Abstract
Rationale: Hypoventilation is typically treated with positive pressure ventilation or, in extreme cases, by phrenic nerve stimulation. This preclinical study explores whether direct stimulation of central chemoreceptors could be used as an alternative method to stimulate breathing.
Objectives: To determine whether activation of the retrotrapezoid nucleus (RTN), which is located in the rostral ventrolateral medulla (RVLM), stimulates breathing with appropriate selectivity.
Methods: A lentivirus was used to induce expression of the photoactivatable cationic channel channelrhodopsin-2 (ChR2) by RVLM Phox2b-containing neurons, a population that consists of central chemoreceptors (the ccRTN neurons) and blood pressure (BP)-regulating neurons (the C1 cells). The transfected neurons were activated with pulses of laser light. Respiratory effects were measured by plethysmography or diaphragmatic EMG recording and cardiovascular effects by monitoring BP, renal sympathetic nerve discharge, and the baroreflex.
Measurements and Main Results: The RVLM contained 600 to 900 ChR2-transfected neurons (63% C1, 37% ccRTN). RVLM photostimulation significantly increased breathing rate (+42%), tidal volume (21%), minute volume (68%), and peak expiratory flow (48%). Photostimulation increased diaphragm EMG amplitude (19%) and frequency (21%). Photostimulation increased BP (4 mmHg) and renal sympathetic nerve discharge (43%) while decreasing heart rate (15 bpm).
Conclusions: Photostimulation of ChR2-transfected RVLM Phox2b neurons produces a vigorous stimulation of breathing accompanied by a small sympathetically mediated increase in BP. These results demonstrate that breathing can be relatively selectively activated in resting unanesthetized mammals via optogenetic manipulation of RVLM neurons presumed to be central chemoreceptors. This methodology could perhaps be used in the future to enhance respiration in humans.
Keywords: medulla oblongata, retrotrapezoid nucleus, breathing, chemoreceptors, optogenetics
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
The retrotrapezoid nucleus (RTN), located within the rostral medulla oblongata, contains neurons suspected to function as central respiratory chemoreceptors. The contribution of RTN neurons to breathing in conscious adult mammals has not been completely elucidated.
What This Study Adds to the Field
This study demonstrates that a vigorous activation of breathing can be produced in conscious rats by photostimulating RTN neurons after their transfection with channelrhodopsin-2 (ChR2). This novel approach could conceivably be applied to the human condition when enhanced respiration is necessary to sustain life.
Sleep apnea is typically treated with positive pressure ventilation (1). Extreme forms, for example congenital central hypoventilation syndrome, can also be managed by electrically stimulating the phrenic nerve (2–5). The purpose of the present study is to test whether brain stimulation could be used to increase breathing in unanesthetized rats, the ultimate goal being to apply this method to the treatment of diseases in which enhanced respiration is necessary to sustain life. This objective presents several challenges. The respiratory centers are located within regions of the pons and medulla oblongata that contain a very heterogeneous cell population as well as the axons of innumerable neurons involved in functions other than breathing (6–9). Electrical or chemical stimulation of this brain region would most likely produce undesirable effects far beyond merely stimulating breathing. Also, to be effective in the context of sleep apnea, brain stimulation should activate breathing without disrupting the regularity of the respiratory rhythm and without impairing the coordination between the various respiratory outflows. The best option would therefore be to target neurons that globally up-regulate the activity of the respiratory controller (the network that generates the respiratory rhythm and the patterns of activity of respiratory muscles) without being a part of this network. The retrotrapezoid nucleus (RTN), located in the rostral ventrolateral medulla (RVLM), seems to fit this profile (10, 11). This nucleus contains a chemically well-defined neuronal population (henceforth called the ccRTN neurons after Reference 12) with central chemoreceptor properties and whose activation in anesthetized rats produces a robust stimulation of breathing that mimics many of the effects of increased Pco2 (11, 13, 14). Although electrical or chemical stimulation are not viable options to selectively target these neurons, the optogenetic approach (15) is promising because the ccRTN neurons make the transcription factor Phox2b throughout life (16) and can be made to express the light-gated cationic channel channelrhodopsin-2 (ChR2) after infection by a lentiviral vector that expresses the transgene under the control of the Phox2-responsive artificial promoter PRSx8 (14). After introducing this vector into the RVLM, the activity of ccRTN neurons can be increased with pulses of laser light delivered from a thin optical fiber, resulting in a substantial increase in breathing (14). A potential limitation of this approach is that the neighboring C1 cells also contain Phox2b; therefore, these cells can also express ChR2 (17). The C1 cells regulate the sympathetic vasomotor tone via their projection to the sympathetic preganglionic neurons (18, 19), and their photostimulation in vivo produces a sympathetically mediated increase in blood pressure (BP) (17). The BP increase produced by activation of ChR2-transfected C1 cells is a priori undesirable if the goal is to produce a selective increase in breathing.
So far, optogenetic activation of RVLM neurons has only been done in anesthetized rats (14, 17). It is therefore essential to determine whether the method can be adapted for use in conscious rats. This technical development represents the first objective of the study. The second objective is to determine what kind of cardiorespiratory effects are produced when the Phox2b-expressing glutamatergic neurons of the RVLM are selectively activated in conscious rats. Specifically, we wished to determine whether a robust stimulation of breathing can be achieved by this optogenetic approach without a major concomitant cardiovascular stimulation.
Some of the results of these studies have been previously reported in the form of an abstract (20).
METHODS
See the online data supplement for additional details.
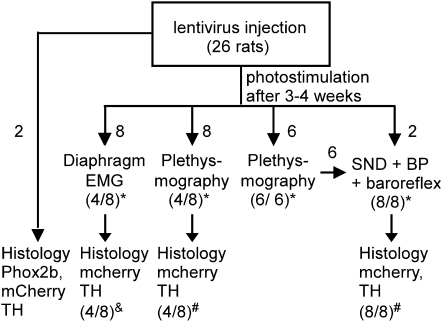
The experiments were done on male Sprague-Dawley rats (n = 26) in accordance with guidelines approved by the University of Virginia Animal Care and Use Committee. The animal use is shown in schematic form in Figure 1. Note that to minimize the number of animals, six of the eight rats that were used for the cardiovascular study had been previously studied by plethysmography.
Figure 1.
Experimental design. * Indicates the fraction of rats in which photostimulation increased breathing or renal sympathetic nerve discharge (SND). & Indicates group of eight rats in which optrode placement was always correct, but only four rats had normal numbers of channelrhodopsin-2 (ChR2)-transfected ccRTN neurons. Photostimulation activated breathing only in the latter rats. # Indicates fraction of rats in which the optrode was correctly placed in relation to the ChR2-transfected cells. The four rats with misplaced optrodes had no response to photostimulation. BP = blood pressure.
Lentivirus Preparation and Injection
A lentivirus featuring the Phox2-responsive artificial promoter PRSx8 and an enhanced version of the photoactivatable cationic channel channelrhodopsin2 (ChR2 H134R) fused to mCherry was prepared as described previously (14, 17). Virus injections (two 200-nL deposits) were made in the left RVLM of 210- to 250-g rats (6–8 wk old) anesthetized with ketamine (75 mg/kg), xylazine (5 mg/kg), and acepromazine (1 mg/kg) (14, 17). A guide cannula was affixed to the skull vertically at midrange between the two injections using metal screws and dental cement. Rats were treated with ampicillin (100 mg/kg, intramuscularly) and ketorolac (0.6 mg/kg, intraperitoneally) for two consecutive days. Animals were studied 3 to 4 weeks later.
