Abstract
Rationale: Distinct sets of corticosteroid-unresponsive genes modulate disease severity in asthma.
Objectives: To identify corticosteroid-unresponsive genes that provide new insights into disease pathogenesis and asthma therapeutics.
Methods: Experimental murine asthma was induced by nasal administration of house dust mite for 5 days per week. Dexamethasone and apolipoprotein E (apo E) mimetic peptides were administered via osmotic minipumps.
Measurements and Main Results: Genome-wide expression profiling of the lung transcriptome in a house dust mite–induced model of murine asthma identified increases in apo E mRNA levels that persisted despite corticosteroid treatment. House dust mite–challenged apo E−/− mice displayed enhanced airway hyperreactivity and goblet cell hyperplasia, which could be rescued by administration of an apo E(130–149) mimetic peptide. Administration of the apo E(130–149) mimetic peptide to house dust mite–challenged apo E−/− mice also inhibited eosinophilic airway inflammation, IgE production, and the expression of Th2 and Th17 cytokines. House dust mite–challenged low-density lipoprotein receptor (LDLR) knockout mice displayed a similar phenotype as apo E−/− mice with enhanced airway hyperreactivity, goblet cell hyperplasia, and mucin gene expression, but could not be rescued by the apo E(130–149) mimetic peptide, consistent with a LDLR-dependent mechanism.
Conclusions: These findings for the first time identify an apo E–LDLR pathway as an endogenous negative regulator of airway hyperreactivity and goblet cell hyperplasia in asthma. Furthermore, our results demonstrate that strategies that activate the apo E–LDLR pathway, such as apo E mimetic peptides, might be developed into a novel treatment approach for patients with asthma.
Keywords: asthma, house dust mite, apolipoprotein E, LDL receptor
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Human and murine studies suggest that distinct sets of corticosteroid-unresponsive genes modulate disease severity in asthma.
What This Study Adds to the Field
This study identifies an apolipoprotein E–low-density lipoprotein receptor pathway as an endogenous negative regulator of airway hyperreactivity and goblet cell hyperplasia in house dust mite–induced asthma. Furthermore, it demonstrates that strategies that activate the apolipoprotein E–low-density lipoprotein receptor pathway, such as apolipoprotein E mimetic peptides, might be developed into a novel treatment approach for patients with asthma.
Asthma is a highly prevalent disease with a complex pathogenesis manifested by airway inflammation, remodeling, and hyperreactivity (1, 2). Corticosteroids, via their ability to suppress airway inflammation, are the primary treatment for asthma (3). Although inhaled corticosteroids effectively control symptoms in most patients, a subset of 5–10% of individuals with asthma has severe disease that is refractory to treatment with high doses of inhaled or oral corticosteroids (4–6). Corticosteroids mediate their effects at the transcriptional and post-transcriptional levels by mechanisms that involve the transrepression of proinflammatory genes and the transcriptional activation of antiinflammatory genes (7). Thus, severe asthma that is refractory to treatment with corticosteroids may be associated with the persistent expression of key disease causality and severity genes. Consistent with this concept, dysregulated gene expression has been documented in peripheral blood mononuclear cells and bronchoalveolar lavage cells from corticosteroid-resistant patients with asthma (8–10). Murine asthma models have also identified corticosteroid-unresponsive genes and the dissociation of airway inflammation from airway hyperreactivity (AHR) and remodeling responses in response to corticosteroid treatment (11–13). Taken together, these human and murine studies suggest that distinct sets of corticosteroid-unresponsive genes modulate disease severity in severe asthma that is refractory to corticosteroid therapy.
We hypothesized that the identification of corticosteroid-unresponsive genes may provide new insights into disease pathogenesis and identify novel therapeutic approaches for patients with asthma. Genome-wide profiling of the lung transcriptome from a clinically relevant house dust mite (HDM) challenge model of asthma identified the up-regulated expression of apolipoprotein E (apo E), which remained persistently elevated despite treatment with corticosteroids. Here, we show that an apo E–low-density lipoprotein receptor (LDLR) pathway functions as an endogenous negative regulator of AHR and goblet cell hyperplasia in asthma. Furthermore, these findings demonstrate that therapeutic strategies that activate the apo E–LDLR pathway, such as apo E mimetic peptides, may represent a novel treatment approach for patients with asthma.
METHODS
Mice
Female A/J, C57BL/6, apo E−/−, LDLR−/− (6–8 wk old) were obtained from Jackson Laboratories (Bar Harbor, ME). Both the apo E−/−and LDLR−/− mice were maintained on a C57BL/6 background. Experimental protocols were approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute, Bethesda, MD.
HDM-Induced Asthma and Therapeutic Interventions
Asthma was induced by nasal inhalation of HDM (Dermatophagoides pteronyssinus) extract (Greer, Lenoir, NC), 25 μg of protein in 10 μl of saline, for 5 days each week (14). The HDM extract contained 0.05 units per μl of endotoxin. Selected groups of mice had osmotic minipumps (Model 2004; Alzet, Cupertino, CA) implanted to administer dexamethasone (1 mg/kg/d), saline, apo E(130–149) mimetic peptide (1 mg/kg/d), or the apo E(scrambled) control peptide (1 mg/kg/d) (Genescript USA, Piscataway, NJ). The sequence of the apo E(130–149) mimetic peptide was TEELRVRLASHLRKLRKRLL, whereas the sequence of the apo E(scrambled) control peptide was LREKKLRVSALRTHRLELRL (15). In the treatment model, mice were challenged with nasal HDM for 6 weeks and osmotic minipumps containing dexamethasone or saline were implanted for Weeks 4 through 6. In the apo E mimetic peptide experiments, osmotic minipumps containing the apo E(130–149) mimetic peptide or the apo E(scrambled) control peptide were implanted coincident with administration of nasal HDM, which was administered for 5 consecutive weeks.
