Abstract
Rationale: IL-5 is a T helper 2 cytokine important in the trafficking and survival of eosinophils. Because eosinophils can be found in malignant pleural effusions (MPE) from mice and humans, we asked whether IL-5 is involved in the pathogenesis of MPE.
Objectives: To determine the role of IL-5 in MPE formation.
Methods: The effects of IL-5 on experimental MPE induced in C57BL/6 mice by intrapleural injection of syngeneic lung (Lewis lung cancer [LLC]) or colon (MC38) adenocarcinoma cells were determined using wild-type (il5+/+) and IL-5–deficient (il5−/−) mice, exogenous administration of recombinant mouse (rm) IL-5, and in vivo antibody-mediated neutralization of endogenous IL-5. The direct effects of rmIL-5 on LLC cell proliferation and gene expression in vitro were determined by substrate reduction and microarray.
Measurements and Main Results: Eosinophils and IL-5 were present in human and mouse MPE, but the cytokine was not detected in mouse (LLC) or human (A549) lung and mouse colon (MC38) adenocarcinoma-conditioned medium, suggesting production by host cells in MPE. Compared with il5+/+ mice, il5−/− mice showed markedly diminished MPE formation in response to both LLC and MC38 cells. Exogenous IL-5 promoted MPE formation in il5+/+ and il5−/− mice, whereas anti–IL-5 antibody treatment limited experimental MPE in il5+/+ mice. Exogenous IL-5 had no effects on LLC cell proliferation and gene expression; however, IL-5 was found to be responsible for recruitment of eosinophils and tumor-promoting myeloid suppressor cells to MPE in vivo.
Conclusions: Host-derived IL-5 promotes experimental MPE and may be involved in the pathogenesis of human MPE.
Keywords: pleural disease, lung cancer, allergy, eosinophils, myeloid suppressor cells
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Malignant pleural effusion (MPE) is a common and severe clinical problem, for which no disease-specific therapy is available. Although tumor-specific biologic pathways that promote MPE formation have been previously identified, no host factors had been implicated in MPE formation to date.
What This Study Adds to the Field
Here we show how host-derived IL-5 enhances MPE progression by modulating the host inflammatory response to intrapleural adenocarcinoma. The effects of IL-5 bioactivity on MPE formation are amenable to exogenous manipulation. Hence, IL-5 can be a therapeutic target against MPE.
Malignant pleural effusions (MPE) occur frequently (∼500 new cases per million population per yr) in patients with cancer, predominantly lung and breast adenocarcinoma (1–4). MPEs imply systemic spread of disease and reduce life expectancy and quality (5–8). Current treatment options, including needle aspiration, pleurodesis (chemical-induced pleural fibrosis), or indwelling pleural catheters, are aimed at draining or eliminating the pleural space. These treatments are nonspecific, often ineffective, associated with morbidity and mortality, and do not benefit a significant number of patients (9–11). Improved understanding of MPE formation and development of new treatments targeting this pathobiology may yield safer and more effective therapeutic options (12).
To aid in elucidating MPE pathobiology, we have developed an immunocompetent model of MPE using syngeneic mice (13–15). Immunocompetent models are vital, because recent evidence has linked inflammation with enhanced formation, growth, and metastasis of tumors (16–19). In this connection, mouse MPEs display a robust immune response, including varying degrees of mononuclear and eosinophil infiltration, similar to human MPEs (13–15, 20, 21). We and others have identified tumor-specific pathways that promote MPE formation, including vascular endothelial growth factor (VEGF), IL-6/signal transducer and activator of transcription-3, and nuclear factor (NF)-κB/tumor necrosis factor (TNF)-α/monocyte chemoattractant protein (MCP)-1 (13–15, 22–24). However, host-derived factors that regulate MPE formation and progression have not been identified.
IL-5 is important for eosinophil differentiation, trafficking, and survival (25). Eosinophils are important effectors of allergy and of host defense against fungi, parasites, and protozoa (25). Eosinophils also have been associated with aggressive behavior of human malignancies (26, 27). IL-5 is involved in the eosinophilia occasionally observed with hematologic and solid tumors (28–30); however, a specific role for IL-5 in tumor progression has not been identified. In addition to eosinophil recruitment, IL-5 may have important effects on other components of the host inflammatory response, including lymphocytes and macrophages (31, 32).
