | 1,124 (460) | 27 | 1,118 (487) |
| Study end | 19 | 730 (230) | 21 | 1,017 (369) |
Definition of abbreviations: 6MWD = 6-minute-walk distance; CO = cardiac output; PAPm = mean pulmonary artery pressure; PVR = pulmonary vascular resistance.
Baseline data were missing for two patients.
Figure 2.
Mean change from baseline in pulmonary hemodynamics after 6 months of treatment with imatinib or placebo. (A) Mean pulmonary artery pressure (PAPm). (B) Cardiac output (CO). (C) Pulmonary vascular resistance (PVR). (D) 6-minute walking distance (6MWD).
TABLE 3.
SIX-MINUTE-WALK DISTANCE AND PULMONARY HEMODYNAMICS AT BASELINE AND END OF STUDY AFTER IMATINIB AND PLACEBO THERAPY IN PATIENTS WITH PAH (STRATIFIED POST HOC BY MEDIAN BASELINE PVR ≥1,000 DYNES · S · CM−5 OR ≤1,000 DYNES · S · CM−5)*
| Imatinib |
Placebo |
|||
|---|---|---|---|---|
| Patients (n) | Absolute Value | Patients (n) | Absolute Value | |
| PVR ≥1,000 dynes · s · cm−5 | ||||
| 6MWD, m (SD) | ||||
| Baseline | 14 | 352 (74) | 15 | 352 (138) |
| Study end | 9 | 400 (103) | 10 | 386 (100) |
| PAPm, mm Hg (SD) | ||||
| Baseline | 14 | 68 (16) | 16 | 63 (9) |
| Study end | 8 | 55 (10) | 12 | 59 (11) |
| CO, L/min (SD) | ||||
| Baseline | 14 | 3.3 (0.9) | 16 | 3.7 (1.1) |
| Study end | 8 | 4.3 (0.8) | 12 | 4.1 (1.8) |
| PVR, dynes · s · cm−5 (SD) | ||||
| Baseline | 14 | 1,493 (322) | 16 | 1,404 (407) |
| Study end | 8 | 855 (214) | 12 | 1,215 (354) |
| PVR <1,000 dynes · s · cm−5 | ||||
| 6MWD, m (SD) | ||||
| Baseline | 13 | 440 (86) | 11 | 385 (91) |
| Study end | 12 | 433 (69) | 9 | 416 (80) |
| PAPm, mm Hg (SD) | ||||
| Baseline | 13 | 55 (12) | 11 | 55 (13) |
| Study end | 12 | 51 (12) | 9 | 52 (12) |
| CO, L/min (SD) | ||||
| Baseline | 13 | 5.1 (1.1) | 11 | 4.6 (1.8) |
| Study end | 12 | 5.3 (1.3) | 9 | 4.7 (1.4) |
| PVR, dynes · s · cm−5 (SD) | ||||
| Baseline | 13 | 728 (133) | 11 | 703 (218) |
| Study end | 11 | 639 (204) | 8 | 748 (190) |
Definition of abbreviations: 6MWD = 6-minute-walk distance; CO = cardiac output; PAPm = mean pulmonary artery pressure; PVR = pulmonary vascular resistance.
To investigate the effect of the drop-outs and missing data on the conclusions, further analysis of 6MWD data was performed. The first of these analyses used the last observation carried forward method. The second analysis was a repeated-measures analysis of 6MWD and used data from all patients. These analyses resulted in a conclusion similar to that from the analysis already presented (i.e., a nonsignificant difference of approximately 20 m).
There was an increase in arterial and mixed venous oxygen saturation (P < 0.05) with imatinib. Systemic arterial oxygen saturation increased from 88 ± 9% to 93 ± 5% with imatinib treatment compared with no change with placebo (92 ± 4% at baseline versus 92 ± 3% at end of study; mean treatment difference 2.4%; 95% CI, 0.5–4.3). Mixed venous oxygen saturation increased from 58 ± 10% to 65 ± 7% with imatinib treatment (consistent with the increase in CO) and decreased with placebo (61 ± 6% at baseline versus 57 ± 9% at end of study; mean treatment difference 7%; 95% CI, 2.1–11.9).
There was no significant change in FC, heart rate, or blood pressure over time with imatinib or placebo.
