Abstract
Rationale: Apelin, a potent vasodilator and angiogenic factor, may be a novel therapeutic agent in neonatal chronic lung disease, including bronchopulmonary dysplasia.
Objectives: To determine the beneficial effect of apelin in neonatal rats with hyperoxia-induced lung injury, a model for premature infants with bronchopulmonary dysplasia.
Methods: The cardiopulmonary effects of apelin treatment (62 μg/kg/d) were studied in neonatal rats by exposure to 100% oxygen, using two treatment strategies: early concurrent treatment during continuous exposure to hyperoxia for 10 days and late treatment and recovery in which treatment was started on Day 6 after hyperoxic injury for 9 days and continued during the 9-day recovery period. We investigated in both models the role of the nitric oxide–cyclic guanosine monophosphate (cGMP) pathway in apelin treatment by specific inhibition of the nitric oxide synthase activity with Nω-nitro-l-arginine methyl ester (l-NAME, 25 mg/kg/d).
Measurements and Main Results: Parameters investigated include survival, lung and heart histopathology, pulmonary fibrin deposition and inflammation, alveolar vascular leakage, lung cGMP levels, right ventricular hypertrophy, and differential mRNA expression in lung and heart tissue. Prophylactic treatment with apelin improved alveolarization and angiogenesis, increased lung cGMP levels, and reduced pulmonary fibrin deposition, inflammation, septum thickness, arteriolar wall thickness, and right ventricular hypertrophy. These beneficial effects were completely absent in the presence of l-NAME. In the injury-recovery model apelin also improved alveolarization and angiogenesis, reduced arteriolar wall thickness, and attenuated right ventricular hypertrophy.
Conclusions: Apelin reduces pulmonary inflammation, fibrin deposition, and right ventricular hypertrophy, and partially restores alveolarization in rat pups with neonatal hyperoxic lung injury via a nitric oxide synthase–dependent mechanism.
Keywords: alveolarization, bronchopulmonary dysplasia, nitric oxide synthase inhibition, lung inflammation, right ventricular hypertrophy
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Despite significant advances in neonatal intensive care, the incidence of bronchopulmonary dysplasia (BPD) increases, because therapeutic strategies to simultaneously reduce ventilator-related tissue damage and stimulate alveolarization are lacking. The hallmark of BPD is an arrest in alveolar development, resulting in permanently enlarged and simplified airspaces.
What This Study Adds to the Field
Apelin not only exerts beneficial effects on lung development and inflammation in newborn rat pups exposed to hyperoxia but may also modify chronic lung injury in ventilated preterm infants.
Bronchopulmonary dysplasia (BPD) is a chronic lung disease that develops in premature infants treated with oxygen and positive pressure ventilation for respiratory distress syndrome (RDS) and is particularly frequent in infants born at less than 30 weeks of gestation and with a birth weight of less than 1,200 g (1). BPD is characterized by decreased alveolarization and vascularization of the developing lung, resulting in enlarged alveoli, and is complicated by inflammation, abnormal coagulation, and fibrinolysis with intraalveolar fibrin accumulation, oxidative stress, and at later stages by pulmonary hypertension (1–4). In animal models, neonatal exposure to hyperoxia results in progressive lung disease, which strongly resembles BPD in premature infants (5–7).
Because apelin is a potent vasodilator and angiogenic factor, apelin treatment may be a novel therapeutic strategy in BPD (8–12). In search of unidentified genes involved in the pathogenesis of BPD, we performed a DNA array experiment and identified an expressed sequence tag (accession number: AA945996) with a high differential expression in rat lung with experimental BPD, as being APJ, a 380–amino acid seven-transmembrane domain spanning G protein–coupled receptor (7). Apelin was identified as an endogenous ligand of APJ. In humans, rats, and mice, the apelin gene encodes a 77–amino acid preproprotein, which, after enzymatic cleavage, produces biologically active apelin-36 in vitro (10, 13, 14). Shorter C-terminal sequences of apelin-36 are also functionally active, of which the N-terminal pyroglutamate form ([Pyr1] apelin-13) is the most effective ligand (13–15). Binding of apelin to APJ activates signaling cascades after coupling to either inhibitory G proteins (Gi), which results in activation of extracellular-regulated kinases (ERK) and Akt-dependent phosphorylation pathways (12, 16), or to Gq proteins, which involve activation of phospholipase C and protein kinase C leading to activation of nuclear factor-κB (17) and increased intracellular calcium concentrations (18). mRNA expression of APJ and apelin has been detected in human, rat, and mouse in heart, lung, and neural tissue (10, 13, 15, 19–24), suggesting a functional role of apelin/APJ in these tissues. APJ is expressed in the lung in bronchial and alveolar epithelial cells, in small pulmonary blood vessels in the endothelium and in smooth muscle cells (25), and in macrophages (26). Apelin is a vasodilator and a nitric oxide (NO)-dependent depressor of blood pressure (8–10), promotes angiogenesis (11, 12), and is a neuropeptide influencing food and water intake and pituitary hormone release (27, 28). Although high levels of expression of the APJ receptor and its ligand apelin in the lung are observed, a functional role of these proteins during normal lung development and under pathological conditions is unknown.
In this study we investigated the cardiopulmonary effects of apelin treatment in experimental BPD in neonatal rats by exposure to hyperoxia, using two different treatment strategies: a prophylactic strategy (early concurrent treatment) and a more clinically relevant strategy in which treatment was started after injury was induced in a lung injury-recovery model (late treatment and recovery). In addition, we investigated in both models the role of the NO–cyclic guanosine monophosphate (cGMP) pathway in apelin treatment of experimental BPD by specific inhibition of the nitric oxide synthase (NOS) activity with Nω-nitro-l-arginine methyl ester (l-NAME). After early concurrent treatment we observed beneficial effects of apelin, such as increased lung cGMP levels, reduced pulmonary inflammation, extravascular fibrin deposition, and vascular-alveolar leakage, improved alveolarization, and angiogenesis, and less right ventricular hypertrophy (RVH). These effects were completely abolished in the presence of l-NAME. In the late treatment and recovery model apelin improved alveolarization and vascular development and attenuated RVH. Some of the results of these studies have been previously reported in the form of an abstract (29).
METHODS
Full methodological details are available in the online supplement.
Animals
The research protocol was approved by the Institutional Animal Care and Use Committee of the Leiden University Medical Center. Adult Wistar rats (6 mo old; n = 6) were exsanguinated after induction of anesthesia with an intraperitoneal injection of ketamine (50 mg/kg) and xylazine (50 mg/kg). Organs were stored at −80°C until isolation of RNA for real time reverse transcriptase–polymerase chain reaction (RT-PCR).
Neonatal rat pups were pooled and distributed over four experimental groups (n = 12): an oxygen, oxygen-apelin, oxygen–l-NAME, and oxygen–l-NAME–apelin group, and four room air-exposed control groups injected either with saline, apelin, and/or l-NAME. The oxygen concentration, body weight, evidence of disease, and mortality were monitored daily.
Early Concurrent Treatment
Pups were continuously exposed to 100% oxygen for 10 days. From Day 2, pups received either 62 μg/kg/d of apelin-13 (preproapelin 65–77) in 0.9% saline, 0.9% saline (age-matched control), 25 mg/kg/d of l-NAME in 0.9% saline, or l-NAME plus apelin in 0.9% saline (100 μl, subcutaneously). Except for the survival experiments, lung and heart tissue were collected on Day 10. Separate experiments were performed for (1) median survival (n = 12), (2) histology (n = 8), (3) frozen lung and heart tissue (n = 10), and (4) collection of bronchoalveolar lavage fluid (n = 10). Median survival was studied in those experimental BPD groups that showed significant differences in survival at Day 10.