In these studies, we asked whether IL-5 promotes the formation and progression of MPE. To address this question, we used two different models, Lewis lung cancer (LLC) and mouse colon adenocarcinoma (MC38)–induced MPE, in IL-5 competent and deficient mice (il5+/+ and il5−/−, respectively). In addition, we delivered recombinant mouse (rm) IL-5 or neutralizing anti–IL-5 antibody (TRFK5) to mice with LLC-induced MPE. We found that IL-5 promotes MPE formation by adenocarcinoma through effects on the MPE-associated inflammatory response. Thus, IL-5 is the first host-derived mediator shown to play an important role in MPE formation.
METHODS
Detailed description of all methods is provided in the online supplement.
Reagents
Anti–IL-5 antibody was prepared from TRFK5 hybridoma (33).
Human Pleural Effusions
Pleural fluid and serum were obtained during initial diagnostic thoracenteses of 55 patients with MPE (tumors: 35 lung, 6 mesothelioma, 7 breast, 7 other) and 20 with congestive heart failure (CHF) at the General Hospital Evangelismos (September 2006 to May 2008). The study was approved by the hospital's ethics committee and all patients gave written informed consent. MPE was diagnosed by cytology and histology (2, 4) and CHF pleural effusion using clinical, radiologic, and laboratory findings (34).
Cell Culture
LLC mouse and A549 human lung adenocarcinoma, MC38 mouse colon adenocarcinoma, and mouse skin melanoma (B16F10) cells (ATCC, Manassas, VA; NCI, Frederick, MD) were cultured and pNGL MC38 cells stably expressing a NF-κB reporter (NF-κB.GFP.LUC; pNGL) were generated as described previously (13).
Animal Models
il5+/+ and il5−/− (35) C57BL/6 mice (Jackson, Bar Harbor, MN; BSRC Alexander Fleming, Vari, Greece) were inbred at Vanderbilt University and Evangelismos Hospital. Experiments were approved by both Institutional Animal Care and Use Committees. Intrapleural tumor cell injection (1.5 × 105), killing of mice, and specimen collection (Day 14 after LLC and Day 11 after MC38 cells) were described previously (13–15). For flank tumor formation, tumor cells (5 × 105) were subcutaneously injected, tumor dimensions (δ1,δ2,δ3) were measured weekly, and tumor volume (V) was determined (V = π×[δ1×δ2×δ3]/6).
Bioluminescence Imaging
Bioluminescence imaging of mice bearing pNGL cells was done using Xenogen IVIS (Alameda, CA).
Cytokine Determinations
Mouse and human IL-5 (detection limits, 7 and 3 pg/ml, respectively) were determined by ELISA (R&D, Minneapolis, MN).
Exogenous rmIL-5 Treatment
A total of 40 ng rmIL-5/100 μl phosphate-buffered saline (PBS) or PBS alone were delivered to the retroorbital veins of mice every other day after LLC cells, a regimen that reconstitutes il5−/−mice (36).
IL-5 Neutralization
Mice received 1 mg/kg intraperitoneal TRFK5, which provides prolonged bioactivity (37), in “prevention” (Days 0 and 8) and “regression” (Day 8) protocols, the latter aimed at therapy of established MPEs developing around Day 8 (13, 38).
Histology
Tissue sections were immunolabeled as described previously (13–15, 38), or stained with Biebrich scarlet for eosinophils (39).
Polymerase Chain Reaction and Gene Expression Profiling
Total RNA was isolated from LLC cells treated with PBS or rmIL-5 (22 pM) for 24 hours, reverse transcribed, and cDNA was labeled using amino-allyl cDNA. Primers and conditions for polymerase chain reaction have been described (40). Independent triplicate samples were mixed with isomolar reference sample and hybridized to Affymetrix GeneChip Mouse Gene 1.0 ST Arrays (Santa Clara, CA) interrogating 28,853 genes. Differentially expressed genes were selected by analysis of variance without sample selection or filtering. Microarray data are available in the ArrayExpress database (www.ebi.ac.uk/arrayexpress) under accession number E-MEXP-2490.