Quantitative Real-Time Polymerase Chain Reaction
Lungs were minced into 1-mm pieces in RNAlater (Ambion, Austin, TX) and stored at −80°C before isolation of total RNA using the mirVana kit (Ambion). RNA was treated with 10 units of DNase I per 20 μg of RNA before reverse transcription using the High-capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). Polymerase chain reaction (PCR) was performed on duplicate 1μg cDNA samples using TaqMan Universal PCR Master Mix, FAM dye-labeled Taqman MGB probes, and a 7500 Real Time PCR System running Sequence Detector version 2.1 software. Gene expression was quantified relative to expression of 18S rRNA using the control sample as calibrator to calculate the difference in Ct values (ΔΔCt) and presented as relative mRNA expression.
Lung Genome-Wide Transcriptome Analysis
Total RNA was incubated with oligo dT/T7 primers and reverse transcribed into double-stranded cDNA. In vitro transcription and biotin labeling of the purified cDNA was performed using T7 RNA polymerase at 37°C for 16 hours. The yield and integrity of the biotin-labeled cRNA were determined using a nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE) and an Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA). A total of 20 μg of biotin-labeled RNA were fragmented to approximately 200 base pairs by incubation in fragmentation buffer (0.2 M tris-acetate pH 8.2, 0.5 M potassium acetate, 0.5 M magnesium acetate) for 35 minutes at 94°C before hybridization. The size of fragmented RNA was assessed using an Agilent 2100 bioanalyzer (Agilent Technologies). RNA was hybridized to mouse genome 430 2.0 gene chips for 16 hours, followed by washing and staining on a fluidics station (Affymetrix, Palo Alto, CA).
Affymetrix GCOS version 1.4 was used to calculate the signal intensity and the percent present calls (Affymetrix). The signal intensity values obtained for probe sets were transformed using an adaptive variance-stabilizing, quantile-normalizing transformation (http://abs.cit.nih.gov/geneexpression.html). Transformed data were subjected to a principal component analysis to detect outliers. One-way analysis of variance and appropriate post hoc tests were performed on each probe set. Probe sets that were twofold elevated above control by HDM-challenge and decreased to less than twofold elevated by corticosteroid treatment were classified as corticosteroid-responsive genes. Probe sets that were twofold elevated above control after HDM-challenge and remained twofold elevated despite corticosteroid treatment were classified as corticosteroid-unresponsive genes. Classifications were based on P less than 0.001 and a twofold change cutoff. Fold cutoff filters and false discovery rate analysis filters were used to address multiple comparisons. Two-way hierarchical clustering was used to compile sample and gene sets with similar expression patterns. The hierarchical cluster was performed with the JMP5.1 statistical software package (SAS Institute, Cary, NC) using the ward method.
Bronchoalveolar Lavage and Lung Histopathologic Examination
Bronchoalveolar lavage was performed with 0.5 ml phosphate-buffered saline (PBS) × 3 and cells were suspended in 0.3 ml RPMI with 10% fetal bovine serum after lysis of red blood cells with ACK buffer for 2 minutes at 4°C. Total cell counts were performed using a hemocytometer and bronchoalveolar lavage fluid (BALF) differentials were performed on Diff-Quik-stained cytospin slides (Siemens, Healthcare Diagnostics, Inc., Newark, DE). Lungs were then inflated with 1 ml 10% formalin at a pressure of 25 cm H2O, fixed in 10% formalin for at least 24 hours, dehydrated through gradient ethanol, embedded in paraffin, and the sagittal sections were cut at a thickness of 5 μm. Sections were stained with hematoxylin and eosin and periodic acid–Schiff (PAS).
Inspection of lung sections revealed intraanimal and interanimal heterogeneity regarding the presence of goblet cell hyperplasia within individual airways. To quantify goblet cell hyperplasia throughout the entire lung of each animal, all the airways present (large [conducting], medium [central], and small [distal]) within a representative lung section were inspected and the number of airways that contained PAS-positive cells was recorded. Goblet cell hyperplasia is presented as the percentage of airways that contained PAS-positive cells. The number of airways inspected in each animal is also presented.
Measurement of AHR
Airway resistance to increasing doses of methacholine was measured in anesthetized mice using an Elan RC Fine Pointe system (Buxco, Wilmington, NC). After tracheal cannulation with a 19-gauge beveled metal catheter, mice were mechanically ventilated with a constant inspiratory flow before nebulization of PBS or increasing doses of methacholine. Airway resistance was recorded at 10-second intervals for 3 minutes and average values are presented as cm H2O/ml/s.
Measurement of Serum IgE
Total serum IgE was measured with an OptEIA (BD Biosciences Pharmingen, San Diego, CA).
Confocal Microscopy
Lungs were instilled with OCT (Tissue-Tek; Sakura Finetek, Torrance, CA) and snap-frozen in liquid nitrogen. Cryostat lung tissue sections were cut into 10 μm thickness, mounted on Plus(+) slides and stored at −80°C. For immunofluorescence analysis, slides were air dried, fixed in 4% paraformaldehyde in PBS for 15 minutes, washed three times in PBS, permeabilized in 0.1% Triton X-100 in PBS for 10 minutes, washed three times in PBS, and incubated in blocking buffer (Aurion Blocking Solution; Electron Microscopy Sciences, Hatfield, PA) for 1 hour before incubation overnight at 4°C with primary antibodies diluted in 0.1% Aurion BSA-c (Electron Microscopy Sciences). Primary antibodies were used at the following concentrations: goat polyclonal anti–apo E antibody (Santa Cruz, Santa Cruz, CA), 1:25 dilution; rabbit polyclonal anti-LDLR antibody (Novus Biologicals, Littleton, CO), 1:100 dilution; rat polyclonal anti-CD68 antibody, 1:50 dilution (Abcam, Cambridge, MA); mouse monoclonal anti-acetylated α-tubulin, 1:50 dilution (Abcam). Sections were washed three times in PBS and incubated with species-specific secondary antibodies conjugated to Alexa Fluor 488 or Alexa Fluor 568 (Molecular Probes, Carlsbad, CA) at a 1:400 dilution. Sections were mounted using Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and visualized using a Leica SP laser scanning confocal microscope (Leica, Heidelberg, Germany). Immunofluorescence was absent when lung sections were reacted with the appropriate control antibodies, which included nonimmune rabbit, rat, and goat serum, and a mouse IgG2b isotype control.