Physiological Experiments
The physiological experiments were done in 24 rats (Figure 1). The rats were 10 to 12 weeks old when the physiological experiments were performed, which corresponds to 4 to 14 years in human life depending on which factors are chosen for comparison (life span versus age at sexual maturity, etc. [21]). The respiratory effects caused by photostimulation of ChR2-transfected RVLM neurons were examined in 22 conscious rats using either whole-body plethysmography (PLY 3223; Buxco Inc., Wilmington, NC; 14 rats) or diaphragmatic EMG recordings (8 rats; Figure 1). Ten of 14 rats studied by plethysmography responded to photostimulation (Figure 1). The four nonresponders had misplaced optrodes. Diaphragmatic EMG recordings were done in eight rats to ascertain that inspiratory motor activity contributed to the breathing stimulation evoked by photostimulation. The EMG electrodes were implanted using the above-described anesthetic and postsurgical treatment. EMG recordings were made 3 days later. Four of the eight rats so prepared responded to photostimulation (Figure 1). The nonresponders had abnormally low levels of ChR2-transfected neurons (Figure 1).
The cardiovascular effects (BP, rSND) elicited by RVLM photostimulation were examined in eight rats, six of which had been studied by plethysmography a few days before (Figure 1). Femoral arterial and venous catheters and a renal SND electrode were implanted 2 days and 1 day, respectively, before physiological experiments were performed (22). All the animals in this group responded to photostimulation and had adequate optrode placements (Figure 1).
Photostimulation of the Ventrolateral Medulla
Photostimulation was done by inserting a fiber optic through the preimplanted guide cannula (17, 23). The light intensity was set at 12 mW at the tip of the optical fibers based on our prior studies in anesthetized rats (14). In experiments in which light pulses were applied at different frequencies (20, 10, 5, and 2 Hz), there was a significant effect of photostimulation frequency on breathing rate (P = 0.0002), tidal volume (P = 0.0036), minute volume (P < 0.0001), and all other parameters (see Table E1 in the online data supplement). A higher frequency (50 Hz) was examined in two rats only and provided no greater breathing stimulation than 20 Hz. We settled on 10-ms pulses delivered at 20 Hz, which appeared to produce maximal effects and required only a 20% duty cycle of illumination (10 ms every 50 ms). All breathing data reported in the Results section used the same stimulation parameters (10 ms, 20 Hz, 12 mW) and the stimulation was maintained for 1 minute (plethysmography studies) or 30 seconds (dEMG studies).
In the rats in which rSND and BP were recorded (n = 8, Figure 1), the RVLM was stimulated for 30 seconds with the same parameters (10 ms, 20 Hz, 12 mW). In these rats, we also examined the effect of single 10-ms light pulses delivered every 5 seconds and the effects of twin pulses delivered every 10 seconds (double-pulse paradigm; interpulse interval: 0.1, 0.3, 0.5, 1, 1.5, 2, and 3 s). Such low-frequency stimulation produces no effect on breathing, but this test was made to assess the ability of the sympathetic outflow to follow repetitive stimuli and for comparison with similar experiments previously done in anesthetized rats (17). Single- and twin-pulse stimulation were repeated 110 times for each interpulse interval and the rectified evoked rSND was signal-averaged using the laser pulse as trigger (for details see Reference 17). Finally, the baroreflex control of rSND was also tested in seven of the eight rats used for the cardiovascular studies (Figure 1), the test having failed in one of these rats for technical reasons. The baroreflex was studied before and during RVLM photostimulation using sequential administration of sodium nitroprusside and phenylephrine (22).
After the experiment, rats were killed with sodium pentobarbital (60 mg/kg), perfused transcardially with 4% paraformaldehyde, and 30-μm–thick transverse sections were prepared for histology.
Data Acquisition and Processing
The ventilatory parameters measured by plethysmography included respiratory frequency, inspiratory time, expiratory time, tidal volume, minute volume, peak inspiratory flow, peak expiratory flow, and expiration relaxation time. BP, rSND, and EMG data were acquired, digitized, and analyzed as described previously (24). The baroreflex relationship between resting mean BP and rSND was determined by fitting a sigmoid function to data points (22).
Histology
Two rats that were not subjected to any physiological procedure were used to verify that the lentivirus batch used in the present experiments produced expression of ChR2 selectively in the Phox2b-expressing neurons (Figure 1). The rest of the histology was done on the rats that had been subjected to physiological experiments (Figure 1). The purpose was to determine the number and distribution of the ChR2-expressing neurons and the distance of the tip of the fiber optic with respect to the transfected neurons. The lentiviral transgene produces an mCherry-ChR2 fusion protein; the location of mCherry therefore indicates where the photosensitive channel is expressed (14, 17). Tyrosine hydroxylase (TH) is a marker of the BP-regulating C1 neurons. As per our previous work, the ChR2-transfected ccRTN neurons were identified as TH-negative and mCherry-positive neurons (14, 17). TH, mCherry, and Phox2b were detected by fluorescence immunohistochemistry using previously described procedures and antibodies of established specificity (14, 25). In the present experiments, the Phox2b rabbit antibody was revealed with an anti–rabbit-IgG Alexa 488 secondary (appears green) with an excitation filter of 500 and an emission filter of 535. TH mouse antibody was revealed with an antimouse IgG Cy5 secondary (pseudo-colored blue) with the immunoreactivity captured with excitation filter of 640 and emission filter of 690. The mCherry has an endogenous red fluorescence with an excitation filter of 545 and an emission filter of 605.
The histological material was examined and photographed as described previously (14, 25). The number and phenotype of transfected neurons was determined from a one-in-six series of 30-μm–thick transverse sections that encompassed the transfected brain area (26).
Statistics
Statistical analysis was done using SigmaStat software (version 3.1; Systat Software, Point Richmond, CA). To compare two groups of data we used the unpaired or paired Student t test when the data were normally distributed or the Wilcoxon signed rank test when the normality test failed. To determine the effect of photostimulation on breathing parameters at multiple time points during and after the stimulus we used repeated measures analysis of variance followed by pairwise multiple comparisons (Student-Newman-Keuls). Values are reported as mean ± SE.
RESULTS
Photostimulation of ChR2-transfected RVLM Neurons Activates Breathing: Plethysmography Studies
The state of consciousness of the rats was not assessed beyond the fact that they were not moving. The rats could have been quietly resting or asleep. Photostimulation was only administered when the breathing rate was low and regular (76 ± 3 breaths/min, Table 1).
TABLE 1.