Flow Cytometry
After red blood cell lysis, cells were suspended in PBS 1% bovine serum albumin, stained with phycoerythrin-Cy7-conjugated anti-CD11b and allophycocyanin-conjugated anti-Gr1 antibodies (0.1 μg/106 cells; 60 minutes), and analyzed on a BD FACS-SCAN (Palo Alto, CA). Data were analyzed using WinMDIv2.8 (J. Trotter, The Scripps Institute, La Jolla, CA; http://facs.scripps.edu/software.html).
Statistics
All values represent mean ± SEM. Intergroup differences in frequencies, means, and medians were examined by χ2 test, t test, or one-way analysis of variance with least square difference post hoc tests, and Mann-Whitney or Kruskal-Wallis test with Dunn post hoc tests, respectively. Two-tailed P values less than 0.05 were considered significant. All analyses were performed using SPSSv.13.0.0 (Chicago, IL).
RESULTS
Eosinophils and IL-5 are Present in Human and Mouse MPE
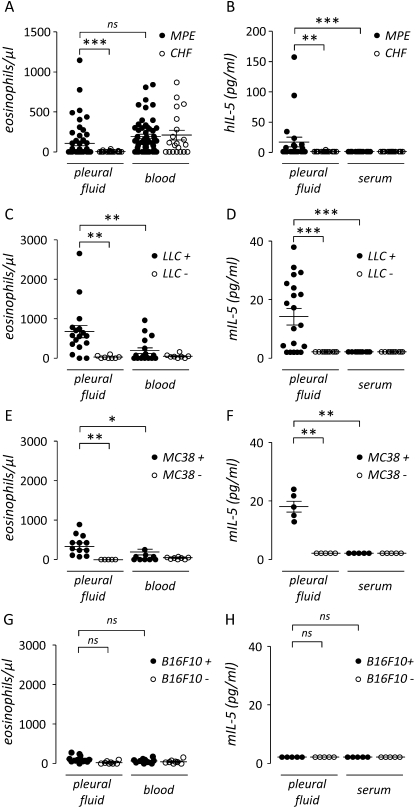
Initially, we determined the abundance of eosinophils in pleural fluid and blood from patients with MPE using patients with pleural effusions caused by CHF as a control group. We found equivalent eosinophil numbers in peripheral blood from patients in these two groups; however, significantly more eosinophils were present in pleural fluid from patients with MPE compared with pleural effusions from patients with CHF (Figure 1A). Although eosinophils were detected in 40 of 55 patients with MPE, only 7 (13%) met the clinical definition of eosinophilic pleural effusion, usually defined as greater than 10% eosinophils (3, 22). Because IL-5 is important in eosinophil homeostasis, we went on to assess the levels of this cytokine in the previously mentioned biologic samples. IL-5 was detected in 9 of 22 human MPEs tested, but in only 1 of 16 effusions caused by CHF (detection limit, 3 pg/ml; χ2 = 5.74; P = 0.017). IL-5 levels were significantly elevated in MPE compared with pleural effusions caused by CHF and with serum from either patient group. The increased IL-5 concentration in MPE compared with serum suggests local production in the malignancy-affected pleural space (Figure 1B). We subsequently sought to assess eosinophils and IL-5 in a mouse model of lung adenocarcinoma–induced MPE (13–15, 38). Pleural accumulation of eosinophils was observed 14 days after intrapleural injection of LLC cells into C57BL/6 mice (Figure 1C). In this model, 6.8 ± 0.8% of inflammatory cells in pleural fluid were eosinophils, and 3 of 17 effusions would be classified as eosinophilic using the clinical definition. Consistent with data from human MPE, IL-5 was also found to be present in mouse MPE; however, IL-5 was not detected in matched serum or in pleural fluid or serum from PBS-treated controls without MPE (Figure 1D). To determine whether this was unique to the LLC model of MPE, we developed an additional mouse model of MPE using intrapleural delivery of MC38 colon adenocarcinoma cells (1.5 × 105 cells, 11-day latency). Similar to our observations with LLC cells, eosinophils and IL-5 locally accumulated in MPEs induced by MC38 cells. In the MC38 model, 5.3 ± 1.2% of inflammatory cells in MPE were eosinophils, and 2 of 12 effusions would be classified as eosinophilic (Figure 1E). IL-5 was also present in MC38-induced MPE, but not in matched serum or in pleural fluid or serum from PBS-treated controls without MPE (Figure 1F). In contrast to our findings with LLC and MC38 cell injections, intrapleural injection of live mouse skin melanoma (B16F10) cells, which are also syngeneic to the C57BL/6 mouse strain, did not result in substantial MPE formation (15), eosinophil recruitment, or IL-5 production (Figures 1G and 1H). In separate experiments, IL-5 was not detected in media conditioned by mouse (LLC) and human (A549) lung and mouse colon (MC38) adenocarcinoma cells by ELISA (data not shown). Collectively, these results implicated intrapleural IL-5 production by the host with associated eosinophil recruitment in response to intrapleural dissemination of adenocarcinoma during MPE formation.