Late Treatment and Recovery
Lung injury and recovery were investigated by exposing pups to hyperoxia for 9 days followed by recovery in room air for 9 days. After 6 days of hyperoxia, daily injections with 0.9% saline, 62 μg/kg/d apelin, 25 mg/kg/d l-NAME, or l-NAME plus apelin were started and continued throughout the 9-day recovery period in room air. Lung and heart tissue were collected on Day 9, after 9 days of hyperoxic lung injury (n = 8) and on Day 18, after a 9-day recovery period in room air (n = 8).
Tissue Preparation
Lungs and heart were snap-frozen in liquid nitrogen for real-time RT-PCR, cGMP ELISA, or fibrin deposition assay, and fixed in formalin for histology studies as previously described (30).
Histology
Formalin-fixed, paraffin-embedded, 4-μm–thick heart and lung sections were stained with hematoxylin and eosin. Lungs were immunostained additionally with 59D8 (β-fibrin; 1:5,000), anti-apelin (1:5,000) anti-APJ (1:5,000), anti–ED-1 (monocytes and macrophages; 1:5), anti-MPO (1:1,500), anti-ASMA (1:10,000), or anti–von Willebrand factor (1:4,000) using standard methods (7, 30–33). Quantitative morphometry was performed by two independent researchers blinded to the treatment strategy as previously described (30, 34).
In situ hybridization
Formalin-fixed, paraffin-embedded, 7-μm–thick lung sections were hybridized with 35S-CTP and 35S -UTP double-labeled antisense cRNA probes to rat APJ, made by in vitro transcription using pCR4-TOPO as a vector, according to the method described by Wilkinson (35, 36). Primers are listed in Table E1 in the online supplement.
Fibrin Detection Assay
Quantitative fibrin deposition in lung tissue homogenates was determined by Western blotting as described previously (7, 30).
Bronchoalveolar Lavages, Protein Assay, and cGMP Assay
Lung lavages, protein, and cGMP assays were performed as previously described (30).
Real-Time RT-PCR
Total RNA isolation from lung and heart tissue homogenates, first-strand cDNA synthesis, and real-time quantitative PCR were performed as described previously (7, 30). Primers are listed in Table E2.
Statistical Analysis
Values are expressed as mean ± SEM. Differences between groups were analyzed with analysis of variance followed by Tukey multiple comparison test. For comparison of survival curves, Kaplan-Meier analysis followed by a log rank test was performed. Differences at P values less than 0.05 were considered statistically significant.
RESULTS
Expression of Apelin and APJ
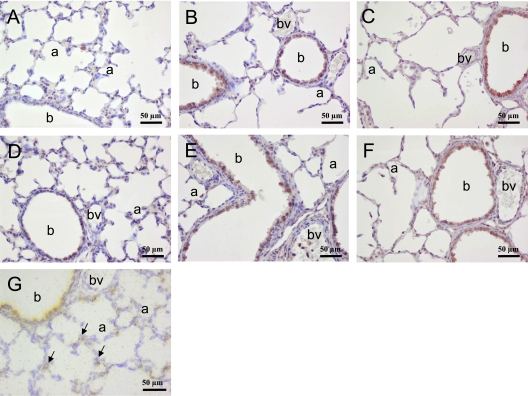
In adult rats, highest mRNA expression of apelin was observed in the heart, lung, and brain, and of APJ in heart and lung (Figure E1). In the neonatal rat lung on Day 10, apelin protein (Figure 1A) expression was low in bronchial and alveolar epithelial cells. Highest APJ protein (Figure 1D) and mRNA (Figure 1G) expression was observed in the bronchial epithelial layer, and low mRNA and protein expression was observed in the alveolar blood vessels and alveolar type 2 cells. In addition, a low level of APJ protein was observed in alveolar type 1 cells, which could not be visualized at the mRNA level. After exposure to 100% oxygen for 10 days, apelin expression was induced in bronchial epithelial cells (Figure 1B) and APJ expression was decreased in the alveoli and vasculature resulting in a high level of expression in bronchial epithelial cells for both genes (Figure 1E). In addition, APJ was expressed in white blood cells. Apelin treatment for 10 days did not alter the patterns of expression of apelin (Figure 1C) and APJ (Figure 1F) in hyperoxia-induced neonatal lung injury.
Figure 1.
Lung sections stained immunohistochemically for (A–C) apelin or (D–F) APJ after early concurrent treatment on Day 10 in (A, D) room air and age-matched O2-exposed pups daily injected with (B, E) saline or (C, F) apelin. (G) APJ mRNA expression by in situ hybridization in a room air–exposed pup on Day 10. Arrows in G indicate APJ mRNA expression in alveolar capillaries and epithelial type 2 cells. a = alveolus; b = bronchus; bv = blood vessel.
Effects of Apelin on Growth and Survival
Early Concurrent Treatment.
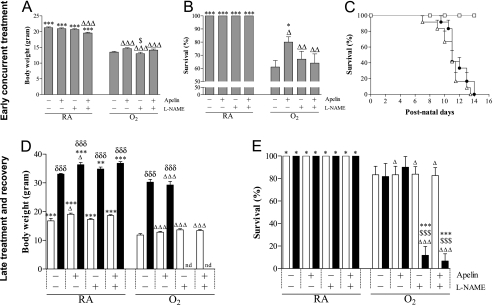
On Day 10, mean body weight of pups was 21.2 ± 0.3 g (Figure 2A). Treatment with apelin or l-NAME showed no adverse effects on mean body weight compared with room air control pups, whereas room air control pups treated with apelin and l-NAME showed a small decrease in body weight. Mean body weight 10 days after hyperoxia exposure was 13.5 ± 0.3 g. Treatment with apelin and/or l-NAME had no additional effect on body weight compared with oxygen-exposed control pups. Exposure to hyperoxia resulted in a 61% survival, which was significantly prolonged to 80% after treatment with apelin on Day 10 (P < 0.05; Figure 2B). l-NAME completely abolished the protective effect of apelin on survival, whereas l-NAME had no additional effect on survival compared with oxygen-exposed control pups. Because an effect on survival on Day 10 was only observed in the experimental BPD groups between the nontreated and the apelin-treated pups, we studied median survival in these experimental groups and compared them with room air–exposed control pups. Median survival was similar in oxygen-exposed control pups and pups treated with apelin under hyperoxia (11 d; Figure 2C). Room air–exposed pups showed no signs of illness or mortality during the first 4 weeks after birth.
Figure 2.
(A, D) Growth and(B, C, E) survival at Day 10 after early concurrent treatment (n = 12, shaded bars; A, B) and after late treatment and recovery (n = 8; D, E) on Days 9 (open bars) and 18 (solid bars) in room air (RA) and age-matched O2-exposed pups (O2) daily injected with either saline, apelin, Nω-nitro-l-arginine methyl ester (l-NAME), or a combination of apelin and l-NAME. Kaplan-Meier survival curve of apelin-treated rat pups (solid circles), age-matched O2-exposed control pups (open triangles) and RA-exposed control pups (open squares) during the first 14 days after birth (n = 12; C). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus age-matched O2-exposed control pups. ΔP < 0.05, ΔΔP < 0.01, ΔΔΔP < 0.001 versus own room air–exposed control pups. $P < 0.05 and $$$P < 0.001 versus apelin-treated O2 pups. δδδP < 0.001 versus own treatment control pups in hyperoxic period. nd = not determined.
Late Treatment and Recovery.