Exploratory Post Hoc Subgroup Analyses
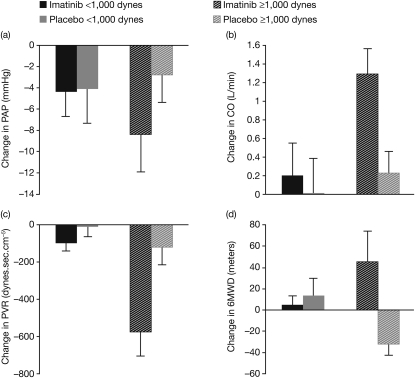
In patients with a baseline PVR greater than or equal to 1,000 dynes · s · cm−5 (median of the baseline data), there was a greater improvement between baseline and study end for PAPm, CO, and PVR in the imatinib group compared with placebo (Figure 3). Compared with the intention-to-treat population, the increase in 6MWD for the group with PVR greater than or equal to 1,000 dynes · s · cm−5 was statistically significant with a mean treatment difference of 74 m (95% CI, 3–144.1). The differences between imatinib and placebo in CO and 6MWD for subjects with PVR greater than or equal to 1,000 dynes · s · cm−5 were statistically significant at P less than 0.05. However, among patients with a baseline PVR less than 1,000 dynes · s · cm−5, no major differences between baseline and study end for PAPm, CO, PVR, or 6MWD were observed (Figure 3).
Figure 3.
Mean change from baseline to study end in pulmonary hemodynamics in patients randomized to imatinib or placebo, stratified by baseline pulmonary vascular resistance (PVR) greater than or equal to 1,000 dynes · s · cm−5 (imatinib n = 8; placebo n = 12) or less than 1,000 dynes · s · cm−5 (imatinib n = 12; placebo n = 9). (A) Mean pulmonary artery pressure (PAPm). (B) Cardiac output (CO). (C) PVR. (D) 6-minute walking distance (6MWD).
Safety and Tolerability
The most common AEs observed in this clinical study were as expected for this population and this drug. The most common AEs reported in the imatinib group were nausea (n = 14; 50%); headache (n = 10; 36%); and peripheral edema (n = 7; 25%). These AEs did not lead to discontinuation of study drug. Nausea was controlled by taking the medication with food. A total of 21 (75%) patients in the imatinib group and 24 (77%) patients in the placebo group reported AEs of mild intensity; 20 (71%) in the imatinib group and 19 (61%) in the placebo group patients reported AEs of moderate intensity; and 9 (32%) patients in the imatinib group and 5 (16%) patients in the placebo group reported AEs of severe intensity. Serious AEs were reported for 11 imatinib recipients (39%) and 7 placebo recipients (23%). Serious AEs in the imatinib group included cardiac arrest (n = 2); vertigo (n = 1); pancreatitis (n = 1); catheter-related complication (n = 1); liver dysfunction (n = 2); dizziness (n = 1); presyncope (n = 1); syncope (n = 1); hemoptysis (n = 1); worsening pulmonary hypertension (n = 3); and pulmonary arterial rupture (n = 1). Serious AEs in the placebo group included atrial flutter (n = 1); cardiac arrest (n = 1); right ventricular failure (n = 2); general physical health deterioration (n = 1); fluid retention (n = 1); dizziness (n = 1); cardiac tamponade secondary to pulmonary arterial rupture (n=1); and worsening pulmonary hypertension (n = 3).
Skin rash or pruritis without rash has been found in more than 10% of patients treated with imatinib for oncology indications. In this study, skin rash was reported in three patients (two mild and one moderate intensity) and pruritis in five patients (four mild and one moderate intensity).
Overall there was a fall in mean (SD) hemoglobin levels with imatinib (151 ± 14 to 128 ± 16 g/L) and a rise in hemoglobin levels with placebo (143 ± 25 to 152 ± 25 g/L). The cause of this fall in hemoglobin is not understood; however, in 16 subjects who have received imatinib for over 1 year there has been no further decline in hemoglobin.