Statistics
Results are presented as mean ± SEM. A one-way analysis of variance followed by Bonferroni multiple comparison test was used to determine statistical significance, except for analyses of AHR, which used a two-way analysis of variance with a Bonferroni posttest test (GraphPad Prism version 5.0a). A P value less than 0.05 was considered significant.
RESULTS
Corticosteroid Treatment Dissociates AHR From Airway Inflammation and Goblet Cell Hyperplasia in a HDM Model of Asthma
A treatment model of HDM-induced asthma was established to identify pulmonary genes that demonstrate persistently up-regulated expression despite corticosteroid therapy. Wild-type A/J mice received saline or HDM by nasal administration 5 days per week for 6 weeks. The A/J strain was selected for these experiments based on its phenotype of enhanced susceptibility to the induction of asthma and allergen-mediated AHR (16–18). Dexamethasone (1 mg/kg) or saline was administered by an osmotic minipump during weeks 4 through 6. HDM-induced asthma was associated with significant increases in the total number of inflammatory cells in BALF (see Figures E1A and E1B in the online supplement), which were suppressed to baseline levels by dexamethasone. Similarly, lung histology showed a reduction in peribronchial inflammatory cell infiltration in dexamethasone-treated mice (Figure E1C). Dexamethasone significantly reduced goblet cell hyperplasia in HDM-challenged mice to levels that were similar to control mice (Figures E1C and E1D). Dexamethasone also suppressed pulmonary mRNA levels of MUC5AC and CLCA3 (Gob5) (Figures E2A and E2B). Corticosteroid treatment was associated with a small, but statistically significant decrease in AHR in HDM-challenged mice; however, airway resistance remained significantly higher than control mice (Figure E2C). These data demonstrate that AHR is dissociated from airway inflammation and goblet cell hyperplasia in response to corticosteroid treatment in this HDM-induced model of asthma.
Identification of Apo E as a Corticosteroid-Unresponsive Gene in HDM-Induced Asthma
Genome-wide expression profiling of the asthmatic lung transcriptome was performed to identify corticosteroid-unresponsive genes, which were defined as transcripts whose expression remained twofold elevated after HDM-challenge despite corticosteroid treatment. Sixty-eight genes were corticosteroid-unresponsive (Figure 1A; Table E1), whereas 352 genes were corticosteroid-responsive (Figure 1B; Table E2). From this data set, apo E was selected as a candidate corticosteroid-unresponsive gene in asthma. Quantitative real-time PCR (qRT-PCR) confirmed that lung expression of apo E mRNA was significantly increased in HDM-challenged mice and remained greater than twofold elevated despite corticosteroid treatment (Figure 1C). Finally, confocal immunofluorescence microscopy demonstrated that apo E was expressed primarily by CD68+ alveolar macrophages in the lungs of HDM-challenged mice (Figure 1D).
Figure 1.
Identification of apolipoprotein E (apo E) as a corticosteroid-unresponsive gene in a treatment model of house dust mite (HDM)–induced asthma. (A–C) Asthma was induced in wild-type A/J mice by daily nasal administration of HDM or saline (Control) 5 days per week for 6 consecutive weeks. Treatment with 1 mg/kg dexamethasone (Dex) or saline (Vehicle) was administered via osmotic minipump during weeks 4–6 in selected groups. (A and B) Hierarchical cluster analysis of lung mRNA transcripts that were up-regulated twofold or greater in the HDM + Vehicle group compared with the Control + Vehicle group. mRNA transcripts with expression levels that remained greater than or equal to twofold increased compared with the Control + Vehicle group were considered corticosteroid-unresponsive (A), whereas mRNA transcripts that were suppressed by corticosteroid treatment to levels less than twofold below that of the Control + Vehicle group were considered corticosteroid-responsive (B). Expression levels were sorted based on the mean intensity of genes in the first column of the Control + Vehicle group (n = 5 mice per group). (C) Lung mRNA expression of apo E was assessed by quantitative real-time polymerase chain reaction and presented as relative mRNA expression (n = 10 mice; *P < 0.01 compared with Control + Vehicle; **P < 0.05 HDM + Vehicle vs. HDM + dexamethasone). (D) Sections of lungs from wild-type C57BL6 mice that had been challenged with HDM, 5 days per week for 5 weeks, were reacted with antibodies against CD68 and apo E, and secondary antibodies conjugated with Alexa Fluor 488 (green) or Alexa Fluor 568 (red), followed by immunofluorescence confocal laser scanning microscopy.
Apo E is an Endogenous Negative Regulator of AHR and Goblet Cell Hyperplasia in HDM-Induced Asthma
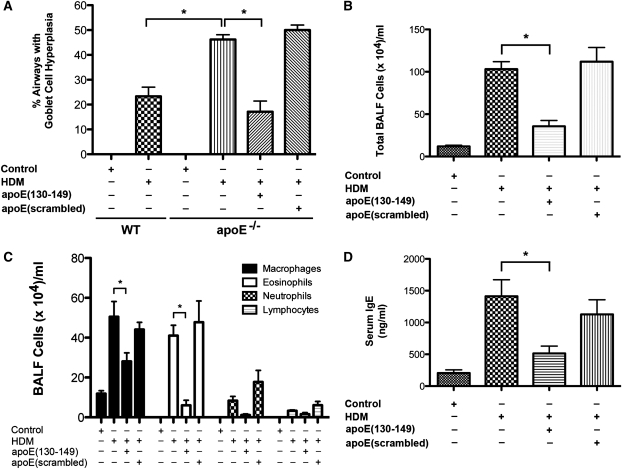
Having identified that apo E gene expression was increased in the lungs of asthmatic mice, apo E−/− mice were used to assess its role in disease pathogenesis. Apo E mRNA levels were increased in the lungs of wild-type C57BL/6 mice in response to HDM-challenge, whereas apo E mRNA was not detected in the lungs of apo E−/− mice (Figure 2A). AHR was significantly increased in HDM-challenged apo E−/− mice compared with HDM-challenged wild-type mice (Figure 2B). Furthermore, the basal level of AHR in saline-challenged apo E−/− mice was increased to a level similar to that of HDM-challenged wild-type mice. This demonstrates that apo E functions as an endogenous negative regulator of AHR, both at baseline and in asthma. MUC5AC (Figure 2C) and CLCA3 (Figure 2D) mRNA levels were significantly increased in the lungs of HDM-challenged apo E−/− mice compared with wild-type mice, which correlated with a significant increase in goblet cell hyperplasia (Figure 3A). In contrast, there was no difference in airway inflammation between HDM-challenged apo E−/− mice and wild-type mice based on lung histology (Figure 2E) and BALF cell counts (see Figure E3). These data are consistent with the conclusion that apo E functions as an endogenous negative regulator of AHR and goblet cell hyperplasia in HDM-induced asthma.