On Day 9, mean body weight of pups was 16.8 ± 0.9 g (Figure 2D). On day 18, mean body weight had increased to 33.1 ± 0.2 g. Treatment with apelin and/or l-NAME had no adverse effects on mean body weight compared with room air control pups, but room air control pups treated with apelin showed a small increase in body weight on Days 9 and 18 (P < 0.05). Mean body weight after 9 days of hyperoxia exposure was 11.8 ± 0.5 g. Treatment with apelin and/or l-NAME had no additional effect on body weight compared with oxygen-exposed control pups on Day 9. Although apelin treatment had no additional effect on growth on Day 18, body weight could not be determined in the l-NAME and apelin–l-NAME–treated pups due to a significant increase in mortality (P < 0.001; Figure 2E). On Days 9 and 18 (Figure 2E) all room air–exposed pups survived. Exposure to hyperoxia resulted in an 80% survival, whereas treatment with apelin and/or l-NAME for 3 days had no additional effect on survival compared with oxygen-exposed control pups on Day 9. Eighty percent of the pups that recovered in room air on Day 18 after hyperoxic lung injury survived. Survival was not affected by apelin treatment. However, treatment with l-NAME in the absence or presence of apelin during the 9-day recovery period resulted in a survival of less than 15% at Day 18. Due to the high mortality rate after injection with l-NAME in the late treatment and recovery experiments, parameters could not be determined on Day 18 after treatment with l-NAME or a combination of apelin and l-NAME in the subsequent experiments.
Effects of Apelin on Lung Airway Development and Inflammation
Early Concurrent Treatment.
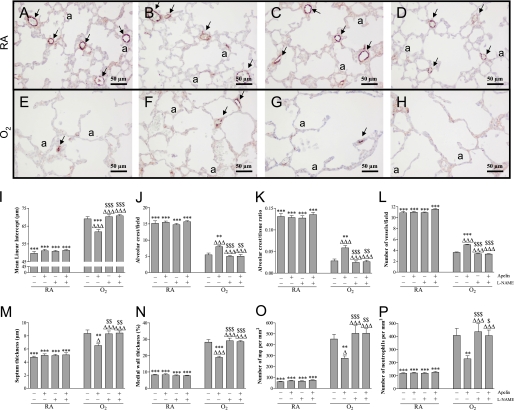
Lung development proceeds from the saccular stage at birth toward the alveolar stage on Day 10 (Figure 3A). Treatment with apelin (Figure 3B), l-NAME (Figure 3C), or apelin and l-NAME (Figure 3D) did not have adverse effects on mean linear intercept (Figure 3I), alveolar crest (Figures 3J and 3K), pulmonary vessel density (Figure 3L), alveolar septum thickness (Figure 3M), medial wall thickness (Figures 3N and E2), and influx of macrophages (Figures 3O and E4) and neutrophilic granulocytes (Figure 3P), compared with room air control pups, demonstrating normal postnatal alveolar and vascular lung development. Oxygen exposure for 10 days (Figure 3E) resulted in edema, a heterogeneous distribution of enlarged air spaces with increased mean linear intercept (1.4-fold, P < 0.001; Figure 3I) and decreased number of alveolar crests (2.8- and 4.7-fold, P < 0.001; Figures 3J and 3K, respectively), which were surrounded by septa with increased thickness (1.8-fold, P < 0.001; Figure 3M), reduced pulmonary vessel density (3.0-fold, P < 0.001; Figures 3E and 3L), and increased pulmonary arteriolar medial wall thickness (3.4-fold, P < 0.001; Figure 3N). Hyperoxia led to a massive inflammatory reaction, characterized by an overwhelming influx of inflammatory cells, including macrophages (7.4-fold, P < 0.001; Figure 3O) and neutrophils (3.4-fold, P < 0.001; Figure 3P), compared with room air–exposed control pups. Apelin treatment improved alveolarization and angiogenesis and reduced inflammation during hyperoxia exposure by decreasing mean linear intercept (10.5%, P < 0.001; Figure 3I) and increasing the number of alveolar crests (47% and 106%, P < 0.01; Figures 3J and 3K, respectively), thinning of alveolar septa (22%, P < 0.01; Figure 3M), increasing pulmonary vessel density (39%, P < 0.001; Figures 3F and 3L), reducing arteriolar medial wall thickness (33%, P < 0.001; Figure 3N) and the influx of macrophages (39%, P < 0.01; Figure 3O) and neutrophilic granulocytes (44%, P < 0.01; Figure 3P) compared with oxygen exposure for 10 days. l-NAME had no additional effect compared with oxygen-exposed control pups (Figure 3G), but l-NAME completely abolished the protective effect of apelin on alveolarization (Figures 3I–K and 3M), angiogenesis (Figures 3H and 3L), pulmonary hypertension (Figures 3N and E2), and inflammation (Figures 3O and 3P, and E4) in hyperoxia-induced neonatal lung injury.
Figure 3.
Lung sections stained for von Willebrand Factor (vWF; A–H) and lung morphometry (I–P) of room air– (RA; A–D) and O2-exposed pups (O2; E–H) daily injected with either saline (A, E), apelin (B, F), Nω-nitro-l-arginine methyl ester (l-NAME; C, G) or a combination of apelin and l-NAME (D, H) at 10 days of age after early concurrent treatment. Lung morphometry, including the quantification of mean linear intercept (I), alveolar crest (per field in J and per tissue ratio in K), number of pulmonary vessels (L), septum thickness (M), medial wall thickness (N) and influx of macrophages (O) and neutrophilic granulocytes (P) was determined on paraffin sections in RA and O2 pups daily injected either with saline, apelin, and/or l-NAME. Values are expressed as mean ± SEM (n = 8). Arrows in A–H indicate vWF-positive blood vessels. **P < 0.01 and ***P < 0.001 versus age-matched O2-exposed control pups. ΔP < 0.05 and ΔΔΔP < 0.001 versus own RA control pups. $P < 0.05, $$P < 0.01, $$$P < 0.001 versus apelin-treated O2 pups. a = alveolus.
Late Treatment and Recovery.
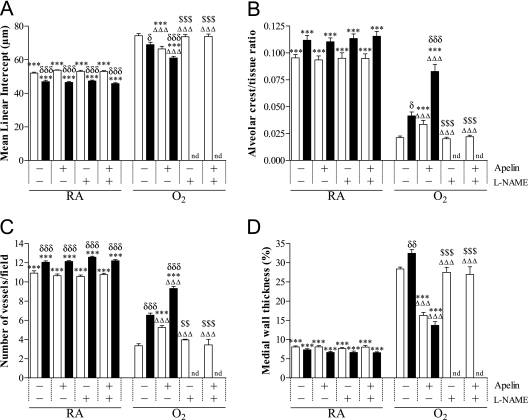
Treatment of room air–exposed pups with apelin and/or l-NAME showed no adverse effects on alveolar (Figures 4A and 4B) and vascular development (Figures 4C and 4D) on Day 9. Continuous neonatal exposure to hyperoxia for 9 days resulted in enlarged alveoli demonstrated by a 1.4-fold increase in mean linear intercept (P < 0.001; Figure 4A) and a 4.7-fold decrease in the number of alveolar crests (P < 0.001; Figure 4B), disturbed vascular development demonstrated by a 3.2-fold increase in medial wall thickness (P < 0.001; Figure 4D), and a 3.2-fold reduction in blood vessel density (P < 0.001; Figures 4C and E3) compared with room air control pups. Apelin treatment during the last 3 days of the injurious hyperoxic period on Day 9 improved alveolarization by decreasing mean linear intercept by 11% (P < 0.001; Figure 4A) and by increasing the number of alveolar crests by 60% (P < 0.001; Figure 4B), and improved vascular development by increasing blood vessel density by 52% (P < 0.001; Figure 4C) and by decreasing medial wall thickness by 43% (P < 0.001; Figure 4D). A recovery period of 9 days in room air after hyperoxia-induced lung injury on Day 18 had a minor beneficial effect on mean linear intercept (P < 0.05; Figure 4A), the number of alveolar crests (P < 0.05; Figure 4B), and blood vessel density (P < 0.001; Figure 4C), but aggravated medial wall thickness (P < 0.01; Figure 4D). Alveoli continued to be enlarged (Figures 4A and E3). Treatment with apelin reduced mean linear intercept by 13.6% (P < 0.001; Figure 4A) and medial wall thickness by 58% (P < 0.001; Figure 4D), and increased alveolar crests by 102% (P < 0.001; Figure 4B) and blood vessel density by 42% (P < 0.001; Figures 4C and E3), compared with nontreated experimental BPD pups at the end of the recovery period on Day 18.