Figure 1.
Eosinophils and IL-5 in mouse and human malignant pleural effusion (MPE). (A) Pleural fluid and blood eosinophils in humans with pleural effusion caused by malignancy (MPE, n = 55) and heart failure (congestive heart failure [CHF], n = 20). Pleural eosinophils are increased in patients with MPE, compared with CHF. (B) Pleural fluid and serum IL-5 levels in humans with MPE (n = 22) and CHF (n = 16). IL-5 is locally increased in the pleural fluid of patients with MPE. (C) Pleural fluid and blood eosinophil numbers in C57BL/6 mice 14 days after intrapleural delivery of LLC cells (LLC+, n = 17) or phosphate-buffered saline (PBS) control (LLC−, n = 8). Pleural eosinophil accumulation is observed after tumor cell injection only. (D) Pleural fluid and serum IL-5 levels in C57BL/6 mice 14 days after intrapleural delivery of LLC cells (LLC+, n = 20) or PBS control (LLC−, n = 10). (E) Pleural fluid and blood eosinophil numbers in C57BL/6 mice 11 days after intrapleural delivery of MC38 cells (MC38+, n = 12) or PBS control (MC38−, n = 5). Pleural eosinophil accumulation is observed after tumor cell injection only. (F) Pleural fluid and serum IL-5 levels in C57BL/6 mice 11 days after intrapleural delivery of MC38 cells (MC38+, n = 5) or PBS control (MC38−, n = 5). (G) Pleural fluid and blood eosinophil numbers in C57BL/6 mice 14 days after intrapleural delivery of B16F10 cells (B16F10+, n = 12) or PBS control (B16F10−, n = 8). (H) Pleural fluid and serum IL-5 levels in C57BL/6 mice 14 days after intrapleural delivery of B16F10 cells (B16F10+, n = 5) or PBS control (B16F10−, n = 5). Dots = raw data points; lines = mean; bars = SE. *P < 0.05, **P < 0.01, ***P < 0.001. B16F10 = mouse skin melanoma; CHF = congestive heart failure; h = human; LLC = Lewis lung cancer; m = mouse; MC38 = mouse colon adenocarcinoma; MPE = malignant pleural effusion; ns = not significant.
Host-Derived IL-5 Promotes MPE Formation
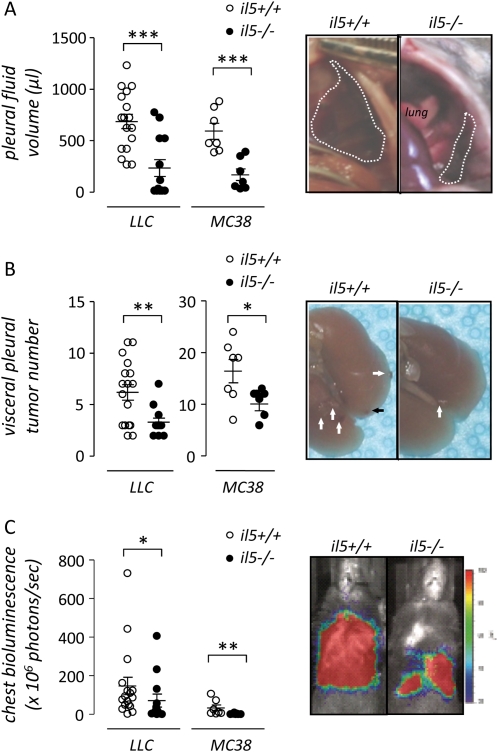
Because IL-5 was present in MPE, we investigated whether host-derived IL-5 contributes to MPE formation. For these studies, C57BL/6 il5+/+ and il5−/− mice (35) received intrapleural LLC cells (or MC38 cells) and MPEs were evaluated at Day 14 (or Day 11 after MC38 cell injection). Compared with il5+/+ mice, il5−/− mice exhibited a marked reduction in MPE formation (6 of 13 il5−/− and 17 of 17 il5+/+ mice developed MPE in response to LLC cells, χ2 = 11.94, P = 0.001; 3 of 7 il5−/− and 7 of 7 il5+/+ mice developed MPE in response to MC38 cells, χ2 = 5.6, P = 0.018). Of mice that developed MPEs, the volume of fluid was reduced in il5−/− mice (mean MPE volume in il5−/− and il5+/+ mice after LLC cells, 217 ± 81 and 662 ± 70 μl, respectively, P < 0.001; mean MPE volume in il5−/− and il5+/+ mice after MC38 cells, 173 ± 47 and 593 ± 65 μl, respectively, P < 0.001) (Figure 2A). In addition, the number of individual pleural tumors was decreased in il5−/− mice (mean number of LLC pleural tumors in il5−/− and il5+/+ mice, 3.2 ± 0.4 and 