Figure 2.
Apolipoprotein E (apo E) is a negative regulator of airway hyperreactivity and mucin gene expression in house dust mite (HDM)–induced asthma. Asthma was induced in wild-type (WT) C57BL6 and apo E−/− mice by nasal administration of HDM or saline (Control) 5 days per week for 5 consecutive weeks. (A) Lung mRNA levels of apo E in WT and apo E−/− mice were assessed by quantitative real-time polymerase chain reaction and presented as relative mRNA expression (n = 6 mice; P < 0.001; WT Control vs. WT HDM). (B) Airway resistance (cm H2O/ml/s) was measured after nebulization of increasing doses of methacholine (n = 9 mice; *P < 0.05 compared with WT Control; **P < 0.01 WT HDM vs. apo E−/− HDM). (C and D) Quantification of lung mRNA levels for MUC5AC and CLCA3 by quantitative real-time polymerase chain reaction presented as relative mRNA expression (n = 6–10 mice; *P < 0.001 compared with WT Control; **P < 0.01, WT HDM vs. apo E−/− HDM). (E) Histologic sections of lung were stained with hematoxylin and eosin (H&E) or periodic acid–Schiff (PAS) stains and images were obtained at ×200 or ×1,000.
Figure 3.
Administration of an apolipoprotein E (apo E)(130–149) mimetic peptide to apo E−/− mice attenuates house dust mite (HDM)–induced asthma. An osmotic minipump containing either apo E(130–149) or an apo E(scrambled) peptide was implanted before the induction of asthma in apo E−/− mice by nasal administration of HDM or saline (Control) 5 days per week for 5 consecutive weeks. Wild-type C57BL6 mice (WT) were also challenged with HDM or saline (Control) 5 days per week for 5 consecutive weeks, where indicated. (A) Goblet cell hyperplasia presented as the percentage of airways containing periodic acid–Schiff positive cells (n = 8–13 mice; *P < 0.0001 vs. HDM-challenged apo E−/− mice). A total of 33 ± 1.2 airways were inspected in each mouse. (B and C) Numbers of total cells (n = 8 mice; *P < 0.001) and inflammatory cell types (n = 8 mice; *P < 0.05, HDM vs. HDM + apo E[130–149]) in bronchoalveolar lavage fluid (BALF). (D) Quantification of serum IgE levels (n = 8 mice; *P < 0.01, HDM vs. HDM + apo E[130–149]).
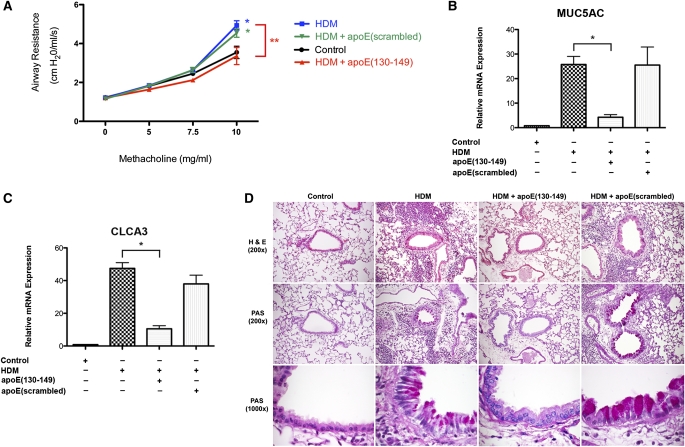
Administration of an Apo E Mimetic Peptide Rescues the Phenotype of Enhanced AHR and Goblet Cell Hyperplasia in HDM-Challenged Apo E−/− Mice
We next assessed whether administration of an apo E mimetic peptide that corresponds to the receptor binding domain (amino acids 130–149) of the native apo E protein could rescue the phenotype of enhanced AHR and mucin gene expression in HDM-challenged apo E−/− mice (15). An apo E(130–149) mimetic peptide or control scrambled apo E peptide were systemically administered to apo E−/− mice via an osmotic minipump before the induction of asthma by daily nasal HDM challenge, 5 days per week for 5 weeks. As shown in Figure 4A, the apo E(130–149) mimetic peptide completely inhibited the induction of AHR in HDM-challenged apo E−/− mice, whereas the control scrambled peptide had no effect. Similarly, the apo E(130–149) mimetic peptide, but not the scrambled control peptide, significantly reduced mRNA levels of MUC5AC and CLCA3 (Figures 4B and 4C), and goblet cell hyperplasia in HDM-challenged apo E−/− mice (Figure 4D; Figure 3A). These results demonstrate that administration of an apo E mimetic peptide rescues the phenotype of asthmatic apo E−/− mice and confirms an important role for apo E as an endogenous negative regulator of AHR and goblet cell hyperplasia in asthma.
Figure 4.
Administration of an apolipoprotein E (apo E)(130–149) mimetic peptide to apo E−/− mice attenuates house dust mite (HDM)–induced asthma. An osmotic minipump containing either apo E(130–149) or an apo E(scrambled) peptide was implanted before the induction of asthma in apo E−/− mice by nasal administration of HDM or saline (Control) 5 days per week for 5 consecutive weeks. (A) Airway resistance (cm H2O/ml/s) was measured after nebulization of increasing doses of methacholine (n = 10 mice; *P < 0.001 vs. Control; **P < 0.001 HDM vs. HDM + apo E[130–149]). (B and C) Quantification of lung mRNA levels for MUC5AC and CLCA3 by quantitative real-time polymerase chain reaction presented as relative mRNA expression (n = 6 mice; *P < 0.01 HDM vs. HDM + apo E[130–149]). (D) Histologic sections of lung were stained with hematoxylin and eosin (H&E) or periodic acid–Schiff (PAS) stains and images obtained at ×200 or ×1,000.