Figure 4.
Quantification of (A) mean linear intercept, (B) alveolar crest, (C) number of pulmonary vessels, and (D) medial wall thickness determined on paraffin sections after late treatment and recovery on Days 9 (open bars) and 18 (solid bars) in room air (RA) and O2-exposed pups (O2) daily injected either with saline, apelin, Nω-nitro-l-arginine methyl ester (l-NAME), or a combination of apelin and l-NAME. Values are expressed as mean ± SEM (n = 8). ***P < 0.001 versus age-matched O2-exposed control pups. ΔΔΔP < 0.001 versus own RA control pups. $P < 0.05, $$P < 0.01, $$$P < 0.001 versus apelin-treated O2 pups. δP < 0.05, δδP < 0.01, δδδP < 0.001 versus own treatment control pups in hyperoxic period. nd = not determined.
Effects of Apelin on Lung Coagulation and Vascular Leakage
Early Concurrent Treatment.
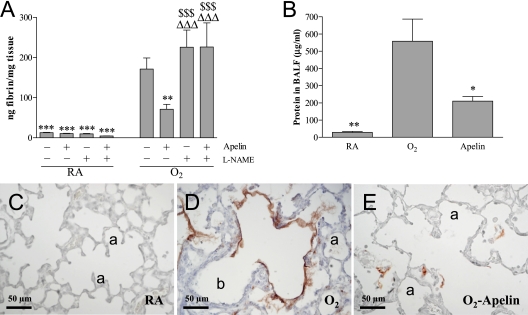
Pulmonary fibrin deposition, a sensitive marker for tissue damage in hyperoxia-induced neonatal lung disease, was studied in homogenates as a read-out for lung damage (Figure 5A). Fibrin deposition was at reference levels during normal neonatal pulmonary development on Day 10 in the absence or presence of apelin and/or l-NAME treatment (< 25 ng fibrin/mg tissue) and increased 13-fold to 170 ± 28 ng fibrin/mg tissue in lungs of pups exposed to 100% oxygen for 10 days (P < 0.001). Apelin therapy attenuated hyperoxia-induced fibrin deposition by 58% to 71 ± 12 ng fibrin/mg tissue (P < 0.01), whereas l-NAME had no additional effect compared with oxygen-exposed control pups. l-NAME completely abolished the protective effect of apelin on hyperoxia-induced fibrin deposition. Extravascular fibrin deposits were demonstrated on paraffin sections in alveolar septa and in the alveolar lumen, but not in the bronchial lumen after exposure to 100% oxygen for 10 days (Figure 5D), suggesting hyperoxia-induced capillary-alveolar leakage of fibrinogen in alveoli and subsequent local conversion into fibrin by thrombin. Fibrin deposits were minor or even absent in oxygen-exposed pups treated with 62 μg/kg/d of apelin (Figure 5E) and absent in normoxia (Figure 5C).
Figure 5.
Quantification of (A) fibrin deposition in lung homogenates, (B) total protein concentration in bronchoalveolar lavage fluid (BALF), and (C–E) expression of β-fibrin on lung sections after early concurrent treatment on Day 10 in room air (RA) and O2-exposed pups (O2) daily injected either with saline, apelin, l-NAME, or a combination of apelin and Nω-nitro-l-arginine methyl ester (l-NAME). Values are expressed as mean ± SEM (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001 versus age-matched O2-exposed control pups. ΔΔΔP < 0.001 versus own RA control pups. $$$P < 0.001 versus apelin-treated O2 pups. a = alveolus; b = bronchus.
Total protein concentration in bronchoalveolar lavage fluid (BALF) was measured to establish the inhibitory effect of apelin on pulmonary edema by capillary-alveolar leakage (Figure 5B). Protein concentration on postnatal Day 10 increased 20-fold after hyperoxia and decreased by 62% after treatment with apelin under hyperoxia (P < 0.05).
Effects of Apelin on Pulmonary cGMP Levels
Early Concurrent Treatment.
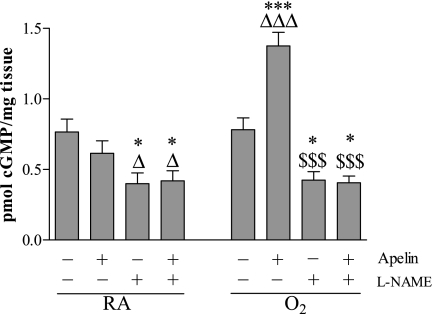
Exposure to hyperoxia for 10 days did not change cGMP levels in lung homogenates compared with room air control pups (Figure 6). Treatment with apelin resulted in a significant increase in cGMP by 76% (P < 0.001) compared with oxygen-exposed control pups, but did not increase cGMP levels during normal development in room air control pups. Inhibition of NOS activity with l-NAME significantly reduced cGMP levels under normoxia and after exposure to hyperoxia to a similar level in both groups (P < 0.05). l-NAME completely abolished the apelin-induced increase in cGMP levels.
Figure 6.
Quantification of cyclic guanosine monophosphate (cGMP) in lung homogenates after early concurrent treatment on Day 10 in room air–exposed (RA) and O2-exposed pups (O2) daily injected either with saline, apelin, Nω-nitro-l-arginine methyl ester (l-NAME), or a combination of apelin and l-NAME. Values are expressed as mean ± SEM (n = 10). *P < 0.05, ***P < 0.001 versus age-matched O2-exposed control pups. ΔP < 0.05, ΔΔΔP < 0.001 versus own room air-exposed control pups. $$$P < 0.001 versus apelin-treated O2 pups.
mRNA Expression in Lung Tissue
Early Concurrent Treatment.
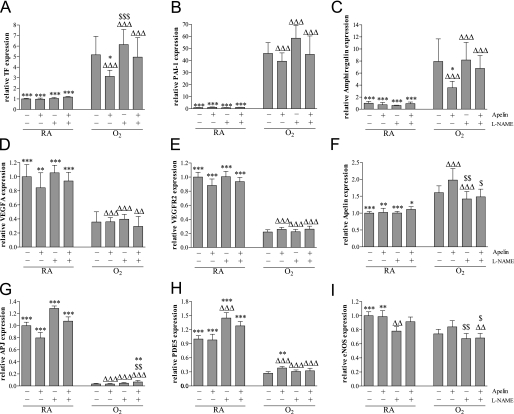
Treatment with apelin and/or l-NAME for 10 days during normal neonatal development did not result in significant differences in mRNA expression (Figure 7) of the procoagulant protein tissue factor (TF; Figure 7A), the anti-fibrinolytic factor plasminogen activator inhibitor-1 (PAI-1; Figure 7B), the growth factor amphiregulin (Figure 7C), vascular endothelial growth factor A (VEGFA; Figure 7D), its receptor vascular endothelial growth factor receptor-2 (VEGFR2; Figure 7E), apelin (Figure 7F), and APJ (Figure 7G) compared with room air–exposed control pups, whereas treatment with l-NAME significantly increased phosphodiesterase type 5 (PDE5; Figure 7H) expression and decreased endothelial nitric oxide synthase (eNOS; Figure 7I) expression compared with room air–exposed control pups. Ten days of oxygen exposure resulted in an increase in mRNA expression of TF by 4.9-fold (P < 0.001), PAI-1 by 45.8-fold (P < 0.001), amphiregulin by 8.5-fold (P < 0.001), and apelin by 1.6-fold (P < 0.001), and a decrease in the expression of VEGFA by 2.0-fold (P < 0.001), VEGFR2 by 4.5-fold (P < 0.001), APJ by 33-fold (P < 0.001), PDE5 by 3.7-fold (P < 0.001), and eNOS by 1.3-fold (P < 0.001) in lungs of oxygen-exposed pups compared with room air–exposed control pups. Treatment with apelin of oxygen-exposed pups for 10 days resulted in a reduction of the mRNA expression of TF by 37% (P < 0.05) and amphiregulin by 63% (P < 0.01), and an increase in mRNA expression of PDE5 by 41% (P < 0.01) compared with oxygen-exposed pups. l-NAME had no additional effect compared with oxygen-exposed control pups, whereas it completely abolished the effect of apelin on the oxygen-induced differential expression of TF, amphiregulin, and PDE5.