EFFECTS OF ROSTRAL VENTROLATERAL MEDULLA PHOTOSTIMULATION ON BREATHING PARAMETERS MEASURED BY WHOLE-BODY PLETHYSMOGRAPHY
| Photostimulation |
Recovery |
||||
|---|---|---|---|---|---|
| Baseline | First 5 s | Last 5 s | First 5 s | >40 s | |
| Breathing rate, breath/min | 76 ± 3 | 108 ± 5* | 92 ± 4*† | 70 ± 2* | 76 ± 2 |
| Tidal volume, ml | 1.72 ± 0.13 | 2.07 ± 0.14* | 1.79 ± 0.15† | 1.46 ± 0.12* | 1.67 ± 0.13 |
| Minute volume, ml/min | 129 ± 9 | 216 ± 18* | 166 ± 17*† | 102 ± 7* | 128 ± 9 |
| Inspiratory duration, ms | 250 ± 10 | 220 ± 10* | 220 ± 10* | 234 ± 12* | 246 ± 9 |
| Peak inspiratory flow, ml/s | 11.1 ± 0.8 | 14.3 ± 1.1* | 12.9 ± 1.2*† | 10.4 ± 0.8* | 11.0 ± 0.8 |
| Expiratory duration, ms | 566 ± 26 | 368 ± 25* | 461 ± 28*† | 651 ± 36* | 563 ± 26 |
| Peak expiratory flow, ml/s | 7.9 ± 0.6 | 11.4 ± 1.2* | 8.8 ± 0.8† | 6.9 ± 0.7* | 7.9 ± 0.6 |
| Expiratory relaxation time,‡ ms | 283 ± 14 | 189 ± 14* | 221 ± 14*† | 278 ± 15 | 291 ± 15 |
Values are means ± SEM (N = 10 rats). Photostimulation was 20 Hz, 10-ms pulses. One-way analysis of variance for repeated measures was done for each breathing parameter. This test indicated that photostimulation produced a significant change of all the parameters. All pairwise multiple comparisons were done by the Student-Newman-Keuls method.
P < 0.05 vs. values at baseline.
P < 0.05 vs. values during the first 5 seconds of photostimulation.
Expiration relaxation time measures the time when expiration flow has decayed to 36% of its maximum value.
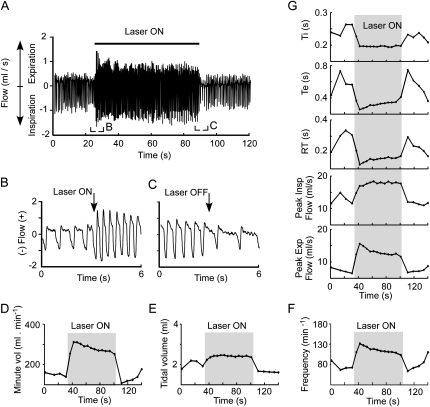
A strong breathing response was observed in all 10 rats in which the tip of the optrode was appropriately close to the transfected neurons (see histological details below). Figure 2 illustrates the effect of the standard 1-minute period of photostimulation in a representative rat, and Table 1 contains the group data. Breathing stimulation was virtually immediate (Figure 2B). All breathing parameters were changed significantly by the stimulation (Figures 2D–2G; Table 1). The changes were largest immediately after the onset of the photostimulation and decreased thereafter to reach a new equilibrium during the latter part of the stimulus (Figures 2D–2G; Table 1). After the end of the photostimulation, most parameters abruptly changed in the direction opposite to that elicited by the light (Figure 2, Table 1). All parameters recovered their prestimulation baseline levels within 30 to 45 seconds (Figure 2, Table 1). Of note, breathing frequency and amplitude remained very regular during and immediately after the stimulus (Figures 2A and 2B). The lack of breathing lability during and after the stimulus plus the complete and quick recovery to baseline of all parameters provided objective evidence that the stimulation did not elicit motor behavior or a persistent change in the state of vigilance.
Figure 2.
Photostimulation of channelrhodopsin-2–transfected rostral ventrolateral medulla neurons activates breathing: representative plethysmography example. (A) Original trace showing the effect of a 1-minute period of photostimulation (20 Hz, 10-ms pulses, 12 mW) on inspiratory and expiratory flow in a conscious, resting rat. (B) Excerpt from A showing the initial phase of the breathing response. (C) Excerpt from A showing the end of the photostimulation period. Note that the breathing rate and flows were smaller toward the end of the stimulation period than at the beginning. Note also that the frequency and flows were reduced below the prestimulus baseline after interruption of the photostimulation. (D) Change in minute volume caused by the stimulus. (E) Change in tidal volume. (F) Change in breathing frequency. (G) Change in other plethysmography parameters (from top to bottom: inspiratory duration [Ti], expiratory duration [Te], expiratory relaxation time [RT], peak inspiratory flow, and peak expiratory flow).
All numerical values are shown in Table 1, but a few points deserve to be highlighted. First, all breathing parameters were significantly different from control values during the first 5 seconds of the 1-minute stimulus. Second, most of these parameters were still above baseline during the last 5 seconds of the stimulus except tidal volume and peak expiratory flow. Third, the increase in rate was caused by a slight reduction in the duration of inspiration (Ti) and a more substantial reduction of expiration time (Te) (e.g., 12 ± 2% vs. 35 ± 4% during the first 5 s of stimulation; Table 1). Finally, during the first 5 seconds after the end of the photostimulation, every breathing parameter was significantly different from the baseline except expiratory relaxation time, which was normal despite the reduced tidal volume.
Photostimulation of the RVLM Increases Diaphragmatic EMG Frequency and Amplitude
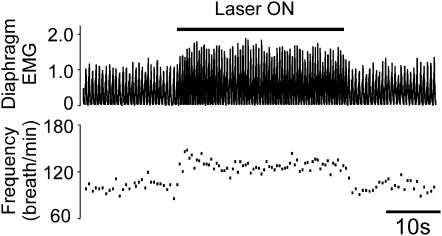
There are diverging views in the literature concerning which aspect of breathing (rate, inspiratory amplitude, active expiration) is regulated by the retrotrapezoid nucleus; some authors have even proposed that this region regulates active expiration exclusively (27, 28). Diaphragmatic EMG (dEMG) was therefore recorded to test whether the increase in tidal volume caused by RVLM photostimulation is at least in part due to an increase in the activity of the diaphragm. In the four successful animals (Figure 1), photostimulation increased the frequency of dEMG bursts (from 104 ± 5 to 125 ± 6 discharges/min, P < 0.01) and their amplitude (from 93 ± 9 to 112 ± 16% baseline, P = 0.082). A representative case is shown in Figure 3. In the four unsuccessful cases (Figure 1), the optical fiber was correctly placed, but far fewer ccRTN neurons were transfected than in the successful placements (117 ± 28 transfected neurons per rat vs. 261 ± 22, P < 0.01 by t test).
Figure 3.
Photostimulation of channelrhodopsin-2–transfected rostral ventrolateral medulla neurons activates diaphragmatic activity in conscious rats: representative example. Top trace: rectified and integrated diaphragmatic EMG with amplitude normalized to 1 during resting period. Bottom trace: breathing frequency. Photostimulation was done for 30 seconds with 10-ms light pulses delivered at 20 Hz.
Photostimulation of the RVLM Increases BP and Renal SND
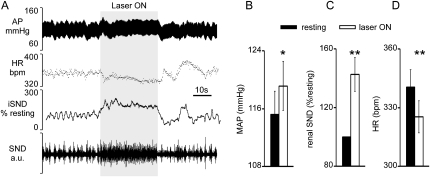
Figure 4A shows a representative example of the cardiovascular effects elicited by photostimulation of ChR2-transfected RVLM neurons in a quiet awake rat (30 s, 20 Hz, 10-ms pulses, 12 mW). Renal SND was elevated, BP increased only slightly, and heart rate (HR) decreased slightly. When photostimulation was interrupted, BP and rSND decreased below baseline before recovering to the prestimulus level. On average (eight rats; Figures 4B–4D), photostimulation of the RVLM increased mean BP (measured during the last 20 s of a 30-s stimulus) by only 4 mm Hg, but the effect was significant (from 115 ± 3 mm Hg to 119 ± 3, P < 0.05). Renal SND increased notably during the stimulus (from 100 to 143 ± 12% of baseline, P < 0.01) and a small HR decrease was consistently observed (from 340 ± 9 to 325 ± 8 bpm, P < 0.001).