Administration of the apo E(130–149) mimetic peptide also significantly inhibited the induction of allergen-mediated airway inflammation. HDM-challenged apo E−/− mice treated with the apo E(130–149) mimetic peptide, but not the scrambled control peptide, had a significant reduction in total BALF cells (Figure 4B) and BALF eosinophils and macrophages (Figure 3C). Similarly, lung histology showed a marked reduction in peribronchial inflammation in HDM-challenged apo E−/− mice that received the apo E(130–149) mimetic peptide (Figure 4D). Treatment with the apo E(130–149) mimetic peptide, but not the scrambled control, also significantly reduced plasma IgE levels (Figure 3D), and mRNA levels of Th2 (IL-4 and IL-13) and Th17 (IL-17A) cytokines (Figure 5). Similarly, treatment with the apo E(130–149) mimetic peptide, but not the scrambled control peptide, also inhibited mRNA levels of chemokines with chemotactic activity toward eosinophils and T cells (CCL7, CCL11, and CCL24), and products of alternatively activated macrophages (arginase 1, chitinase 3-like 3 [Chi3L3, YM1], and Fizz1 [resistin-like α]) (2, 19–22). Taken together, these data demonstrate that administration of an apo E(130–149) mimetic peptide to apo E−/− mice inhibits the induction of key manifestations of asthma, including eosinophilic airway inflammation, AHR, goblet cell hyperplasia, and IgE production.
Figure 5.
Administration of an apolipoprotein E (apo E)(130–149) mimetic peptide to apo E−/− mice attenuates house dust mite (HDM)–induced asthma. An osmotic minipump containing either apo E(130–149) or an apo E(scrambled) peptide was implanted before the induction of asthma in apo E−/− mice by nasal administration of HDM or saline (Control) 5 days per week for 5 consecutive weeks. Quantification of lung mRNA levels for IL-4, IL-13, IL-17A, CCL7, CCL11, CCL17, arginase 1 (Arg1), Chi3L3, and Fizz1 (resistin-like α) by quantitative real-time polymerase chain reaction presented as relative mRNA expression (n = 6 mice; *P < 0.05, Control vs. HDM; **P < 0.05, HDM vs. HDM + apo E[130–149]).
Apo E Modulates AHR and Goblet Cell Hyperplasia Via a LDLR-Dependent Pathway
Because apo E binds to receptors belonging to the LDLR family, we investigated whether HDM-induced asthma is mediated via the LDLR. First, we assessed whether the LDLR is expressed in asthmatic airways. Confocal immunofluorescence microscopy demonstrated that LDLR is expressed by ciliated airway epithelial cells, as indicated by coexpression with cells positive for acetylated α-tubulin (Figure 6A) (23). LDLR expression was localized to cilia and punctate cytoplasmic vesicular structures. Next, we hypothesized that if apo E regulates the induction of AHR and goblet cell hyperplasia via the LDLR, then HDM-challenged LDLR−/− mice should display a similar phenotype as apo E−/− mice, with the exception that the apo E(130–149) mimetic peptide should be incapable of attenuating the manifestations of asthma in LDLR−/− mice. As shown in Figure 6B, the phenotype of HDM-challenged LDLR−/− mice resembled that of apo E−/− mice, with enhanced AHR as compared with wild-type asthmatic mice. LDLR−/− mice also demonstrated enhanced basal levels of AHR, with increases in airway resistance similar to that of HDM-challenged wild-type mice. Similarly, mRNA levels of MUC5AC and CLCA3 (Figure 7A) and goblet cell hyperplasia (Figures 7B and 7C) were increased in HDM-challenged LDLR−/− mice compared with wild-type mice. Also similar to apo E−/− mice, there was no difference in peribronchial inflammatory cell infiltration (Figure 7C) or BALF inflammatory cell counts (see Figure E4) between HDM-challenged wild-type and LDLR−/− mice. However, in contrast to apo E−/− mice, administration of the apo E(130–149) mimetic peptide to HDM-challenged LDLR−/− mice did not attenuate the induction of AHR (Figure 6B), mRNA levels of MUC5AC and CLCA3 (Figure 7A), goblet cell hyperplasia (Figures 7B and 7C), or airway inflammation (Figure 7C; see Figure E4). These findings demonstrate that apo E negatively regulates the induction of AHR and goblet cell hyperplasia via a LDLR-dependent mechanism.
Figure 6.
Low-density lipoprotein receptor (LDLR)−/− mice recapitulate the asthma phenotype of apolipoprotein E (apo E) −/− mice, but are resistant to rescue by the apo E(139–149) mimetic peptide. (A) Sections of lungs from wild-type C57BL6 (WT) mice were reacted with antibodies against the low-density lipoprotein receptor (LDLR) and acetylated α-tubulin and secondary antibodies conjugated with Alexa Fluor 488 (green) or Alexa Fluor 568 (red), respectively, before immunofluorescence confocal laser scanning microscopy. Differential interference contrast (DIC) images are shown in the bottom right panel. The arrow indicates ciliated airway epithelial cells, whereas the scale bar denotes 8.23 μm. (B) An osmotic minipump containing either apo E(130–149) or an apo E(scrambled) peptide was implanted before the induction of asthma in LDLR−/− mice by nasal administration of house dust mite (HDM) or saline (Control) 5 days per week for 5 consecutive weeks. WT were similarly challenged with HDM or saline (Control) 5 days per week for 5 consecutive weeks. Airway resistance (cm H2O/ml/s) was measured after nebulization of increasing doses of methacholine (n = 9 mice; *P < 0.05 vs. WT + Control; **P < 0.001 vs. WT + HDM). The WT + HDM and LDLR−/− + Control groups are superimposed. The LDLR−/− + HDM, LDLR−/− + HDM + apo E(scrambled), and LDLR−/− + HDM + apo E(130–149) groups are also superimposed.
Figure 7.