6.1 ± 0.8, respectively, P = 0.001; mean number of MC38 pleural tumors in il5−/− and il5+/+ mice, 11 ± 1.1 and 16.4 ± 1.9, respectively, P = 0.04) (Figure 2B). We next used intrapleurally injected LLC and MC38 cells stable expressing a constitutive NF-κB reporter (pNGL), to verify noninvasively the reduced MPE formation and integrate pleural tumor load in il5−/− mice (13–15). Indeed, in these models designed to monitor tumor-specific NF-κB and hence tumor mass (13), il5−/− mice bearing intrapleural pNGL LLC or pNGL MC38 cells displayed markedly reduced chest photon emission before sacrifice compared with il5+/+ mice, consistent with decreased intrapleural tumor load (Figure 2C). The concentration of several tumor cell–derived mediators important in MPE formation, including VEGF, TNF-α, MCP-1, and IL-6, was similar in pleural fluid from both genotypes (data not shown). These results indicate that IL-5 deficiency in the host reduces intrapleural fluid accumulation and tumor dissemination of experimental MPE.
Figure 2.
IL-5 promotes experimental lung and colon adenocarcinoma–induced malignant pleural effusion (MPE). (A) Pleural fluid volume, (B) pleural tumor number, and (C) bioluminescence of MPEs generated in wild-type (il5+/+, n = 27) and IL-5 knock-out (il5−/−, n = 23) mice on the C57BL/6 background 14 and 11 days after intrapleural delivery of LLC (n = 30) and MC38 (n = 14) cells, respectively. Genetic IL-5 deficiency limits pleural fluid accumulation and intrapleural adenocarcinoma dissemination. Photographs show representative images of MPEs (A, transdiaphragmatic views), of pleural tumors (B, post-lung explantation from thorax; arrows point toward visceral pleural tumors), and of bioluminescence emission (C, day of sacrifice) from il5+/+ and il5−/−mice bearing intrapleural LLC cells. Dots = raw data points; lines = mean; bars = SE. *P < 0.05, ** P < 0.01, ***P < 0.001. LLC = Lewis lung cancer; MC38 = mouse colon adenocarcinoma.
IL-5 Does Not Directly Enhance Tumor Growth
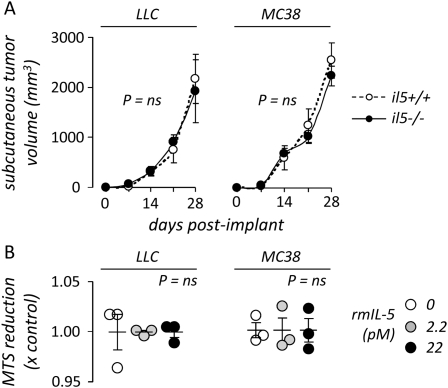
Based on the finding of decreased tumor burden in the pleural space of il5−/− mice compared with il5+/+ mice, we evaluated whether host-derived IL-5 directly impacts tumor growth in vivo using the flank tumor growth model. For these studies, il5+/+ and il5−/− mice received subcutaneous LLC or MC38 cells and the volume of the resulting tumors was measured weekly. We found no difference in the growth of subcutaneous LLC and MC38 tumors between il5+/+ and il5−/− mice (Figure 3A). We also made observations regarding the proliferation rate of LLC and MC38 cells exposed to rmIL-5 in vitro. Exogenous IL-5 did not alter LLC or MC38 cell proliferation even after prolonged exposure times (4 days) (Figure 3B). These findings support the conclusion that the impact of IL-5 on tumor dissemination and effusion formation in the pleural space are dependent on local microenvironmental factors rather than a general effect of IL-5 on tumor cell proliferation or survival.