Figure 4.
Photostimulation of channelrhodopsin-2–transfected rostral ventrolateral medulla neurons activates renal sympathetic nerve activity and blood pressure in conscious rats. (A) Representative example. The traces from top to bottom are blood pressure (descending aorta, AP), heart rate (HR), integrated renal sympathetic nerve discharge (iSND; rectified and integrated with 2-s time constant; trace intentionally moved by 2 s to the right to correct for integration time and proper alignment with the other traces), and raw renal SND (100–3,000 Hz). Photostimulation was applied for 30 s (gray area) with 10-ms light pulses delivered at 20 Hz. (B) Average effect of photostimulation on blood pressure (n = 8). (C) Average effect of photostimulation on renal SND (n = 8). (D) Average effect of photostimulation on heart rate. * P < 0.05; ** P < 0.01. MAP = mean arterial pressure.
An analysis of variance with repeated measures showed a significant effect of the frequency of photostimulation (20, 10, 5, and 2 Hz) on mean BP (P < 0.05), rSND (P < 0.05), and HR (P < 0.05) (Table E2).
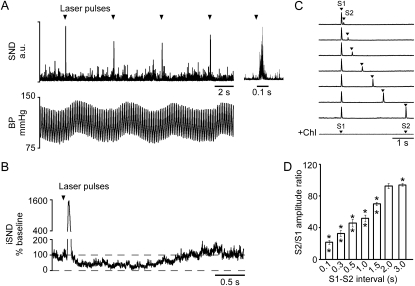
Very low-frequency photostimulation of the RVLM (0.1 Hz, 10-ms pulses) produced a large evoked response in the renal nerve (peak = 2,051 ± 228% of baseline) with an onset latency of 40.8 ± 0.9 ms and a peak latency of 66.8 ± 1.2 ms (Figure 5A). This peak was followed by a period of reduced sympathetic nerve discharge (down to 14 ± 5% of baseline) which recovered to baseline in 1.75 ± 0.03 seconds (Figure 5B).
Figure 5.
Low-frequency photostimulation of channelrhodopsin-2–transfected rostral ventrolateral medulla neurons evokes large amplitude response in renal sympathetic nerve discharge (SND). (A) Representative example of the effect of 10-ms light pulses, applied every 10 seconds, on renal SND and blood pressure (BP). The far right of the trace shows an expanded trace of the same stimulus. (B) A laser pulse-triggered waveform average of a representative case. This case shows that after the initial burst, renal SND falls below baseline before slowly returning to prestimulus levels. (C) Paired-pulse paradigm. Each trace represents the evoked responses caused by a pair of 10-ms light pulses delivered at a fixed interpulse interval (S1 and S2). Small interpulse intervals (top trace) cause the second evoked response to be dramatically reduced. This reduction becomes smaller as the interpulse interval is increased (following three traces). The bottom trace illustrates that the evoked responses are abolished after administration of the ganglionic blocker chlorisondamine. (D) Attenuation of the second evoked response as a function of the interval between the pulses (eight rats; the asterisk indicates that the second evoked response of each pair was significantly smaller than the first).
The double-pulse paradigm indicated that the amplitude of the second evoked peak of rSND was drastically reduced (down to 22 ± 3% of the amplitude of the first stimulus) when photostimulation was applied 100 ms after the first stimulus (Figures 5C and 5D). The full effect of photostimulation gradually recovered when the interpulse interval was increased to between 2 to 3 seconds (Figure 5D). In other words, although very low-frequency stimulation of the RVLM produces a large transient sympathetic response, high-frequency stimulation produces a modest increase in rSND and only a minimal increase in BP.
Photostimulation of the RVLM Enhances the Sympathetic Baroreflex
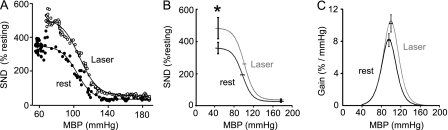
The baroreflex control of rSND was examined in seven rats before and during RVLM photostimulation (20 Hz, 10-ms pulses, ∼12 mW). The baroreflex test (Figure 6A) was repeated at least twice in each condition and averaged afterward. A four-parameter sigmoid equation could be satisfactorily fitted to all BP-rSND baroreflex relationships (Figure 6A) as demonstrated by high coefficients of determination (Table 2). Photostimulation increased the maximal level of rSND observed during complete unloading of the baroreceptors with sodium nitroprusside (Figures 6A and 6B, Table 2) but had no effect on the lower plateau observed at saturation of the baroreflex (Figures 6A and 6B, Table 2).
Figure 6.
Photostimulation of channelrhodopsin-2–transfected rostral ventrolateral medulla (RVLM) neurons increases the range of the sympathetic baroreflex. (A) Second-by-second relationship between mean blood pressure and renal SND during the administration of sodium nitroprusside followed by administration of phenylephrine. The solid circles show the relationship at rest and the open circles show the relationship during photostimulation of the RVLM (20 Hz, 10-ms pulses). The best fit logistic curves are also shown. (B) Average baroreflex logistic curve for eight rats at rest and during photostimulation of the RVLM. Asterisk indicates that the lower plateau (maximum sympathetic nerve discharge [SND] observed when the baroreceptors are unloaded) was significantly (P < 0.05) elevated by RVLM photostimulation. (C) Average baroreflex gain plotted as a function of the mean blood pressure (MBP) for eight rats. The maximum gain was not significantly greater during photostimulation.
TABLE 2.
EFFECTS OF ROSTRAL VENTROLATERAL MEDULLA PHOTOSTIMULATION ON THE BAROREFLEX CONTROL OF RENAL SYMPATHETIC NERVE DISCHARGE IN CONSCIOUS RATS
| Baseline | Photostimulation | |
|---|---|---|
| R2 | 0.969 ± 0.006 | 0.980 ± 0.004 |
| Higher plateau, P1+ P4; % baseline | 361 ± 38 | 484 ± 70* |
| Lower plateau, P1; % baseline | 29 ± 4 | 39 ± 6 |
| Mean BP at half SND range, P3; mm Hg | 95 ± 4 | 99 ± 3 |
| Max gain, % baseline/mm Hg | 8.1 ± 0.9 | 10.2 ± 1.2 |
Definition of abbreviations: BP = blood pressure; SND = sympathetic nerve discharge. P = constant in the sigmoid function defined by the curve fitting.
Values are means ± SEM (N = 7 rats). The relationship between renal SND and BP was fitted to the following sigmoid function: SND = P1/(1 + exp[P2(BP−P3)]) − P4 (see Methods in the online supplement ). R2, coefficient of determination (observed vs. predicted values); maximum gain is the value of the first derivative of the above equation at P3 with sign reversed.
P < 0.05 vs. baseline condition.
The maximum baroreflex gain was increased in six of seven rats but it was decreased in one animal such that no overall statistical difference was observed (P = 0.078; Figure 6C, Table 2). The increase in baroreflex gain was marginally correlated with the increase in rSND observed at rest (n = 7; r2 = 0.53; P = 0.053). The BP corresponding to the midrange of the reflex was unchanged (Figure 6B, Table 2).
In summary, the operating range of the baroreflex (difference between maximum SND and SND at saturation of the baroreflex) was enhanced by photostimulation of the RVLM. The gain and the midrange of the baroreflex also tended to be elevated but the difference did not reach statistical significance.