Low-density lipoprotein receptor (LDLR)−/− mice recapitulate the asthma phenotype of apolipoprotein E (apo E) −/− mice, but are resistant to rescue by the apo E(139–149) mimetic peptide. An osmotic minipump containing either apo E(130–149) or an apo E(scrambled) peptide was implanted before the induction of asthma in LDLR−/− mice by nasal administration of house dust mite (HDM) or saline (Control) 5 days per week for 5 consecutive weeks. Wild-type C57BL6 (WT) mice were similarly challenged with HDM or saline (Control) 5 days per week for 5 consecutive weeks. (A) Quantification of lung mRNA levels by quantitative real-time polymerase chain reaction presented as relative mRNA expression (n = 6 mice; *P < 0.05 vs. WT + Control; **P < 0.01 WT + HDM vs. LDLR−/− + HDM; P = NS, LDLR−/− + HDM vs. LDLR−/− + HDM + apo E[130–149] mimetic peptide). (B) Goblet cell hyperplasia presented as percent of airways with periodic acid–Schiff positive airway epithelial cells (n = 10–15 mice; *P < 0.0001 vs. WT + Control; **P < 0.0001 WT + HDM vs. LDLR−/− + HDM; P = NS, LDLR−/− + HDM vs. LDLR−/− + HDM + apo E[130–149] mimetic peptide). A total of 29 ± 1 airways were inspected in each mouse. (C) Histologic sections of lung were stained with hematoxylin and eosin (H&E) or periodic acid–Schiff (PAS) stains and images obtained at ×200 or ×1,000.
DISCUSSION
Approximately 5–10% of individuals with asthma have severe disease that is refractory to treatment with high-doses of inhaled or oral corticosteroids (5, 6). These individuals with severe asthma have a clinical phenotype characterized by persistent symptoms and increased use of health care resources (6). Here, we sought to identify corticosteroid-resistant genes that modulate disease severity in asthma. We used a clinically relevant murine model of asthma induced by repeated exposure to HDM (D. pteronyssinus), a common aeroallergen that is an environmental trigger and risk factor for the development of persistent asthma (14, 24). HDM is a complex mixture of proteins and lipopolysaccharide, which can induce airway inflammation via allergic and nonallergic mechanisms (25–27). Furthermore, the mechanism of HDM-induced asthma, involves Toll-like receptor 4 signaling in airway epithelial cells, with resultant activation of innate and adaptive immune responses (28). Here, we show that administration of systemic corticosteroids to mice with established HDM-mediated asthma significantly suppressed airway inflammation and goblet cell hyperplasia, but not AHR, which demonstrates dissociation of these responses. We next performed a genome-wide screen of lung transcriptome expression in corticosteroid-treated asthmatic mice, which led to the identification of apo E as a novel corticosteroid-unresponsive gene in HDM-induced asthma that is primarily expressed by CD68+ alveolar macrophages.
Apo E plays a key role in lipoprotein metabolism by serving as a high-affinity ligand for the LDLR (29, 30). Consistent with its important role in cholesterol homeostasis, apo E knockout mice develop hypercholesterolemia and spontaneous atherosclerosis (30). The role of apo E in disease pathogenesis is not limited to cholesterol metabolism, because the ɛ4 allele of the human apo E gene modulates synthesis and clearance of the amyloid-β peptide and has been linked to Alzheimer disease (31, 32). Apo E modulates host susceptibility to infection by malaria, HIV, and herpes simplex virus (32). Apo E has also been shown to facilitate the presentation of lipid antigens to antigen-presenting cells via a process that involves the endocytosis of apo E–lipid-antigen complexes via the LDLR (33). Here, we identify a new function for apo E in modulating the pathogenesis of HDM-induced asthma. Not only was AHR increased in apo E−/− mice after the induction of asthma, but basal levels of airway resistance in apo E−/− mice were similar to those of HDM-challenged wild-type mice. Goblet cell hyperplasia, and mRNA levels of the MUC5AC mucin gene and CLCA3 (Gob5), a calcium-activated chloride channel that is associated with goblet cell hyperplasia, were also elevated in HDM-challenged apo E−/− mice (34). These findings identify apo E as an endogenous negative regulator of AHR and goblet cell hyperplasia in HDM-induced asthma.
Apo E is a 34-kD, 299 amino acid protein that folds into a helical horseshoe configuration that contains a distinct LDLR-binding domain, corresponding to amino acids 134 to 150 of the amino-terminus, and a major lipid-binding domain, corresponding to amino acids 244 to 272 of the carboxy-terminus (32). The amino-terminus of apo E is comprised of a four amphipathic α-helices, with the LDLR-binding domain located in helix 4 (35). Apo E mimetic peptides, which correspond to the LDLR-binding domain, have been shown to bind LDLR family members and thereby suppress inflammation and neurotoxicity, and to mediate antiviral effects (15, 36–39). We confirmed that apo E functions as a negative regulator of AHR and goblet cell hyperplasia in asthma by demonstrating that the phenotype of HDM-challenged apo E−/− mice could be rescued by systemic administration of an apo E mimetic peptide. The apo E(130–149) mimetic peptide also significantly attenuated the induction of eosinophilic airway inflammation, IgE production, and mRNA levels of Th2 and Th17 cytokines, and chemokines for eosinophils and T cells (CCL7, CCL11, CCL17) and products of alternatively activated macrophages (arginase 1, Chi3L3 [YM1], and Fizz1 [resistin-like α]) (2, 19–22, 40). This demonstrates that apo E pathways also suppress the initiation of airway inflammation in asthma, which is consistent with prior reports describing antiinflammatory and immune modulatory functions of apo E in animal models of bacterial infection, endotoxemia, and septic shock (41, 42).