Figure 7.
Relative mRNA expression in lung homogenates after early concurrent treatment of (A) tissue factor (TF), (B) plasminogen activator inhibitor type 1 (PAI-1), (C) amphiregulin, (D) vascular endothelial growth factor (VEGFA), (E) vascular endothelial growth factor receptor type 2 (VEGFR2), (F) apelin (F), (G) APJ, (H) PDE5, and (I) endothelial nitric oxide synthase (eNOS) on Day 10 in room air–exposed (RA) and O2-exposed pups (O2) daily injected either with saline, apelin, Nω-nitro-l-arginine methyl ester (l-NAME), or a combination of apelin and l-NAME. Values are expressed as mean ± SEM (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001 versus age-matched O2-exposed control pups. ΔP < 0.05, ΔΔP < 0.01, ΔΔΔP < 0.001 versus own RA control pups. $P < 0.05, $$P < 0.01, $$$P < 0.001 versus apelin-treated O2 pups.
Heart Development and RVH
Early Concurrent Treatment.
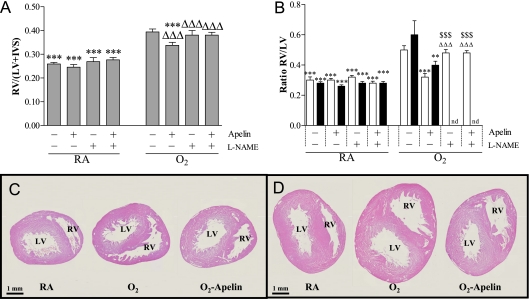
Treatment with apelin and/or l-NAME for 10 days during normal neonatal development did not result in any differences in cardiac characteristics (Table E3 and Figure 8A). Exposure to hyperoxia for 10 days resulted in RVH as demonstrated by a 1.5-fold increase in the weight ratio RV/(left ventricle [LV] + interventricular septum [IVS]) (Figure 8A) and increased RV weight and free wall thickness (Table E3 and Figure E5) compared with room air control pups (P < 0.001). Treatment with apelin resulted in a significant regression of RVH (P < 0.001; Figure 8A) due to a decrease of the RV weight and free wall thickness by 16% and 22%, respectively, compared with the oxygen-exposed control pups (P < 0.01). l-NAME had no additional effect compared with oxygen-exposed control pups, but completely abolished the protective effect of apelin on hyperoxia-induced RVH. Left ventricular weight and free wall thickness were not affected by oxygen exposure, apelin, and/or l-NAME. The ratio of heart weight to body weight was similar in all experimental groups (not shown).
Figure 8.
Right ventricular hypertrophy depicted as (A) the weight ratio RV/(LV+IVS) at Day 10 after early concurrent treatment (n = 10, shaded bars) and (B) as RV/LV wall thickness ratio after late treatment and recovery (n = 8) on Days 9 (open bars) and 18 (solid bars) in room air–exposed control pups (RA) and age-matched O2-exposed control pups (O2) daily injected with either saline, apelin, Nω-nitro-l-arginine methyl ester (l-NAME), or a combination of apelin and l-NAME. Data are expressed as mean ± SEM. Cardiac characteristics are presented in Table E3. **P < 0.01, ***P < 0.001 versus age-matched O2-exposed control pups. ΔΔΔP < 0.001 versus room air–exposed control pups. $$$P < 0.001 versus apelin-treated O2 pups. Paraffin heart sections stained with hematoxylin and eosin after late treatment and recovery on Days 9 (C) and 18 (D) in room air–exposed control pups (RA) and age-matched O2-exposed control pups (O2) daily injected with either saline or apelin. IVS = interventricular septum; LV = left ventricle; nd = not determined; RV = right ventricle.
Late Treatment and Recovery.
Nine days of hyperoxic lung injury resulted in a 1.7-fold increase in the ratio RV/LV wall thickness compared with room air control pups (P < 0.001; Figures 8B and 8C), which was attenuated after apelin treatment for 3 days (35%, P < 0.05) on Day 9. l-NAME had no additional effect compared with oxygen-exposed control pups, but completely abolished the protective effect of apelin on hyperoxia-induced RVH on Day 9. A recovery period of 9 days did not reduce RVH in the nontreated pups, but the RV/LV wall thickness ratio was decreased after apelin treatment on Day 18 (Figures 8B and 8D).
Cardiac mRNA Expression
Early Concurrent Treatment.
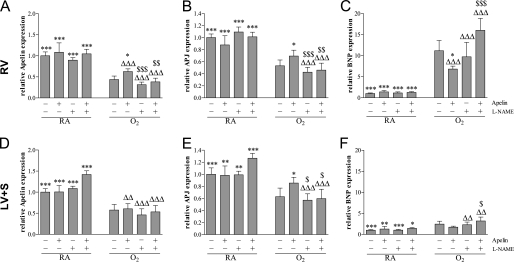
Treatment with apelin and/or l-NAME for 10 days during normal neonatal development did not result in differences in mRNA expression profiles of apelin, APJ, and brain natriuretic peptide (BNP) in the heart (Figure 9). After exposure to hyperoxia for 10 days, mRNA expression in the right ventricle was decreased for apelin (2.3-fold, P < 0.001; Figure 9A) and APJ (1.9-fold, P < 0.001; Figure 9B), whereas expression was increased for the natriuretic peptide BNP (11-fold, P < 0.001; Figure 9C) as compared with room air control pups. Treatment with apelin for 10 days under hyperoxia decreased the expression of BNP (39%, P < 0.05) and increased the expression of both apelin (43%, P < 0.05) and APJ (30%, P < 0.05) compared with oxygen-exposed control pups, whereas l-NAME had no additional effect compared with oxygen-exposed control pups. l-NAME completely abolished the protective effect of apelin on hyperoxia-induced differential mRNA expression of BNP, apelin, and APJ in the right ventricle.
Figure 9.
Relative mRNA expression in (A–C) the right ventricle (RV) and (D–F) left ventricle and interventricular septum (LV+S) after early concurrent treatment of (A, D) apelin, (B, E) APJ, and (C, F) brain natriuretic peptide (BNP) in room air–exposed control pups (RA) and age-matched O2-exposed control pups (O2) daily injected with either saline, apelin, Nω-nitro-l-arginine methyl ester (l-NAME), or a combination of apelin and l-NAME. Data are expressed as mean ± SEM (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001 versus age-matched O2-exposed control pups. ΔΔP < 0.01, ΔΔΔP < 0.001 versus own room air–exposed control pups. $P < 0.05, $$P < 0.01, $$$P < 0.001 versus apelin-treated O2 pups.