Histology
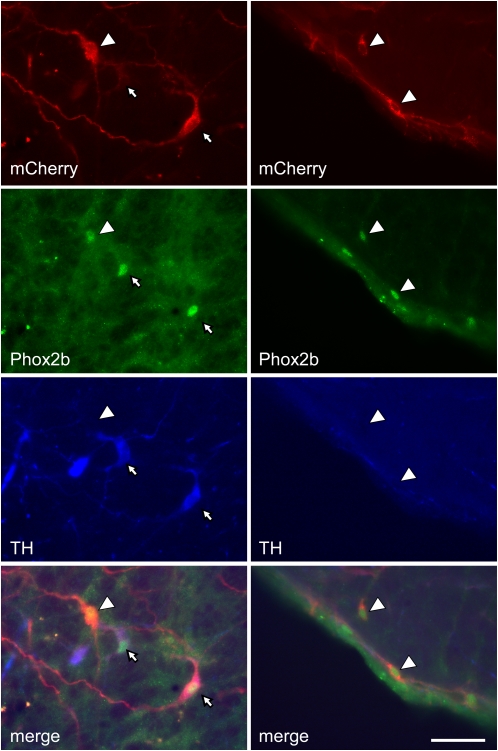
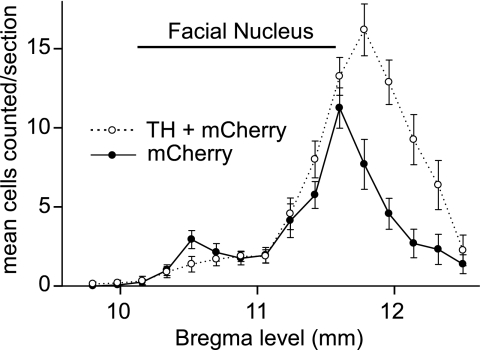
The left RVLM of the rats used to examine the effect of photostimulation on breathing contained 649.4 ± 72.4 mCherry-immunoreactive (i.e., ChR2-transfected) neurons (total cells counted in a one-in-six series of section multiplied by 6), of which 63 ± 2% were TH-immunoreactive (ir). The rats used to examine the effect of RVLM photostimulation on BP and rSND contained 833.3 ± 90.2 transfected neurons, of which 63.8 ± 2% were TH-ir. These values were not significantly different. Examples of TH-ir and TH-negative ChR2-transfected Phox2b-ir neurons are shown in Figure 7. The rostrocaudal distribution of the two transfected cell types (C1 and ccRTN neurons) was the same in both groups of rats and the pooled data are illustrated in Figure 8. Two rats that had been injected with lentivirus but had no further experimentation were used to determine the percentage of transfected (i.e., mCherry-containing) cells that were Phox2b positive. Intact rats were used for this determination because fiberoptic insertion decreases tissue Phox2b immunoreactivity. Out of a total of 114 mCherry cells counted within the RVLM over seven to eight sections in these 2 rats, 109 were Phox2b positive. The percentage of Phox2b-positive neurons (96%) was therefore similar to our prior observations with different batches of the same virus.
Figure 7.
Examples of transfected catecholaminergic and noncatecholaminergic Phox2b-positive neurons in the rostral ventrolateral medulla (RVLM). Left column illustrates the region immediately caudal to the facial motor nucleus. Almost all transfected cells (mCherry positive, red) have a Phox2b-immunoreactive nucleus (green) and most (small white arrows) contain tyrosine hydroxylase (TH, blue). The large white arrow points to a Phox2b-positive neuron that is transfected (mCherry positive) but does not contain TH. Right column illustrates the region immediately under the caudal end of the facial motor nucleus (the RTN). All transfected neurons in this region have a Phox2b-positive nucleus but they are devoid of TH (ccRTN neurons, white arrowheads). Rat brain tissue was cut into 30-μm sections through the RVLM and processed for immunohistochemistry as described in the Methods section. The scale bar at lower right (50 μm) applies to all panels.
Figure 8.
Distribution of channelrhodopsin-2–transfected catecholaminergic (C1) and noncatecholaminergic (ccRTN) neurons in the rostral ventrolateral medulla (RVLM). Mean numbers of channelrhodopsin-2 (ChR2)-expressing neurons counted on the left side of the RVLM in a one-in-six series of 30-μm coronal sections from 15 rat brains. Rats had been injected with ChR2-mCherry lentivirus and subjected to physiological experiments described in the current study. Cell numbers are plotted against the relative rostrocaudal position of the coronal section relative to bregma. These levels were determined relative to the landmark of the most caudal end of the facial motor nucleus designated as 11.6-mm caudal to bregma after the atlas of Paxinos and Watson (54). Error bars represent the SEM. Note that the bulk of the ChR2-transfected C1 cells (positive for both mCherry and tyrosine hydroxylase [TH], open circles, dotted line) are slightly caudal to the peak of the ccRTN neurons (positive for mCherry, negative for TH; filled circles, solid line), but the two populations overlap considerably.
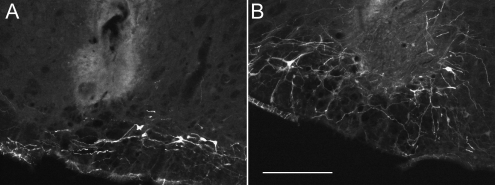
Figure 9 shows the bottom of the optrode insertion in two cases. The tip of the optrode was located an average of 398 ± 62 μm from the bulk of the transfected neuronal cell bodies in the rats in which photostimulation elicited a robust stimulation of breathing and/or cardiovascular stimulation. It should be recalled that the transfected neurons have extensive dendrites within the ventrolateral medulla and axons that ascend vertically (14, 29). Therefore, many of the ChR2-bearing processes from these cells could have been closer to the light source than indicated by the distance from the tip of the optrode to the geometric center of the labeled cell bodies.
Figure 9.
Optrode placement in two representative cases Photomicrographs showing the maximum optrode penetration in two cases with robust physiological responses to laser light stimulation. (A, B) Transverse sections showing mCherry fluorescent cells and processes (denoting the presence of channelrhodopsin-2). (A) Approximately 11.24 mm caudal to bregma (i.e., under the caudal third of the facial motor nucleus). (B) Approximately 11.6 mm caudal to bregma (i.e., under the very caudal end of the facial motor nucleus). The scale bar in B (250 μm) applies to both panels.
DISCUSSION
We show here for the first time that selective activation of a few hundred Phox2b-expressing ventrolateral medullary neurons activates breathing robustly in a conscious laboratory animal while only producing a small increase in BP attributable to an increase in sympathetic tone. The breathing activation includes an increase in the frequency and amplitude of inspiration. Based on prior experiments performed in anesthetized rats with the same optogenetic method, we attribute the respiratory stimulation to the activation of the ccRTN neurons and the cardiovascular stimulation to the activation of the TH-containing C1 neurons. These findings suggest a possible future application of the optogenetic method to the treatment of hypoventilation in humans.
Technical Considerations: Selectivity of the Transfection, Selectivity of the Photostimulus
The selectivity of the transfection procedure has been defined in prior studies (14, 17). In the targeted region, the expression of the transgene is tightly associated with the presence of the transcription factor Phox2b (95–98%) (14, 17), consistent with the fact that the promoter that we used (PRSx8) is a multimer of a Phox2b recognition site (30). Our viral vector does not produce ChR2 expression in the inhibitory neurons of the Bötzinger region, which lack Phox2b, or, fortuitously, in the facial motor neurons, although these cells do express a low level of Phox2b in the adult (17). The pulses of laser light produced no movement of the vibrissae, confirming that facial motor neurons were unaffected by the laser light. As a result, ChR2 is expressed almost exclusively by a population of putatively glutamatergic neurons (i.e., positive for vesicular glutamate transporter-2) that contain Phox2b plus a small number of cholinergic neurons previously estimated at less than 2% of the total transfected population (14, 17). The transfected glutamatergic neurons belong to one of two cell types: the C1 neurons, which are identified by the presence of TH, and the ccRTN neurons that lack this enzyme and reside closer to the ventral surface of the brainstem (14, 17).