Figure 3.
IL-5 does not enhance tumor growth in vivo and in vitro. (A) Volume of subcutaneous flank tumors induced in wild-type (il5+/+, n = 10) and IL-5 knock-out (il5−/−, n = 10) mice after subcutaneous injection of 5 × 105 Lewis lung carcinoma (LLC; n = 10) or colon adenocarcinoma (MC38; n = 10) cells. Dots = mean; bars = SE. (B) In vitro cell proliferation rate of LLC and MC38 cells 4 days after exposure to varying concentrations of recombinant mouse IL-5, as determined by a substrate (MTS) reduction assay. Note that 22 pM is the binding constant (kilodalton) of IL-5 for its receptor. Dots = raw data points; lines = mean; bars = SE. LLC = Lewis lung cancer; MC38 = mouse colon adenocarcinoma; ns = not significant.
Exogenous IL-5 Treatment Enhances MPE Formation
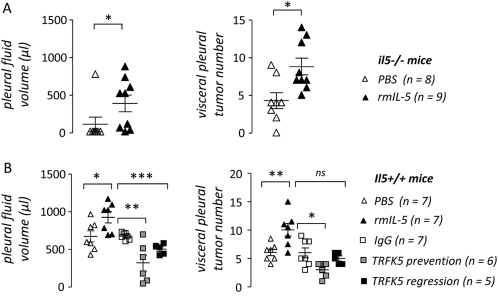
During subsequent studies aimed at identifying a mechanism of MPE promotion by IL-5, we used the better established and characterized LLC-induced MPE model. To show that reduced MPE formation in IL-5–deficient mice was directly related to loss of IL-5 and not a developmental or compensatory effect, we reconstituted il5−/− mice with IL-5 during MPE formation. For this study, il5−/− mice on the C57BL/6 background received intrapleural LLC cells followed by intravenous PBS or rmIL-5 treatment every other day until harvest. Fourteen days later, rmIL-5–treated il5−/− mice exhibited increased MPE formation (mean MPE volume in PBS- and rmIL-5–treated mice, respectively: 94 ± 94 and 368 ± 109 μl; P < 0.05) and intrapleural tumor dissemination (mean number of pleural tumors in PBS- and rmIL-5–treated mice, respectively: 4.3 ± 1 and 8.8 ± 1.1; P < 0.05) compared with PBS-treated il5−/−mice (Figure 4A).
Figure 4.
Exogenous IL-5 directly promotes and IL-5 neutralization limits experimental lung adenocarcinoma–induced malignant pleural effusion (MPE). (A) Pleural fluid volume (left) and pleural tumor number (right) of MPEs generated in IL-5–deficient C57BL/6 (il5−/−) mice 14 days after intrapleural delivery of Lewis lung cancer (LLC) cells, when mice were treated with phosphate-buffered saline (PBS) control or recombinant mouse (rm) IL-5 (n = 8 and 9, respectively). Exogenous IL-5 enhances pleural fluid accumulation and intrapleural adenocarcinoma dissemination in il5−/−mice. (B) Pleural fluid volume (left) and pleural tumor number (right) of MPEs generated in wild-type C57BL/6 (il5+/+) mice 14 days after intrapleural delivery of LLC cells, when mice were treated with PBS control, recombinant mouse (rm) IL-5, IgG control, or anti–IL-5 antibody (TRFK5) in both a prevention (Days 0 and 8) and a regression (Day 8) trial (n = 7, 7, 7, 6, and 5, respectively). Exogenous IL-5 enhances, whereas antibody-mediated IL-5 neutralization decreases pleural fluid accumulation and intrapleural adenocarcinoma dissemination. Points = raw data; lines = mean; bars = SE. *P < 0.05, **P < 0.01, and ***P < 0.001 for comparison with appropriate control. Ig = immunoglobulin; MPE = malignant pleural effusion; PBS = phosphate-buffered saline; rm = recombinant mouse; TRFK5 = anti–IL-5 neutralizing antibody.