Exposure to hyperoxia for 10 days decreased mRNA expression in the LV plus IVS to an extent similar to that observed in the RV for apelin (1.7-fold, P < 0.001; Figure 9D) and APJ (1.6-fold, P < 0.001; Figure 9E) and expression of the natriuretic peptide BNP was only increased marginally in the left ventricle (2.5-fold, P < 0.001; Figure 9F) compared with room air control pups. Treatment with apelin for 10 days under hyperoxia only increased the expression of APJ by 36% (P < 0.05) compared with oxygen-exposed control pups, whereas l-NAME had no additional effect compared with oxygen-exposed control pups. l-NAME completely abolished the apelin-induced differential expression of APJ in hyperoxia-exposed pups in the left ventricle plus IVS.
DISCUSSION
Treatment with apelin in neonatal rat pups exposed to prolonged hyperoxia, an in vivo model for experimental BPD (7), attenuated lung inflammation and RVH and increased cGMP levels, alveolarization, and pulmonary angiogenesis. The beneficial effects of apelin on lung inflammation were demonstrated by a reduction in the influx of macrophages and neutrophils, a lower protein content in bronchoalveolar lavage fluid (a marker for capillary-alveolar leakage), a reduction in alveolar septum thickness, and a decrease in extravascular fibrin deposition, which could be fully abolished by inhibiting NOS activity with l-NAME. Extravascular fibrin deposition in alveoli in experimental BPD is the result of pulmonary inflammation-induced leakage of proteins, including fibrinogen, from the pulmonary capillaries into the alveolar lumen and the subsequent local conversion of fibrinogen into fibrin by thrombin. Reduced pulmonary inflammation and fibrin deposition and improved alveolarization after apelin treatment in experimental BPD are in agreement with the differential mRNA expression patterns of the procoagulant protein tissue factor and the growth factors amphiregulin and VEGFA. Apelin therapy started after the initiation of hyperoxia-induced lung injury improved alveolarization and angiogenesis by attenuating alveolar enlargement and arteriolar medial wall thickness and restoring pulmonary blood vessel density and RVH. These findings demonstrate for the first time that apelin has a beneficial effect on experimental lung disease via a NOS-dependent mechanism and indicate that apelin may be a suitable candidate for therapeutic intervention in neonates suffering from BPD.
Apelin mRNA was highly expressed in the lungs, heart, and brain and its receptor APJ in heart and lungs, confirming previously published data in rats (22). In rat lung, apelin is expressed in endothelial cells of small blood vessels (37), whereas APJ is expressed in bronchial epithelial cells and in small pulmonary blood vessels in the endothelium, in smooth muscle cells (25), and in macrophages (26). In addition, we found that mRNA expression of APJ and apelin is up-regulated soon after birth, suggesting a role for apelin/APJ signaling in neonatal lung development by contributing to angiogenesis (11, 12). Both genes are differentially expressed in hyperoxia-induced lung injury with an increased expression of apelin and a decreased expression of APJ. This adaptive response toward hyperoxia suggests a role for apelin/APJ signaling in the pathophysiology of experimental BPD, in which arrested alveolar and vascular development and pulmonary hypertension play pivotal roles (2, 3, 38). Because apelin is an angiogenic factor (11, 12), a decrease in APJ in experimental chronic lung disease may hamper alveolar and vascular development and worsen pulmonary hypertension, analogous to findings in the heart (8–10). Although pulmonary APJ expression was decreased more than 10-fold after exposure to oxygen for 10 days, beneficial effects of apelin treatment in lungs and heart were observed. Because APJ is the only known receptor for apelin thus far, our results indicate that the gradual decrease in APJ expression in the injured neonatal hyperoxic lung does not restrict apelin/APJ signaling.
The beneficial effects of apelin treatment in experimental BPD may be explained by either an induction of angiogenesis leading to improved alveolarization, a reduction of the inflammatory and coagulation response by attenuating the infiltration and subsequent activation of macrophages (26), or by NO-dependent vasodilation after synthesis and/or activation of eNOS (39) leading to less pulmonary hypertension and less RVH. For apelin/APJ-induced vasodilation an intact endothelial layer is mandatory to generate NO after eNOS activation and induce relaxation of the smooth muscle layer. When the endothelial layer is damaged and apelin can bind directly to APJ on smooth muscle cells, apelin treatment may induce smooth muscle cell proliferation and contraction (20, 40, 41), thereby aggravating pulmonary hypertension. This may explain why a relatively low dosage of apelin had beneficial effects in both rat models of experimental BPD.
NO plays an important role in regulating pulmonary vascular tone and alveolar capillary development and in reducing inflammation in the developing lung (42–44). Inhaled NO can exert its biological effects via S-nitrosylation or by the NO-cGMP pathway (42, 45, 46). Based on our morphological data showing an increased arteriolar wall thickness after exposure to hyperoxia we expected an increase in cGMP levels in vascular smooth muscle cells in the wall of small pulmonary arterioles. In addition, we observed a decrease in PDE5 mRNA expression in hyperoxia-induced lung injury, which was confirmed at the protein level in a mouse model of neonatal hyperoxia-induced lung injury (47), suggesting transcriptional regulation of PDE5 gene expression and increased cGMP levels due to a decreased hydrolysis of cGMP. The absence of a hyperoxia-induced increase in cGMP levels in lung homogenates may be explained by a complex differential regulation of expression and/or activation of factors that regulate the synthesis (including eNOS and iNOS) and degradation of cGMP (multiple PDEs) in multiple cell types in the lung, including epithelium, vascular smooth muscle cells, endothelium, and inflammatory cells. The importance of the NO-cGMP pathway in the treatment of experimental BPD with apelin was demonstrated by specific inhibition of NOS activity with l-NAME, which completely abolished the beneficial effects of apelin on pulmonary inflammation, angiogenesis, medial wall thickness, and RVH. Furthermore, the similarity of beneficial effects we and others have observed with apelin, inhaled NO (44), and sildenafil (30, 48) treatment in the same model of experimental BPD suggests that, besides S-nitrosylation and nitration, the NO-cGMP pathway plays an important role in the pathogenesis of experimental BPD. Apelin treatment was superior during the injury phase in the injury-recovery model compared with sildenafil, as beneficial effects on alveolarization and angiogenesis were already observed 9 days after exposure to hyperoxia with apelin treatment during the last 3 days of the hyperoxic period.
The significance of eNOS in heart and lung development was studied in genetically modified mice. Although eNOS-deficient mice are viable, they show small litter sizes and exhibit minor cardiovascular (49, 50) and major pulmonary abnormalities with marked septal thickening, reduced airspaces, capillaries abutting saccular air spaces, and lamellar bodies in alveolar type 2 cells in the late fetal and early neonatal period (51). The importance of NOS activity for normal lung development was confirmed by inducing similar pulmonary abnormalities in late fetal mice on embryonic Day 19.5 after inhibition of NOS activity by l-NAME from the early fetal period onward (51). The absence of adverse effects of l-NAME on early postnatal lung development suggests that NOS activity may be less important for alveologenesis after birth when the transition from the saccular to the alveolar stage takes place compared with prenatal lung development. In addition, eNOS-deficient mice are more susceptible to hypoxia-induced neonatal lung injury, aggravating alveolar enlargement and blood vessel density reduction (52), which could be completely restored by inhaled NO (53), demonstrating the important role of eNOS in neonatal lung injury. However, we demonstrated that inhibition of NOS activity after birth did not aggravate hyperoxia-induced neonatal lung injury and RVH after early concurrent treatment, suggesting that prenatal defective lung development in eNOS-deficient mice makes the neonatal lung more vulnerable to injurious triggers in the absence of NOS activity. However, prolonged inhibition of NOS activity by l-NAME in experimental BPD was lethal during the recovery period, suggesting that, in contrast to early neonatal hyperoxic lung injury and repair, eNOS is needed during later stages of lung tissue repair. In intact animals l-NAME administration leads to an increase in blood pressure (54), which may be poorly tolerated by a damaged heart. Inhibition of inducible and/or neuronal NOS activity by l-NAME may account for the significant increase in lethality during the recovery period after hyperoxic neonatal lung injury.