Apo E is a ligand for members of the LDLR family, of which LDLR is the prototypical receptor (43). Because apo E functions as a negative regulator of AHR and goblet cell hyperplasia, we investigated whether the LDLR might serve as the relevant receptor for apo E in the asthmatic airway. First, we showed by confocal laser scanning microscopy that ciliated airway epithelial cells express the LDLR. Furthermore, LDLR expression colocalized with acetylated α-tubulin consistent with expression by cilia, and in cytoplasmic vesicular structures. Interestingly, motile cilia, which had previously been thought to serve a purely mechanical role, have recently been shown to express bitter taste receptors and thereby function as chemosensors that interact with the external environment (44). We then hypothesized that the phenotype of LDLR−/− mice in an induction model of asthma should recapitulate that of apo E−/− mice if the LDL receptor were the relevant receptor for apo E in the airway. Consistent with this conclusion, HDM-challenged LDLR−/− mice displayed enhanced AHR, mucin gene expression, and goblet cell hyperplasia compared with wild-type mice, whereas airway inflammatory responses were not altered. In contrast to apo E−/− mice, administration of the apo E(130–149) mimetic did not rescue the phenotype of enhanced AHR and goblet cell hyperplasia in LDLR−/− knockout mice, which is consistent with the conclusion that the LDLR mediates the negative regulatory effects of apo E on AHR and goblet cell hyperplasia. Taken together, this demonstrates that via binding to LDLRs expressed on ciliated airway epithelial cells, apo E functions in a paracrine fashion negatively to modulate AHR, mucin gene expression, and goblet cell hyperplasia. We are currently conducting additional studies to identify the mechanisms by which the apo E–LDLR interaction attenuates these cardinal manifestations of asthma, which may involve the modified expression of key genes or proteins that initiate airway responses after HDM exposure.
It is important to address several points regarding our study. First, our model of HDM-induced asthma demonstrated that AHR remained elevated despite corticosteroid treatment and represented a steroid-resistant end point, whereas airway inflammation and goblet cell hyperplasia were abrogated by corticosteroid treatment. This is consistent with the conclusion that ours is not a model of steroid-resistant inflammation. Therefore, a role for apo E in the pathogenesis of steroid-resistant airway inflammation would need to be defined either in human patients or in murine models of severe, corticosteroid-refractory asthma. Second, we used corticosteroid treatment as a tool to identify candidate genes that modulate asthma pathogenesis, which led to the identification of apo E. Consistent with this, in the absence of steroids, we show that apo E negatively regulates allergen-mediated AHR and goblet cell hyperplasia. Corticosteroid treatment, however, decreased apo E mRNA levels in HDM-challenged mice. Therefore, we do not believe that corticosteroids mediate their beneficial effects in asthma via the modulation of apo E gene expression. Third, HDM-induced airway inflammation was not modified in either apo E−/− or LDLR−/− mice. This is consistent with the conclusion that endogenous apo E does not modulate airway inflammation. Instead, our results suggest that endogenous apo E functions as a disease severity gene that attenuates excessive AHR and goblet cell hyperplasia in HDM-induced asthma. Furthermore, our results show that mechanistic pathways exist that selectively regulate AHR and goblet cell hyperplasia, but are dissociated from those mediating airway inflammation. Lastly, the preparation of HDM that we used contained low levels of endotoxin (0.05 EU/μl), which makes it unlikely that the asthmatic phenotype was solely a consequence of endotoxin-induced inflammation (45).
To the best of our knowledge, we have for the first time identified an apo E–LDLR pathway as an endogenous negative regulator of AHR and goblet cell hyperplasia in asthmatic airways. Furthermore, we show that administration of an apo E mimetic peptide inhibits the induction of HDM-mediated asthma. Thus, our findings establish the novel concept that strategies that activate the apo E–LDLR pathway, such as apo E mimetic peptides, might be used as a novel treatment approach for patients with asthma.
Supplementary Material
Acknowledgments
The authors are extremely appreciative of the staff of the National Heart, Lung, and Blood Institute (NHLBI) Laboratory of Animal Medicine and Surgery, whose commitment, professional advice, and excellent technical support made this study possible. They are very appreciative of the Pathology Core Facility, NHLBI, for their assistance with the histopathologic analyses. The authors are most appreciative of the support provided by Dr. Peter Munson from the Mathematical and Statistical Computing Lab, Division of Computational Biosciences, Center for Information Technology, National Institutes of Health, regarding the analysis of gene expression data. The authors thank the Light Microscopy Core Facility, NHLBI, for their support. They also thank Dr. Myra Mandel for her assistance with assays and Hiren Bhakta, Andrew Crouch, and Milkesso Foge for their assistance with the performance of experiments. The authors are most appreciative of Drs. Joel Moss and Martha Vaughan for their helpful discussions and comments regarding the manuscript.
Supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201002-0308OC on July 9, 2010
Author Disclosure: X.Y. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Z-X.Y. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. X.X. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.J.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.J.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.J.A.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.T.R. received more than $100,001 from KineMed in industry-sponsored grants as a development agreement (CRADA) to develop 5A mimetic peptide for treatment of atherosclerosis and holds a National Institutes of Health licensed patent on 5A peptide to KineMed for future development. S.J.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc 2009;6:301–305. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest 2008;118:3546–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.N.A.E.P. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Bethesda, Maryland: National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services; 2007.