There were no relevant changes in white blood cell count, platelet count albumin, alkaline phosphatase, total bilirubin, calcium, cholesterol, creatinine, g-GT, glucose, lactate dehydrogenase, inorganic phosphorus, lipase, amylase, potassium, total protein, C-reactive protein, glutamate oxalacetate transaminase, glutamate pyruvate transaminase, sodium, triglycerides, urea, or uric acid. There was also no evidence of left ventricular dysfunction on echocardiography. There was no change in mean pulmonary capillary wedge pressure in patients treated with imatinib (9 ± 3 mm Hg at baseline versus 8 ± 3 mm Hg at end of study) or placebo (8 ± 2 mm Hg at baseline versus 9 ± 3 mm Hg at end of study).
There were three deaths in each group. One patient in the imatinib group and one patient in the placebo group had spontaneous rupture of the pulmonary artery (fatal in both cases), not related to study treatment and confirmed on autopsy. An autopsy was also performed on one other patient who received placebo treatment. Right heart failure was found without evidence of pulmonary artery rupture. No autopsy was performed on the other three patients who died. Two additional patients died in the placebo group within 2 months of completing the study.
DISCUSSION
This is the first randomized, double-blind, placebo-controlled trial to assess the safety, tolerability, and efficacy of the tyrosine kinase inhibitor imatinib in patients with PAH. Although imatinib seemed safe and well tolerated over a 6-month period, the primary efficacy parameter (6MWD) did not improve in patients randomized to imatinib compared with placebo, despite significant improvement in secondary end points.
Overall, 59 patients were enrolled. As per the study protocol, only patients on background treatment with at least one PAH-specific drug (i.e., prostacyclin analogs, ERAs, PDE5 inhibitors) who had not adequately improved were enrolled (56% of patients were receiving two drugs and 24% were receiving three drugs at baseline). This may have contributed to the reduced improvement in 6MWD observed in this study compared with previous studies in which only treatment-naive patients were included (24–26). In clinical trials in which background-specific medications have been allowed, the overall improvement in 6MWD has been less than in the treatment-naive trials (27, 28).
It has been suggested that inhibition of the ABL tyrosine kinase pathway may infrequently induce myocardial damage in patients receiving long-term treatment with imatinib for chronic myeloid leukemia (CML) (29, 30). However, a long-term, multicenter study in a large population of patients with CML showed an acceptable safety profile for imatinib (31). A review of all patients receiving imatinib showed that 0.5% of patients per year developed incident congestive cardiac failure (no risk factors present). In patients with CML receiving imatinib, 0.4% of patients per year develop congestive cardiac failure compared with 0.75% per year for patients receiving interferon-γ plus Ara-C (32). Considering the potential for cardiotoxicity (which could be particularly problematic for patients with PAH), regular assessments of cardiac function by echocardiography and measurements of serum cardiac troponin levels were performed in this trial. Overall, there were no signals indicating a potential detrimental effect of imatinib on myocardial function when compared with the overall safety profile of the placebo group. In contrast, some of the beneficial effect of imatinib on PVR reduction seemed to be caused by improvements in CO. Nonetheless, cardiac safety remains a key concern with kinase inhibitors.
There were eight withdrawals (including three deaths) in the placebo group and nine withdrawals (including three deaths) in the imatinib group. This withdrawal rate may be higher than in previous PAH clinical trials. The usual drop out rate for studies in treatment-naive patients is approximately 5–10%; however, these data are from pure placebo-controlled trials or add on to only one background therapy with most such patients (if not all) considered not to need additional therapy for the duration of the trial. However, in our point-of care study, 80% were on two or more PAH-specific therapies, thus the death and drop out rate would be expected to be higher in patients on multiple therapies who remain symptomatic.
The drop in hemoglobin seen in this trial was less than that observed in studies of newly diagnosed CML. With newly diagnosed CML, Grade 3/4 anemia (hemoglobin <80 g/L) was reported in 4% of the subjects. For patients with CML in blast crisis, Grade 3/4 anemia was reported in 50% of patients. In our PAH study no Grade 3/4 anemia was observed. The lowest hemoglobin level was 111 g/L. Patients entered in our 24-week point-of-care study were eligible for inclusion in an extension study. There are 16 patients in the extension study who have been treated with imatinib for 1 to 3 years. The mean hemoglobin (g/L ± SD) for this group is 134 (± 20) and the lowest value is 109 g/L.