Apelin treatment improved hyperoxia-induced RVH in experimental BPD, reduced BNP expression in the RV (a marker that is up-regulated under myocardial stress conditions) (55, 56), increased the mRNA expression of apelin and APJ in the RV, and reduced the thickness of the RV. The beneficial effect of apelin on the heart can be explained by a reduction of pulmonary hypertension resulting in reduced RVH, which is supported by our study by an apelin-induced reduction in pulmonary arteriolar wall thickness. In addition, similar beneficial effects of apelin in experimental models of lung disease, including monocrotaline- and hypoxia-induced pulmonary hypertension, have been described (57, 58). The natriuretic peptide BNP is synthesized and released in response to ventricular overload and plasma concentrations are related to ventricular dysfunction and severity of cardiac pathology (55, 56), whereas a down-regulation of apelin-APJ signaling is associated with heart failure (39). The increased mRNA expression of BNP observed in hyperoxia-induced lung injury in the RV and to a much lesser extent in the LV is associated with RVH and cardiac overload. Improvement of RVH after apelin treatment in experimental BPD is associated with decreased BNP mRNA expression compared with hyperoxia-exposed control pups. These results suggest that BNP expression is a suitable marker for RVH in neonatal hyperoxia-induced lung injury. A similar decrease in mRNA expression of apelin and APJ was observed in hyperoxia-induced lung injury in both RV and LV, which was partially restored after apelin treatment. The differential mRNA expression in the LV of BNP, apelin, and APJ suggests an adaptive response in the LV as well, but this was not associated with left ventricular hypertrophy.
Treatment with the potent vasodilators apelin, NO, and sildenafil had similar beneficial effects on lung pathology in this rat model for experimental BPD (30, 44). Intervention studies in hyperoxic lung injury with inhaled NO, apelin, and (selective) PDE inhibitors have demonstrated less lung inflammation but incomplete restoration of lung development resulting in persistently enlarged alveoli (30, 44, 59). Treatment with sildenafil or apelin showed similar beneficial effects on RVH in experimental BPD. However, apelin only had a small beneficial effect on survival until Day 10, whereas inhaled NO and sildenafil treatment prolonged median survival for 1.5 and 4 days, respectively, compared with oxygen-exposed control pups (30, 44). Reduced diuresis after apelin treatment due to renal malfunction (60) may explain, in part, the marginal effect on survival after apelin treatment in experimental BPD.
Our findings suggest that apelin is a promising treatment option for experimental BPD. The beneficial effects of apelin on alveolarization, lung inflammation, extravascular fibrin deposition, arteriolar wall thickness, and RVH in neonatal rats with hyperoxia-induced lung injury emphasize the potential of apelin administration for therapeutic intervention in chronic inflammatory lung diseases in premature infants and, likewise, possibly in adults suffering from chronic obstructive pulmonary disease or asthma.
Supplementary Material
Acknowledgments
The authors thank Dr. J.C.M. Meijers (Department of Experimental Vascular Medicine, AMC, Amsterdam, the Netherlands) and Dr. T. van der Poll (Center for Experimental and Molecular Medicine, AMC, Amsterdam, the Netherlands) for providing the 59D8 antibody. They also thank Dr. E. de Heer (Department of Pathology, LUMC, Leiden, the Netherlands) for providing the ED-1 antibody, S. van Wijngaarden (Department of Pulmonology, LUMC, Leiden, the Netherlands) for excellent photography, and Dr. P.S. Hiemstra (Department of Pulmonology, LUMC, Leiden, the Netherlands) for critically reading the manuscript.
Supported by National Institutes of Health grants 1R01 HL092158 and 1R01 ES015330 (F.J.W.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI:10.1164/rccm.200909-1361OC on July 9, 2010
Author Disclosure: Y.P.d.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.J.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.H.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.v.d.L. receives royalties from Elsevier Publishers (up to $1,000). G.T.M.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007;357:1946–1955. [DOI] [PubMed] [Google Scholar]
- 2.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–1729. [DOI] [PubMed] [Google Scholar]
- 3.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 1999;46:641–643. [DOI] [PubMed] [Google Scholar]
- 4.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet 2006;367:1421–1431. [DOI] [PubMed] [Google Scholar]
- 5.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol 1998;275:L110–L117. [DOI] [PubMed] [Google Scholar]
- 6.Coalson JJ. Experimental models of bronchopulmonary dysplasia. Biol Neonate 1997;71:35–38. [DOI] [PubMed] [Google Scholar]
- 7.Wagenaar GT, ter Horst SA, van Gastelen MA, Leijser LM, Mauad T, van der Velden PA, de Heer E, Hiemstra PS, Poorthuis BJ, Walther FJ. Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med 2004;36:782–801. [DOI] [PubMed] [Google Scholar]
- 8.Cheng X, Cheng XS, Pang CC. Venous dilator effect of apelin, an endogenous peptide ligand for the orphan APJ receptor, in conscious rats. Eur J Pharmacol 2003;470:171–175. [DOI] [PubMed] [Google Scholar]
- 9.Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, Sugaya T, Matsuzaki H, Yamamoto R, Shiota N, et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem 2004;279:26274–26279. [DOI] [PubMed] [Google Scholar]
- 10.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O'Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem 2000;74:34–41. [DOI] [PubMed] [Google Scholar]
- 11.Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, Hinuma S, Baba A. Apelin is a novel angiogenic factor in retinal endothelial cells. Biochem Biophys Res Commun 2004;325:395–400. [DOI] [PubMed] [Google Scholar]
- 12.Masri B, Morin N, Cornu M, Knibiehler B, Audigier Y. Apelin (65-77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. FASEB J 2004;18:1909–1911. [DOI] [PubMed] [Google Scholar]
- 13.Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta 1999;1452:25–35. [DOI] [PubMed] [Google Scholar]
- 14.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 1998;251:471–476. [DOI] [PubMed] [Google Scholar]
- 15.Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, Kitada C, Honda S, Kurokawa T, Onda H, et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem 2000;275:21061–21067. [DOI] [PubMed] [Google Scholar]
- 16.Masri B, Lahlou H, Mazarguil H, Knibiehler B, Audigier Y. Apelin (65-77) activates extracellular signal-regulated kinases via a PTX-sensitive G protein. Biochem Biophys Res Commun 2002;290:539–545. [DOI] [PubMed] [Google Scholar]
- 17.Ye RD. Regulation of nuclear factor kappaB activation by G-protein-coupled receptors. J Leukoc Biol 2001;70:839–848. [PubMed] [Google Scholar]
- 18.Neves SR, Ram PT, Iyengar R. G protein pathways. Science 2002;296:1636–1639. [DOI] [PubMed] [Google Scholar]
- 19.De Mota N, Lenkei Z, Llorens-Cortes C. Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology 2000;72:400–407. [DOI] [PubMed] [Google Scholar]
- 20.Katugampola SD, Maguire JJ, Matthewson SR, Davenport AP. [(125)I]-(Pyr(1))Apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in man. Br J Pharmacol 2001;132:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto M, Hidaka K, Akiho H, Tada S, Okada M, Yamaguchi T. Low stringency hybridization study of the dopamine D4 receptor revealed D4-like mRNA distribution of the orphan seven-transmembrane receptor, APJ, in human brain. Neurosci Lett 1996;219:119–122. [DOI] [PubMed] [Google Scholar]