- 4.Wenzel SE, Busse WW. Severe asthma: lessons from the severe asthma research program. J Allergy Clin Immunol 2007;119:14–21. [DOI] [PubMed] [Google Scholar]
- 5.American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med 2000;162:2341–2351. [DOI] [PubMed] [Google Scholar]
- 6.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol 2007;119:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark AR. Anti-inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol 2007;275:79–97. [DOI] [PubMed] [Google Scholar]
- 8.Hakonarson H, Bjornsdottir US, Halapi E, Bradfield J, Zink F, Mouy M, Helgadottir H, Gudmundsdottir AS, Andrason H, Adalsteinsdottir AE, et al. Profiling of genes expressed in peripheral blood mononuclear cells predicts glucocorticoid sensitivity in asthma patients. Proc Natl Acad Sci USA 2005;102:14789–14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lane SJ, Adcock IM, Richards D, Hawrylowicz C, Barnes PJ, Lee TH. Corticosteroid-resistant bronchial asthma is associated with increased c-fos expression in monocytes and T lymphocytes. J Clin Invest 1998;102:2156–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung DY, Martin RJ, Szefler SJ, Sher ER, Ying S, Kay AB, Hamid Q. Dysregulation of interleukin 4, interleukin 5, and interferon gamma gene expression in steroid-resistant asthma. J Exp Med 1995;181:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kibe A, Inoue H, Fukuyama S, Machida K, Matsumoto K, Koto H, Ikegami T, Aizawa H, Hara N. Differential regulation by glucocorticoid of interleukin-13-induced eosinophilia, hyperresponsiveness, and goblet cell hyperplasia in mouse airways. Am J Respir Crit Care Med 2003;167:50–56. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, Herbert C, Siegle JS, Vuppusetty C, Hansbro N, Thomas PS, Foster PS, Barnes PJ, Kumar RK. Steroid-resistant neutrophilic inflammation in a mouse model of an acute exacerbation of asthma. Am J Respir Cell Mol Biol 2008;39:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birrell MA, Battram CH, Woodman P, McCluskie K, Belvisi MG. Dissociation by steroids of eosinophilic inflammation from airway hyperresponsiveness in murine airways. Respir Res 2003;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med 2004;169:378–385. [DOI] [PubMed] [Google Scholar]
- 15.Croy JE, Brandon T, Komives EA. Two apolipoprotein E mimetic peptides, apoE(130–149) and apoE(141–155)2, bind to lrp1. Biochemistry 2004;43:7328–7335. [DOI] [PubMed] [Google Scholar]
- 16.Levitt RC, Mitzner W. Expression of airway hyperreactivity to acetylcholine as a simple autosomal recessive trait in mice. FASEB J 1988;2:2605–2608. [DOI] [PubMed] [Google Scholar]
- 17.Wills-Karp M, Ewart SL. The genetics of allergen-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med 1997;156:S89–S96. [DOI] [PubMed] [Google Scholar]
- 18.Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol 2010;184:1663–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003;3:23–35. [DOI] [PubMed] [Google Scholar]
- 20.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 2009;27:451–483. [DOI] [PubMed] [Google Scholar]
- 21.Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, August A. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J Exp Med 2008;205:1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying S, Robinson DS, Meng Q, Barata LT, McEuen AR, Buckley MG, Walls AF, Askenase PW, Kay AB. C-c chemokines in allergen-induced late-phase cutaneous responses in atopic subjects: association of eotaxin with early 6-hour eosinophils, and of eotaxin-2 and monocyte chemoattractant protein-4 with the later 24-hour tissue eosinophilia, and relationship to basophils and other c–c chemokines (monocyte chemoattractant protein-3 and RANTES). J Immunol 1999;163:3976–3984. [PubMed] [Google Scholar]
- 23.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, et al. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol 2004;31:382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med 2003;349:1414–1422. [DOI] [PubMed] [Google Scholar]
- 25.De Alba J, Raemdonck K, Dekkak A, Collins M, Wong S, Nials AT, Knowles RG, Belvisi MG, Birrell MA. HDM induces direct airway inflammation in vivo: implications for future disease therapy? Eur Respir J 2010;35:1377–1387. [DOI] [PubMed] [Google Scholar]
- 26.Thomas WR, Smith WA, Hales BJ, Mills KL, O'Brien RM. Characterization and immunobiology of house dust mite allergens. Int Arch Allergy Immunol 2002;129:1–18. [DOI] [PubMed] [Google Scholar]
- 27.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity 2009;31:412–424. [DOI] [PubMed] [Google Scholar]
- 28.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via toll-like receptor 4 triggering of airway structural cells. Nat Med 2009;15:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pendse AA, Arbones-Mainar JM, Johnson LA, Altenburg MK, Maeda N. Apolipoprotein E knock-out and knock-in mice: atherosclerosis, metabolic syndrome, and beyond. J Lipid Res 2009;50:S178–S182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992;258:468–471. [DOI] [PubMed] [Google Scholar]
- 31.Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 2009;10:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer's disease to AIDS. J Lipid Res 2009;50:S183–S188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Elzen P, Garg S, Leon L, Brigl M, Leadbetter EA, Gumperz JE, Dascher CC, Cheng TY, Sacks FM, Illarionov PA, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature 2005;437:906–910. [DOI] [PubMed] [Google Scholar]
- 34.Nakanishi A, Morita S, Iwashita H, Sagiya Y, Ashida Y, Shirafuji H, Fujisawa Y, Nishimura O, Fujino M. Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma. Proc Natl Acad Sci USA 2001;98:5175–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narayanaswami V, Maiorano JN, Dhanasekaran P, Ryan RO, Phillips MC, Lund-Katz S, Davidson WS. Helix orientation of the functional domains in apolipoprotein E in discoidal high density lipoprotein particles. J Biol Chem 2004;279:14273–14279. [DOI] [PubMed] [Google Scholar]
- 36.Misra UK, Adlakha CL, Gawdi G, McMillian MK, Pizzo SV, Laskowitz DT. Apolipoprotein E and mimetic peptide initiate a calcium-dependent signaling response in macrophages. J Leukoc Biol 2001;70:677–683. [PubMed] [Google Scholar]
- 37.Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT. ApoE genotype and an apoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem 2003;278:48529–48533. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharjee PS, Neumann DM, Foster TP, Clement C, Singh G, Thompson HW, Kaufman HE, Hill JM. Effective treatment of ocular HSK with a human apolipoprotein E mimetic peptide in a mouse eye model. Invest Ophthalmol Vis Sci 2008;49:4263–4268. [DOI] [PubMed] [Google Scholar]
- 39.Dobson CB, Sales SD, Hoggard P, Wozniak MA, Crutcher KA. The receptor-binding region of human apolipoprotein E has direct anti-infective activity. J Infect Dis 2006;193:442–450. [DOI] [PubMed] [Google Scholar]
- 40.Pilette C, Francis JN, Till SJ, Durham SR. Ccr4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. Eur Respir J 2004;23:876–884. [DOI] [PubMed] [Google Scholar]
- 41.de Bont N, Netea MG, Demacker PN, Verschueren I, Kullberg BJ, van Dijk KW, van der Meer JW, Stalenhoef AF. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. J Lipid Res 1999;40:680–685. [PubMed] [Google Scholar]
- 42.Rensen PC, Oosten M, Bilt E, Eck M, Kuiper J, Berkel TJ. Human recombinant apolipoprotein E redirects lipopolysaccharide from Kupffer cells to liver parenchymal cells in rats in vivo. J Clin Invest 1997;99:2438–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009;29:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science 2009;325:1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 2002;196:1645–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.