Because of the relatively small sample size there are limitations to the study. Some of the trends favoring imatinib were not statistically significant. There was no statistical difference in WHO FC between imatinib and placebo at baseline, but despite randomization there was a trend toward more WHO FC III patients and more patients on three specific PAH therapies in the placebo group. This indicates that the placebo patients may have had more severe disease, and this may contribute to the difference seen in pulmonary hemodynamics between the two groups.
Although no significant increases in 6MWD were observed with imatinib compared with placebo, significant improvements in CO and PVR were observed. These observations led us to undertake a post hoc analysis stratifying patients by baseline median PVR. In patients with baseline PVR greater than or equal to 1,000 dynes · s · cm−5, there were improvements from baseline to study end for 6MWD, PVR, and CO in the imatinib group, compared with placebo (Figure 3). This was not observed in the patients with baseline PVR levels less than 1,000 dynes · s · cm−5. The post hoc derived analyses were at an apparently statistically significant level, but because of the difficulty in interpreting “significance” from unplanned post hoc analyses these values should be viewed with caution. In addition, tyrosine kinase inhibitors are not recognized to have any significant vasodilator or inotropic effects (33), with their effects considered antiproliferative and proapoptotic. One hypothesis that could explain the current study results is that, for treatment with imatinib to be effective, a certain degree of disease severity (i.e., vascular proliferation) may be needed. However, because these data are hypothesis generating, it cannot be excluded that patients with less severe PAH may also benefit from long-term imatinib therapy by a preventive mechanism.
The results of this pilot study suggest that imatinib is well tolerated in patients with PAH and may be efficacious as add-on therapy in patients with PAH with inadequate improvement (in the judgment of the investigator) on at least one other PAH-targeted treatment. The study failed to show significant improvement in the primary end point (i.e., 6MWD). However, the hemodynamic improvements suggest that imatinib may be efficacious as add-on therapy. Based on this, a Phase III trial is in progress.
Acknowledgments
Editorial assistance was provided by Paul Hutchin. This assistance was funded by Novartis Pharma AG.
Supported by Novartis Pharma AG, Basel, Switzerland. Novartis Pharma AG participated in the design of the study, collection, analysis, and data interpretation; in writing the study report; and in the decision to submit the paper for publication. This study was supported in part by a Medical Research Council (UK) Experimental Medicine Award to N.W.M., and by the German Research Foundation (DFG, SFB 547) and the Excellence Cluster Cardio-Pulmonary System (ECCPS) to F.G. and H.A.G.
Originally Published in Press as DOI: 10.1164/rccm.201001-0123OC on June 25, 2010
Author Disclosure: H.A.G. received $10,001–$50,000 from Bayer Schering, $10,001–$50,000 from Pfizer, $10,001–$50,000 from Actelion, $10,001–$50,000 from Encysive, and $10,001–$50,000 from Nycomed in consultancy fees; $10,001–$50,000 from Bayer Schering, $10,001–$50,000 from Pfizer, $10,001–$50,000 from Actelion, and $10,001–$50,000 from Encysive in lecture fees; and more than $100,001 from Bayer Schering, $10,001–$50,000 from Encysive (bls June 2009), $10,001–$50,000 from Pfizer, and $10,001–$50,000 from Ergonex in industry-sponsored grants. N.W.M. received more than $100,001 in a research grant from Novartis. M.M.H. received $5,001–$100,000 from Pfizer in consultancy fees; $5,001–$10,000 from Pfizer, $1,001–$5,000 from Actelion, $1,001–$5,000 from Bayer, $1,001–$5,000 from LungRx, and $1,001–$5,000 from GlaxoSmithKline in advisory board fees; and $1,001–$5,000 from Pfizer, $1,001–$5,000 from Actelion, $1,001–$5,000 from Bayer, $1,001–$5,000 from GlaxoSmithKline, and $1,001–$5,000 from Lilly in lecture fees. H.O. received more