Conventional stimulation of the RVLM region with an amino acid or a GABA receptor antagonist increases blood pressure but reduces breathing amplitude and rate (e.g., References 31, 32). The inhibitory effects on respiration are attributed to the activation of the inhibitory GABAergic and glycinergic respiratory neurons of the Bötzinger region, which reside dorsal and caudal to the ccRTN neurons and are integral components of the respiratory rhythm and pattern generator (6, 33). Our photostimulation method produced the opposite respiratory effects, consistent with the fact that our viral vector does not cause ChR2 expression in the inhibitory neurons of the Bötzinger region (17).
In anesthetized rats, C1 cells and ccRTN neurons transfected with ChR2 with the same method as in the present study are vigorously activated by pulses of laser light (14, 17). Similar single-unit experiments cannot be performed in conscious rats, which represents a limitation of the present study. However, the assumption that the C1 cells and the ccRTN neurons were activated to a comparable degree in conscious versus anesthetized rats is legitimate given the similarity of the cardiorespiratory effects that were produced under both conditions.
The fact that no physiological effect was observed when the tip of the fiberoptic was misplaced controlled for the unlikely possibility that the rats might have been mounting some form of emotional response to the sight of the laser light passing through the optical fiber. In prior publications we have also excluded the possibility that heat generated by the tip of the fiber optic could have produced the observed physiological effects for the following reasons (14, 17): photostimulation of the nontransfected side of the brain produces no effect, stimulation of the RVLM after transfection with a lentiviral vector that expresses a photo-insensitive transgene in the same population of neurons is ineffective, light pulses entrain single identified C1 and ccRTN neurons on a pulse-by-pulse basis, and, as shown here also, each 10-ms light pulse evokes a temporally precise sympathetic response most unlikely to be caused by a temperature change (14, 17).
Photostimulation of ChR2-transfected RVLM Neurons Produce a Vigorous Activation of Breathing
Photostimulation of ChR2-transfected neurons produced a robust 68% increase in minute volume on average (range 19–105%). This effect was due to an increase of both breathing frequency and tidal volume. These effects are discussed in the context of the three existing theories on the role of the RTN in breathing: (1) RTN neurons regulate the breathing rate only (34), (2) RTN regulates breathing amplitude only (13, 35), and (3) RTN regulates only active expiration (27, 28). Based on the present study in conscious rats and our prior work on anesthetized rats, we conclude that the glutamatergic Phox2b-expressing neurons of the RTN region (the ccRTN neurons) control both the breathing rate and the amplitude of inspiration. Our data suggest that these neurons could also regulate active expiration, but without direct electromyographic evidence of increased abdominal muscle activity our interpretation must remain tentative.
The notion that ccRTN neurons regulate the breathing rhythm selectively derives from studies performed in rodents before or immediately after birth (36–38). This interpretation may be valid only for neonates. Also, it could be biased by the fact that these studies were performed with an in vitro preparation that generates a respiratory-like outflow of essentially invariant amplitude (i.e., only subject to frequency modulation under the influence of pH, potassium, and drugs such as opiates) (39). Our experiments on anesthetized and conscious adult rats support the notion that the ccRTN neurons control the breathing rate but they do not support the idea that these neurons control the breathing rate selectively.
The notion that RTN controls breathing amplitude selectively derives from experiments conducted by Li and colleagues (13) and Li and Nattie (35) in conscious rats to explore the response of the RTN region to local parenchymal acidification. In these experiments, acidification of the RTN region produced small changes in tidal volume without modification of the frequency of breathing. Our results are in agreement with these studies to the extent that we also observed significant increases in tidal volume both under anesthesia (14) and in conscious rats (present study). The discrepancy between our results (effects on rate and amplitude) and those of Li and colleagues (13) and Li and Nattie (35) (amplitude changes only) could have several explanations. One possibility is that RTN acidification, as performed by Li and colleagues (13) and Li and Nattie (35), affects several types of neurons simultaneously, not just the ccRTN neurons. Another possibility is that the ccRTN neurons, as we define them, include subpopulations that differentially control the breathing rate, inspiratory amplitude, and active expiration. Our experiments targeted neurons located predominantly at the caudal end of the RTN, whereas the others (13, 35) generally targeted a more rostral region of the nucleus that could conceivably contain predominantly amplitude-regulating neurons. The next possibility is that the rate-regulating ccRTN neurons are acid-insensitive and hence are not affected by local parenchymal acidification. This possibility is extremely unlikely because all ccRTN neurons recorded so far, including those suspected to regulate the breathing rate early in development, are activated by acidification (38, 40, 41). A final possibility is that the breathing rate could be responsive to activation of the C1 neurons, which were also stimulated in our present experiments and probably were not activated in Li and Nattie's work because the C1 cells do not appear to be directly acid sensitive (40). This interpretation is also unsatisfactory because stimulation of ChR2-transfected ccRTN neurons in rats in which extremely few C1 neurons also express the transgene is still capable of increasing the breathing frequency robustly in anesthetized rats (14).
The final notion, that the RTN governs active expiration only, derives from experiments done in juvenile rats treated with an N-methyl-D-aspartic acid receptor antagonist plus an opiate agonist (27) and from experiments conducted in an artificially perfused rat preparation (28). These studies suggest that the parafacial region of the RVLM contains a network of neurons that regulate active expiration with some selectivity and may retain oscillatory properties under specific pharmacological conditions that eliminate the inspiratory motor outflow. The individual neuronal components of this expiratory oscillator have not been identified. Specifically, there is no evidence yet that the cells that are hypothesized by these authors to selectively control expiration are the ccRTN neurons. Our plethysmography data revealed that ccRTN neuron stimulation increased peak expiratory flow with concomitant reduction in expiratory relaxation time. Increased elastic recoil forces triggered by stronger inspiratory efforts and/or decreased airway resistance from less laryngeal adductor motor activity probably account for these changes. However, one possible interpretation is that the photostimulation had increased active expiration. These different possibilities will have to be sorted out by recording the activity of abdominal and airway muscles.
The power of the excitatory drive contributed by the ccRTN neurons can be appreciated from the fact that a robust stimulation of breathing could be produced by activating at most 16% of the total population. Indeed, 220 to 330 ccRTN neurons out of a total population of 2,000 (∼ 1,000 per side [42]) were detectably transfected with ChR2, and only a subset of the transfected cells may have been photoactivated given the presumed limited spread of the light emanating from the optical fiber. Moreover, this robust breathing activation occurred despite the countervailing influence of hypocapnia that should have developed during the forced hyperventilation. The hypocapnia induced by the forced period of hyperpnea is the most likely cause of the reduced respiratory activation that was observed toward the end of the photostimulation period and of the rebound hypoventilation seen just after the photostimulation period. Hypocapnia should have gradually reduced the activity of the central chemoreceptors that were not being activated by the laser pulses, thereby partially compensating for the breathing stimulation caused by the ChR2-transfected neurons that were directly activated by the light pulses. This interpretation is also consistent with our prior observations that the activation of ccRTN or C1 neurons by trains of laser light was sustained during the entire duration of the stimulus (14, 17).