- 22.Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, Lawrie KW, Hervieu G, Riley G, Bolaky JE, et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem 2003;84:1162–1172. [DOI] [PubMed] [Google Scholar]
- 23.O'Carroll AM, Selby TL, Palkovits M, Lolait SJ. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim Biophys Acta 2000;1492:72–80. [DOI] [PubMed] [Google Scholar]
- 24.O'Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 1993;136:355–360. [DOI] [PubMed] [Google Scholar]
- 25.Kleinz MJ, Skepper JN, Davenport AP. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept 2005;126:233–240. [DOI] [PubMed] [Google Scholar]
- 26.Leeper NJ, Tedesco MM, Kojima Y, Schultz GM, Kundu RK, Ashley EA, Tsao PS, Dalman RL, Quertermous T. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol 2009;296:H1329–H1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunter D, Hewson AK, Dickson SL. Intracerebroventricular injection of apelin-13 reduces food intake in the rat. Neurosci Lett 2003;353:1–4. [DOI] [PubMed] [Google Scholar]
- 28.Taheri S, Murphy K, Cohen M, Sujkovic E, Kennedy A, Dhillo W, Dakin C, Sajedi A, Ghatei M, Bloom S. The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochem Biophys Res Commun 2002;291:1208–1212. [DOI] [PubMed] [Google Scholar]
- 29.de Visser YP, Walther FJ, Laghmani EH, van der Laarse A, Wagenaar GT. Apelin attenuates pulmonary inflammation and fibrin deposition in neonatal hyperoxic lung injury. Am J Respir Crit Care Med 2009;179:A2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Visser YP, Walther FJ, Laghmani EH, Boersma H, van der Laarse A, Wagenaar GT. Sildenafil attenuates pulmonary inflammation and fibrin deposition, mortality and right ventricular hypertrophy in neonatal hyperoxic lung injury. Respir Res 2009;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao L, Ning Q, Li Y, Wang W, Wang A, Wei W, Liu X, Auten RL, Tanswell AK, Luo X. CXCR2 blockade reduces radical formation in hyperoxia-exposed newborn rat lung. Pediatr Res 2006;60:299–303. [DOI] [PubMed] [Google Scholar]
- 32.Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology 1985;54:589–599. [PMC free article] [PubMed] [Google Scholar]
- 33.Hui KY, Haber E, Matsueda GR. Monoclonal antibodies to a synthetic fibrin-like peptide bind to human fibrin but not fibrinogen. Science 1983;222:1129–1132. [DOI] [PubMed] [Google Scholar]
- 34.Yi M, Jankov RP, Belcastro R, Humes D, Copland I, Shek S, Sweezey NB, Post M, Albertine KH, Auten RL, et al. Opposing effects of 60% oxygen and neutrophil influx on alveologenesis in the neonatal rat. Am J Respir Crit Care Med 2004;170:1188–1196. [DOI] [PubMed] [Google Scholar]
- 35.ter Horst SA, Fijlstra M, Sengupta S, Walther FJ, Wagenaar GT. Spatial and temporal expression of surfactant proteins in hyperoxia-induced neonatal rat lung injury. BMC Pulm Med 2006;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilkinson DG. In situ hybridization. A practical approach. Oxford: IRL Press at Oxford University Press, 1992.
- 37.Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept 2001;99:87–92. [DOI] [PubMed] [Google Scholar]
- 38.Koppel R, Han RN, Cox D, Tanswell AK, Rabinovitch M. Alpha 1-antitrypsin protects neonatal rats from pulmonary vascular and parenchymal effects of oxygen toxicity. Pediatr Res 1994;36:763–770. [DOI] [PubMed] [Google Scholar]
- 39.Chandrasekaran B, Dar O, McDonagh T. The role of apelin in cardiovascular function and heart failure. Eur J Heart Fail 2008;10:725–732. [DOI] [PubMed] [Google Scholar]
- 40.Japp AG, Newby DE. The apelin-APJ system in heart failure: pathophysiologic relevance and therapeutic potential. Biochem Pharmacol 2008;75:1882–1892. [DOI] [PubMed] [Google Scholar]
- 41.Kojima Y, Kundu R, Cox CM, Leeper NJ, Anderson JA, Chun HJ, Ali ZA, Ashley EA, Krieg PA, Quertermous T. Upregulation of the apelin-APJ pathway promotes neointima formation in the carotid ligation model in mouse. Cardiovasc Res 2010;87:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res 2005;58:22–29. [DOI] [PubMed] [Google Scholar]
- 43.McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, Kerecman JD, Albertine KH, Winter VT, Coalson JJ, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol 2005;288:L450–L459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ter Horst SA, Walther FJ, Poorthuis BJ, Hiemstra PS, Wagenaar GT. Inhaled nitric oxide attenuates pulmonary inflammation and fibrin deposition and prolongs survival in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 2007;293:L35–L44. [DOI] [PubMed] [Google Scholar]
- 45.Auten RL, Mason SN, Whorton MH, Lampe WR, Foster WM, Goldberg RN, Li B, Stamler JS, Auten KM. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am J Respir Crit Care Med 2007;176:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaston B, Singel D, Doctor A, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med 2006;173:1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woyda K, Koebrich S, Reiss I, Rudloff S, Pullamsetti SS, Ruhlmann A, Weissmann N, Ghofrani HA, Gunther A, Seeger W, et al. Inhibition of phosphodiesterase 4 enhances lung alveolarisation in neonatal mice exposed to hyperoxia. Eur Respir J 2009;33:861–870. [DOI] [PubMed] [Google Scholar]
- 48.Ladha F, Bonnet S, Eaton F, Hashimoto K, Korbutt G, Thebaud B. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med 2005;172:750–756. [DOI] [PubMed] [Google Scholar]
- 49.Lee TC, Zhao YD, Courtman DW, Stewart DJ. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation 2000;101:2345–2348. [DOI] [PubMed] [Google Scholar]
- 50.Feng Q, Song W, Lu X, Hamilton JA, Lei M, Peng T, Yee SP. Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation 2002;106:873–879. [DOI] [PubMed] [Google Scholar]
- 51.Han RN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, Post M, Stewart DJ. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: a model of alveolar capillary dysplasia? Circ Res 2004;94:1115–1123. [DOI] [PubMed] [Google Scholar]
- 52.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase-deficient mouse. Am J Physiol Lung Cell Mol Physiol 2003;284:L964–L971. [DOI] [PubMed] [Google Scholar]
- 53.Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol 2006;291:L119–L127. [DOI] [PubMed] [Google Scholar]
- 54.Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest 1992;90:278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Felker GM, Petersen JW, Mark DB. Natriuretic peptides in the diagnosis and management of heart failure. CMAJ 2006;175:611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshimura M, Yasue H, Okumura K, Ogawa H, Jougasaki M, Mukoyama M, Nakao K, Imura H. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation 1993;87:464–469. [DOI] [PubMed] [Google Scholar]
- 57.Falcao-Pires I, Goncalves N, Henriques-Coelho T, Moreira-Goncalves D, Roncon-Albuquerque R Jr, Leite-Moreira AF. Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 2009;296:H2007–H2014. [DOI] [PubMed] [Google Scholar]
- 58.Andersen CU, Markvardsen LH, Hilberg O, Simonsen U. Pulmonary apelin levels and effects in rats with hypoxic pulmonary hypertension. Respir Med 2009;103:1663–1671. [DOI] [PubMed]
- 59.de Visser YP, Walther FJ, Laghmani EH, van Wijngaarden S, Nieuwland K, Wagenaar GT. Phosphodiesterase-4 inhibition attenuates pulmonary inflammation in neonatal lung injury. Eur Respir J 2008;31:633–644. [DOI] [PubMed] [Google Scholar]
- 60.Principe A, Melgar-Lesmes P, Fernandez-Varo G, del Arbol LR, Ros J, Morales-Ruiz M, Bernardi M, Arroyo V, Jimenez W. The hepatic apelin system: a new therapeutic target for liver disease. Hepatology 2008;48:1193–1201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.