than $100,001 from Unither as a consultant; $10,001–$50,000 from Bayer Schering Pharma, $5,001–$10,000 from GlaxoSmithKline, and $1,001–$5,000 from Novartis in advisory board fees; $10,001–$50,000 from Actelion, $10,001–$50,000 from Bayer Schering, $5,001–$10,000 from Pfizer, and $1,001–$5,000 from GlaxoSmithKline in lecture fees; $50,001–$100,000 from Actelion, $10,001–$50,000 from GlaxoSmithKline, and $10,001–$50,000 from Bayer Schering in industry-sponsored grants; and $50,001–$100,000 from the EU and $50,001–$100,000 from the OENB in sponsored grants. A.J.P. received $1,001–$5,000 from Pfizer, $1,001–$5,000 from GlaxoSmithKline, $1,001–$5,000 from Actelion, and $1,001–$5,000 from Eli Lilly in consultancy fees; $1,001–$5,000 from Bayer, $1,001–$5,000 from GlaxoSmithKline, and $1,001–$5,000 from Pfizer in advisory board fees; $1,001–$5,000 from GlaxoSmithKline and $1,001–$5,000 from United Therapeutics in lecture fees; and $10,001–$50,000 from Actelion, $10,001–$50,000 from GlaxoSmithKline, and $10,001–$50,000 from Pfizer in industry-sponsored grants. R.J.B. received $50,0001–$100,000 from Actelion in consultancy, advisory board, and lecture fees; $10,001–$50,000 from Gilead in consultancy, advisory board, and lecture fees; $1,001–$5,000 from Novartis and $1,001–$5,000 from Pfizer in consultancy and advisory board fees; $10,001–$50,000 from Eli Lilly in consultancy fees and $10,001–$50,000 for serving as an expert witness for diet pill litigation; $5,001–$10,000 from NHLBI in sponsored grants; and $5,001–$10,000 in royalties as the editor of a textbook. S.S. received $5,001–$10,000 from United Therapeutics and $5,001–$10,000 from Gilead for serving as a consultant; $1,001–$5,000 from Actelion in advisory board fees; $5,001–$10,000 from Gilead, $5,001–$10,000 from United Therapeutics, and $1,001–$5,000 from Actelion in lecture fees; and industry-sponsored grants from Novartis, Gilead, United Therapeutics, Pfizer, and Actelion. H.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.G. received $50,001–$100,000 from Altana in consultancy fees; $5,001–$10,000 from Pfizer, $1,001–$5,000 from Schering, $1,001–$5,000 from GlaxoSmithKline, $1,001–$5,000 from Actelion, and $1,001–$5,000 Encysive in lecture fees; and more than $100,001 from Pfizer, $1,001–$5,000 from Schering, more than $100,001 from Bayer, $10,001–$50,000 from Actelion, and $1,001–$50,000 from Encysive in industry-sponsored grants. S.P. is an employee of Novartis and owns stock in Novartis, which make and sell Gleevec (the therapeutic agent in the study).
References
- 1.Badesch DB, Champion HC, Gomez Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeige R, Olschewski H, Oudiz RJ, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:55S–66S. [DOI] [PubMed] [Google Scholar]
- 2.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115:343–349. [DOI] [PubMed] [Google Scholar]
- 3.Barst R, Gibbs JSR, Ghofrani HA, Hoeper MM, McLaughlin VV, Rubin LJ, Sitbon O, Tapson VF, Galiè N. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:78S–84S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009;119:2894–2903. [DOI] [PubMed] [Google Scholar]
- 5.Ghofrani HA, Wilkins MW, Rich S. Uncertainties in the diagnosis and treatment of pulmonary arterial hypertension. Circulation 2008;118:1195–1201. [DOI] [PubMed] [Google Scholar]
- 6.Macchia A, Marchioli R, Marfisi R, Scarano M, Levantesi G, Tavazzi L, Tognoni G. A meta-analysis of trials of pulmonary hypertension: a clinical condition looking for drugs and research methodology. Am Heart J 2007;153:1037–1047. [DOI] [PubMed] [Google Scholar]
- 7.Galiè N, Manes A, Negro L, Palazzini M, Bacchi-Reggiani ML, Branzi A. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J 2009;30:394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:20S–31S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase-deficient mouse. Am J Physiol Lung Cell Mol Physiol 2003;284:L964–L971. [DOI] [PubMed] [Google Scholar]
- 10.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 1999;79:1283–1316. [DOI] [PubMed] [Google Scholar]