Given prior evidence that the activity of the ccRTN neurons in vivo is strongly regulated by the level of arterial Pco2 (40, 43), the powerful respiratory stimulation that these cells are capable of eliciting in conscious mammals provides additional support to the view that these neurons are a major hub for blood gas stabilization through breathing (11).
Photostimulation of ChR2-Transfected RVLM Neurons Produces a Modest Increase in BP
Low-frequency stimulation of ChR2-transfected RVLM neurons (<0.5 Hz) produced the same robust evoked response in the renal nerve (present study) as in the splanchnic nerve under anesthesia (17). This effect can be explained by the synchronous activation of the ChR2-expressing C1 neurons (17). This evoked discharge was followed by a long period of inhibition that drastically attenuated the effect of a second stimulus applied shortly after the first one. The attenuation was similar in amount and duration in conscious rats (this study) and in anesthetized rats (17). This inhibition is probably an intrinsic property of the preganglionic neurons, specifically an after-hyperpolarization (44, 45) (for a more detailed discussion see Reference 17).
The steady-state increase in SNA elicited by higher-frequency stimulation (20 Hz) was far more modest (∼ 40% increase above baseline) and the increase in BP quite small (average of 4 mm Hg). These effects were also similar to those that we have observed previously in chloralose-anesthetized rats except that the change in BP was smaller in the present study (17). Under anesthesia the activity of the ChR2-transfected C1 neurons is faithfully entrained by light pulses delivered up to 20 Hz and the entrainment is maintained throughout the period of stimulation (17). Assuming that this is also the case in conscious rats, the limited increase in rSND observed during high-frequency stimulation of the RVLM must therefore be due to the integrative properties of the downstream network that generates the sympathetic outflow, including the strong frequency adaptation discussed above. The very small increase in BP may have additional causes. First, RVLM stimulation could have produced vasodilation in skeletal muscle beds via inhibition of sympathetic nerve activity or by causing epinephrine release from the adrenal medulla. The known properties of the C1 cells suggest that they differentially regulate regional blood flow in addition to stabilizing BP (18, 46). Second, RVLM photostimulation produced some bradycardia. This bradycardia could have been a manifestation of the baroreflex or it could have resulted from the direct activation of a few cardiovagal parasympathetic efferents, because these cholinergic cells express Phox2b and a small number can be transfected with ChR2 after lentivirus injection into the RVLM (14, 17). Third, the increase in BP may simply have been limited by the baroreflex. The high buffering power of this reflex was revealed by the fact that the sympathetic response was much larger when the baroreceptors were fully unloaded with sodium nitroprusside than at resting or elevated BP. Finally, the ccRTN neurons themselves could also conceivably influence the circulation to cause blood flow redistribution.
In summary, the limited increase in BP caused by stimulating the C1 neurons at 20 Hz is primarily due to the inability of the spinal sympathetic network to follow volleys of synchronous excitatory inputs delivered above 1 Hz, to the BP-buffering effect of the baroreflex, and, possibly, to differential effects of C1 cell stimulation on regional blood flows.
Possible Cross-Talk between C1 and ccRTN Neurons
In anesthetized rats, the respiratory stimulation produced by photoactivating a mixed population of Phox2b-expressing RVLM neurons persisted in rats in which the number of ChR2-transfected C1 cells was severely depleted by administration of a catecholaminergic neuron-selective toxin (14). Accordingly, the ccRTN neurons were clearly producing the bulk of the respiratory stimulation that was elicited under these specific experimental conditions. This evidence does not exclude the possibility that C1 cell activation could stimulate breathing to some degree under other conditions, especially in conscious rats. The C1 cells are activated by a variety of stresses, such as pain, hypotension, hemorrhage, and infection (i.e., conditions that are typically associated with increased breathing) (47). Definitive evidence of synapses between C1 and ccRTN neurons has not been produced, but the RTN region is clearly innervated by C1 neurons (48, 49). An excitatory influence of some C1 cells on the ccRTN neurons is therefore conceivable, especially because the C1 cells have the potential to release glutamate (26).
Conversely, the ccRTN neurons innervate the region of the medulla oblongata that harbors the C1 neurons (14, 48). Again, evidence of direct synaptic contacts is lacking, but many C1 cells are activated by increases in central nervous system Pco2 (50). Because these cells do not seem to be intrinsically pH sensitive (40), their response to CO2 must rely on synaptic inputs, and the ccRTN neurons are a plausible source of CO2-dependent excitation given their sensitivity to hypercapnia and their projection pattern. Conceivably, the subset of ccRTN neurons that contributes to active expiration could also be targeting selected populations of C1 cells, thereby increasing BP and/or contributing to blood flow redistribution to the muscles.
Possible Translational Application
The present experiments provide proof of principle that photoactivation of the ccRTN neurons can robustly activate breathing in an unanesthetized resting or sleeping mammal without causing any overt behavior or notable increase in blood pressure. A group of noncatecholaminergic Phox2b-positive and substance P receptor–expressing neurons that are presumably the homolog to the rodent ccRTN neurons has been recently identified in humans in approximately the same location as in rodents and cats relative to the facial motor nucleus and the ventral medullary surface (51). These neurons could therefore conceivably be activated using some improved version of the optogenetic method used in the present study to stimulate breathing. Although the optogenetic method that we selected also causes the expression of ChR2 in C1 cells, the BP effect resulting from stimulating these cells was modest and may not represent a major impediment to implementing this approach in humans. Also, the bulk of the C1 cells are at a greater distance from the RTN in humans because of the much larger brain size (51, 52); therefore, the C1 cells would be less likely to be stimulated by a fiber optic directed at the RTN.
The most appropriate potential application of this optogenetic methodology cannot be predicted without further basic studies. Nonobstructive sleep apnea comes to mind, but the method could also conceivably find some usefulness in the treatment of obstructive sleep apnea because central chemoreceptors also activate airway dilator muscles (53), and it is very possible that stimulation of the ccRTN neurons would also improve upper airway patency. The congenital central hypoventilation syndrome, an extreme form of hypoventilation caused by polyalanine expansion of Phox2b (4), may be caused in part by the loss of the ccRTN neurons (54). However, in the mouse model, which is a 27-alanine repeat mutation designed to mimic a severe form of the human disease (4), only 70% of the RTN cells are missing (54). The proportion of ccRTN neurons that are lost in patients with congenital central hypoventilation syndrome is unknown and presumably varies according to the severity of the disease. Thus, at this time, the theoretical possibility that benefits could be derived from stimulating the surviving ccRTN neurons is not excluded.
Promoters that are even more selective for ccRTN neurons will most likely become available in the future and ion channels operated by electromagnetic wavelengths that can be targeted noninvasively could perhaps be engineered to avoid the use of invasive optical fibers. Obviously, many additional preclinical studies would be required before one could envision a human application of optogenetics to the treatment of hypoventilation. Most critical would be definitive evidence that ccRTN stimulation increases breathing during sleep and that breathing stimulation can be sustained for extended periods or faithfully repeated when needed. Behavioral tests designed to ascertain that such stimulation does not cause any untoward sensations or side effects would also be required.
Conclusion
Photoactivation of a few hundred ChR2-transfected Phox2b-expressing glutamatergic neurons located in the RVLM in conscious rats produces a robust stimulation of breathing and a small increase in BP. No motor effect other than on the respiratory system was detected. Activation of the ccRTN neurons was presumably responsible for the respiratory stimulation, whereas activation of the C1 cells presumably caused the increase in sympathetic tone and BP. The methodology described in this study may have future applicability to the human condition when enhanced respiration is necessary to sustain life.