Next, we asked whether exogenous IL-5 administration in vivo would impact MPE formation in wild-type (C57BL/6 il5+/+) mice. In these studies, we treated mice as described previously with intravenous PBS or rmIL-5 every other day after intrapleural LLC cells. Fourteen days later, rmIL-5–treated mice exhibited increased MPE formation (mean MPE volume in PBS- and rmIL-5–treated mice, respectively: 662 ± 72 and 911 ± 73 μl; P < 0.001) and intrapleural tumor dissemination (mean number of pleural tumors in PBS- and rmIL-5–treated mice, respectively, 6.1 ± 0.6 and 10.1 ± 1.1; P < 0.001) (Figure 4B). The levels of VEGF, TNF-α, MCP-1, and IL-6 were similar in MPEs from both experimental groups (data not shown). Collectively, these results supported the conclusion that IL-5 directly promotes MPE formation.
IL-5 Neutralization Limits Experimental MPE
We next sought to assess whether neutralization of endogenous IL-5 bioactivity in vivo favorably impacts experimental MPE. For these studies, wild-type (C57BL/6 il5+/+) mice received intrapleural LLC cells followed by 1 mg/kg intraperitoneal IgG2a (control) or anti–IL-5 antibody (TRFK5) in two protocols designated as prevention (Days 0 and 8 after LLC cells) or regression studies (Day 8 after LLC cells). This latter study design was aimed at unveiling potential therapeutic effects of TRFK5 against already established mouse MPE, which develop around Day 8 (13, 38). Fourteen days after LLC cell injection, all mice developed MPE; however, TRFK5-treated mice in both prevention and regression studies displayed decreased MPE formation and intrapleural tumor growth compared with IgG-treated controls (Figure 4B). Again, pleural fluid VEGF, TNF-α, MCP-1, and IL-6 levels were similar between groups (data not shown). In addition to further supporting a MPE-promoting role for IL-5, these studies indicated that targeting IL-5 could have therapeutic benefit in reducing MPE formation.
IL-5 Enhances In Vivo Tumor Cell Survival and MPE Formation through Modulation of the Host Immune Response
To determine whether IL-5 directly affects tumor cells, we studied LLC cells in culture. Cultured LLC cells did not express the IL-5 receptor (IL-5Rα) as determined by reverse transcriptase-polymerase chain reaction (data not shown). As mentioned previously, treatment of LLC cells in vitro with IL-5 at concentrations equaling its binding constant with IL-5Rα (1 ng/ml = 22 pM) had no effect on LLC cell proliferation as determined by MTS assay, NF-κB activation as determined by a relevant reporter, Ras/RhoA expression as determined by Western blot, or apoptosis as determined by terminal deoxynucleotidyl nick-end labeling (TUNEL) (data not shown). Microarray-assisted interrogation of 28,853 genes showed that IL-5 treatment for 24 hours altered the expression of only 139 putative genes with no known function (see Table E1 in the online supplement). The greatest changes in gene expression observed were modest (less than two-fold). Moreover, these modest differences in 139 of 28,853 genes were fewer than those expected to appear by chance: when the threshold of significance (P < 0.05) was divided by the number of comparisons made (28,853), these changes in gene expression were not significant. Most importantly, microarray data and validation at the protein level showed that exogenous IL-5 induced no significant change in the expression of genes previously implicated in MPE pathogenesis (Table 1). These results indicated that host-derived IL-5 does not directly impact MPE formation via effects on tumor cells, but rather seems to facilitate MPE formation through alteration of the microenvironment of the pleural space.
TABLE 1.