- 11.Humbert M, Monti G, Fartoukh M, Magnan A, Brenot F, Rain B, Capron F, Galanaud P, Duroux P, Simonneau G, et al. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur Respir J 1998;11:554–559. [PubMed] [Google Scholar]
- 12.Balasubramaniam V, Le Cras TD, Ivy DD, Grover TR, Kinsella JP, Abman SH. Role of platelet-derived growth factor in vascular remodeling during pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 2003;284:L826–L833. [DOI] [PubMed] [Google Scholar]
- 13.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 2005;115:2811–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young KC, Torres E, Hehre D, Suguihara C, Hare J. Neonatal c-kit mutant mice exhibit decreased susceptibility to hypoxia-induced pulmonary hypertension. Circulation 2009;120:S750–S751. [Google Scholar]
- 15.Day E, Waters B, Spiegel K, Alnadaf T, Manley PW, Buchdunger E, Walker C, Jarai G. Inhibition of collagen induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib. Eur J Pharmacol 2008;599:44–53. [DOI] [PubMed] [Google Scholar]
- 16.de Kogel CE, Schellens JH. Imatinib. Oncologist 2007;12:1390–1394. [DOI] [PubMed] [Google Scholar]
- 17.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med 2005;353:1412–1413. [DOI] [PubMed] [Google Scholar]
- 18.Patterson KC, Weissmann A, Ahmadi T, Farber HW. Imatinib mesylate in the treatment of refractory idiopathic pulmonary arterial hypertension. Ann Intern Med 2006;145:152–153. [DOI] [PubMed] [Google Scholar]
- 19.Souza R, Sitbon O, Parent F, Simonneau G, Humbert M. Long term imatinib treatment in pulmonary arterial hypertension. Thorax 2006;61:736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barst RJ, Ghofrani A, Morrell N, Hoeper M, Olschewski H, Peacock A, Shapiro S, Pascoe S, Quinn DA. Imatinib mesylate treatment for severe pulmonary arterial hypertension: a proposed phase III 24-week double-blind placebo-controlled randomized clinical trial [abstract]. Chest Meeting Abstracts 2009;136:64S. [Google Scholar]
- 21.Ghofrani HA, Morrel NW, Hoeper MM, Olschewski H, Peacock D, Barst RJ, Shapiro S, Golpon H, Toshner M, Grimminger F, et al. Imatinib in patients with severe pulmonary artery hypertension (PAH) refractory to standard therapy [abstract]. Am J Respir Crit Care Med 2009;179:A1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghofrani HA, Morrell NW, Hoeper MM, Olschewski H, Peacock A, Barst RJ, Shapiro S, Quinn DA, Pascoe S. Long term use of imatinib in patients with severe pulmonary arterial hypertension [abstract]. Am J Respir Crit Care Med 2010;181:A2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 24.Rubin LJ, Badesch DB, Barst RJ, Galiè N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002;346:896–903. [DOI] [PubMed] [Google Scholar]
- 25.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, et al., for The Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med 1996;334:296–302. [DOI] [PubMed] [Google Scholar]
- 26.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, et al. Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005;353:2148–2157. [DOI] [PubMed] [Google Scholar]
- 27.Simonneau G, Rubin LJ, Galiè N, Barst RJ, Fleming TR, Frost AE, Engel PJ, Kramer MR, Burgess G, Collings L, et al., PACES Study Group. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med 2008;149:521–530. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, Badesch DB, Barst RJ, Hsu HH, Rubin LJ. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006;174:1257–1263. [DOI] [PubMed] [Google Scholar]
- 29.Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med 2006;12:908–916. [DOI] [PubMed] [Google Scholar]
- 30.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007;370:2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, et al. IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006;355:2408–2417. [DOI] [PubMed] [Google Scholar]
- 32.Hatfield A, Owen S, Pilot PR. In reply to ‘Cardiotoxicity of the cancer therapeutic agent imatinib mesylate’. Nat Med 2007;13:13, author reply 15–16. [DOI] [PubMed]
- 33.Quintás-Cardama A, Kantarjian H, Cortes J. Flying under the radar: the new wave of BCR-ABL inhibitors. Nat Rev Drug Discov 2007;6:834–848. [DOI] [PubMed] [Google Scholar]