Supplementary Material
Supported by National Institutes of Health Grants HL74011, and HL28785 (P.G.G.), and NS054117 (S.J.L.), and by grants from Galleon Pharmaceuticals (S.J.L.). Roy Kanbar received a fellowship from the French Society of Hypertension.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201001-0047OC on July 9, 2010
Author Disclosure: R.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.L.S. has received sponsored grants from NIH ($50,001–$100,000). D.R.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.J.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.G.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 2010;90:47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khong P, Lazzaro A, Mobbs R. Phrenic nerve stimulation: the Australian experience. J Clin Neurosci 2010;17:205–208. [DOI] [PubMed] [Google Scholar]
- 3.Amiel J, Dubreuil V, Ramanantsoa N, Fortin G, Gallego J, Brunet JF, Goridis C. PHOX2B in respiratory control: lessons from congenital central hypoventilation syndrome and its mouse models. Respir Physiol Neurobiol 2009;168:125–132. [DOI] [PubMed] [Google Scholar]
- 4.Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG, Loghmanee DA, Trang H. An official ATS clinical policy statement: congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med 2010;181:626–644. [DOI] [PubMed] [Google Scholar]
- 5.Spengler CM, Gozal D, Shea SA. Chemoreceptive mechanisms elucidated by studies of congenital central hypoventilation syndrome. Respir Physiol 2001;129:247–255. [DOI] [PubMed] [Google Scholar]
- 6.Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol 2008;164:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 2006;101:618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 2006;7:232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 2003;26:239–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol 1989;281:69–96. [DOI] [PubMed] [Google Scholar]
- 11.Guyenet PG. The 2008. Carl Ludwig lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol 2008;105:404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol 2009;517:69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li A, Randall M, Nattie EE. CO(2) microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol 1999;87:910–919. [DOI] [PubMed] [Google Scholar]
- 14.Abbott SBG, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus Phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci 2009;29:5806–5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 2005;8:1263–1268. [DOI] [PubMed] [Google Scholar]
- 16.Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 2006;26:10305–10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbott SB, Stornetta RL, Socolovsky CS, West GH, Guyenet PG. Photostimulation of channelrhodopsin-2 expressing ventrolateral medullary neurons increases sympathetic nerve activity and blood pressure in rats. J Physiol 2009;587:5613–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 2006;7:335–346. [DOI] [PubMed] [Google Scholar]
- 19.Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand 2003;177:209–218. [DOI] [PubMed] [Google Scholar]
- 20.Kanbar R, Stornetta RL, Lewis SJ, Guyenet PG. Photostimulation of channelrhodopsin-transfected rostral medullary Phox2b-expressing neurons activates breathing in unaesthetized rats [abstract]. FASEB J 2010;24:1026.10. [Google Scholar]
- 21.Quinn R. Comparing rat's to human's age: how old is my rat in people years? Nutrition 2005;21:775–777. [DOI] [PubMed] [Google Scholar]
- 22.Kanbar R, Orea V, Barres C, Julien C. Baroreflex control of renal sympathetic nerve activity during air-jet stress in rats. Am J Physiol Regul Integr Comp Physiol 2007;292:R362–R367. [DOI] [PubMed] [Google Scholar]
- 23.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, De Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 2007;450:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 2005;25:8938–8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, Takakura AC, Gwilt JM, Guyenet PG, Stornetta RL. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol 2007;503:627–641. [DOI] [PubMed] [Google Scholar]
- 26.Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/GLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J Comp Neurol 2002;444:207–220. [DOI] [PubMed] [Google Scholar]
- 27.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 2006;570:407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins, and implications for respiratory rhythm generation. J Physiol 2009;587:3539–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol 1997;387:524–536. [DOI] [PubMed] [Google Scholar]
- 30.Hwang DY, Carlezon WA Jr, Isacson O, Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Hum Gene Ther 2001;12:1731–1740. [DOI] [PubMed] [Google Scholar]
- 31.Monnier A, Alheid GF, McCrimmon DR. Defining ventral medullary respiratory compartments with a glutamate receptor agonist in the rat. J Physiol 2003;548:859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyenet PG, Darnall RA, Riley TA. Rostral ventrolateral medulla and sympathorespiratory integration in rats. Am J Physiol 1990;259:R1063–R1074. [DOI] [PubMed] [Google Scholar]
- 33.Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res 2007;165:201–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onimaru H, Homma I. Two modes of respiratory rhythm generation in the newborn rat brainstem-spinal cord preparation. Adv Exp Med Biol 2008;605:104–108. [DOI] [PubMed] [Google Scholar]
- 35.Li A, Nattie EE. Focal central chemoreceptor sensitivity in the RTN studied with a CO2 diffusion pipette in vivo. J Appl Physiol 1997;83:420–428. [DOI] [PubMed] [Google Scholar]
- 36.Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci 2003;23:1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat Neurosci 2009;12:1028–1035. [DOI] [PubMed] [Google Scholar]
- 38.Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci 2009;29:14836–14846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol 1999;59:583–634. [DOI] [PubMed] [Google Scholar]
- 40.Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol 2009;517:69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci 2008;28:12845–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol 2008;586:2975–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 2004;7:1360–1369. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimura M, Polosa C, Nishi S. Noradrenaline-induced after depolarization in cat sympathetic preganglionic neurons in vitro. J Neurophysiol 1987;57:1314–1324. [DOI] [PubMed] [Google Scholar]
- 45.Inokuchi H, Yoshimura M, Polosa C, Nishi S. Tachykinins depress a calcium-dependent potassium conductance in sympathetic preganglionic neurons. Regul Pept 1993;46:367–369. [DOI] [PubMed] [Google Scholar]
- 46.Dampney RAL, Coleman MJ, Fontes MAP, Hirooka Y, Horiuchi J, Li YW, Polson JW, Potts PD, Tagawa T. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol 2002;29:261–268. [DOI] [PubMed] [Google Scholar]
- 47.Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res 2000;122:61–78. [DOI] [PubMed] [Google Scholar]
- 48.Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 2006;499:64–89. [DOI] [PubMed] [Google Scholar]
- 49.Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J Comp Neurol 2006;499:840–859. [DOI] [PubMed] [Google Scholar]
- 50.Moreira TS, Takakura AC, Colombari E, Guyenet PG. Central chemoreceptors and sympathetic vasomotor outflow. J Physiol 2006;577:369–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudzinski E, Kapur RP. PHOX2B immunolocalization of the candidate human retrotrapezoid nucleus. Pediatr Dev Pathol (In press) [DOI] [PubMed]
- 52.Benarroch EE, Smithson IL, Low PA, Parisi JE. Depletion of catecholaminergic neurons of the rostral ventrolateral medulla in multiple systems atrophy with autonomic failure. Ann Neurol 1998;43:156–163. [DOI] [PubMed] [Google Scholar]
- 53.Bruce EN, Mitra J, Cherniack NS. Central and peripheral chemoreceptor inputs to phrenic and hypoglossal motoneurons. J Appl Physiol 1982;53:1504–1511. [DOI] [PubMed] [Google Scholar]
- 54.Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnoea and specific loss of parafacial neurons. Proc Natl Acad Sci USA 2008;105:1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 5th ed. San Diego: Elsevier Academic Press; 2005.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.