CHANGES IN THE EXPRESSION OF GENES (ΔGE) OF PROVEN OR POTENTIAL IMPORTANCE IN MALIGNANT PLEURAL EFFUSION PATHOGENESIS INDUCED IN LEWIS LUNG ADENOCARCINOMA CELLS BY 22 pM RECOMBINANT MOUSE IL-5*
| Gene Symbol | Microarray |
ELISA/CBA |
|||
|---|---|---|---|---|---|
| Gene Name | ΔGE | P | ΔGE | P | |
| Ccl2 | Chemokine (C-C motif) ligand 2 | 1.080 | 0.310 | 1.029 | 0.885 |
| Ccl12 | Chemokine (C-C motif) ligand 12 | 1.080 | 0.330 | 1.101 | 0.741 |
| Cxcl2 | Chemokine (C-X-C motif) ligand 2 | 1.020 | 0.800 | −1.090 | 0.720 |
| Ifng | Interferon-γ | 1.180 | 0.230 | 1.100 | 0.691 |
| Il6 | IL-6 | 1.020 | 0.660 | 1.011 | 0.978 |
| Il10 | IL-10 | −1.020 | 0.770 | 1.038 | 0.776 |
| Il12b | IL-12b | 1.090 | 0.260 | 1.243 | 0.489 |
| Tnf | Tumor necrosis factor | 1.180 | 0.230 | 1.171 | 0.515 |
| Vegfa | Vascular endothelial growth factor A | −1.020 | 0.650 | 1.046 | 0.852 |
Definition of Abbreviations: CBA = cytometric bead array; P = probability.
Determined at the RNA level by microarray and at the protein level by ELISA or CBA.
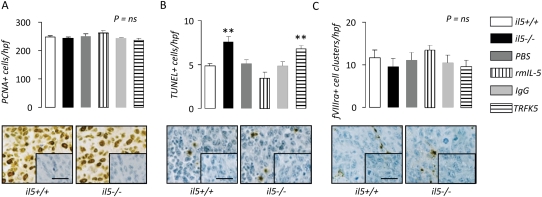
To investigate the microenvironmental impact of host-derived IL-5 in the pleural space, we assessed tumor cell proliferation, apoptosis, and angiogenesis in vivo. We performed immune-labeling of pleural tumor tissue sections obtained from LLC-induced MPE-bearing C57BL/6 il5+/+ and il5−/− mice, and C57BL/6 il5+/+ mice treated with rmIL-5, TRFK5 antibodies, and controls for proliferating cell nuclear antigen, TUNEL, and factor VIII–related antigen, indicators of cell proliferation, apoptosis, and angiogenesis, respectively (Figure 5). We found no significant intergroup differences in tumor cell proliferation and angiogenesis (Figures 5A and 5C). However, pleural tumor cell apoptosis was significantly increased in il5−/− mice compared with il5+/+ mice and in TRFK5-treated compared with IgG-treated mice (Figure 5B).
Figure 5.
IL-5 promotes pleural tumor cell survival. Immunodetection of (A) proliferating cell nuclear antigen, (B) TUNEL, and (C) fVIIIra in pleural tumor tissue obtained from wild-type (il5+/+), IL-5 knock-out (il5−/−), and phosphate-buffered saline–, rmIL-5–, IgG-, and TRFK5-treated il5+/+ C57BL/6 mice (n = 7/group) 14 days after intrapleural delivery of LLC cells. Endogenous IL-5 deficiency enhances, exogenous IL-5 delivery decreases, and IL-5 blockade increases pleural tumor cell apoptosis. Microphotographs show representative images of staining from il5+/+ and il5−/− mice (inlays = isotype controls; bars = 50 μm; original magnification ×400; brown = immunoreactivity; blue = nuclear hematoxylin counterstaining). Columns = mean; bars = SE. *P < 0.05, **P < 0.01, and ***P < 0.001, respectively, for comparison with appropriate control. fVIIIra = factor VIII–related antigen; Ig = immunoglobulin; LLC = Lewis lung cancer; PBS = phosphate-buffered saline; PCNA = proliferating cell nuclear antigen; rm = recombinant mouse; TRFK5 = anti–IL-5 neutralizing antibody; TUNEL = terminal deoxynucleotidyl nick-end labeling.
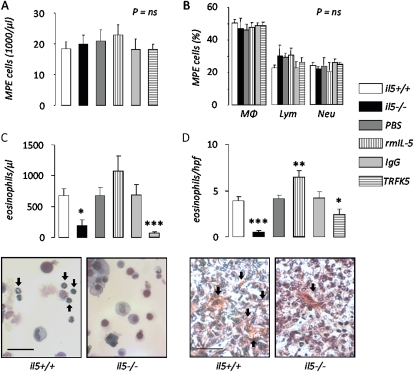
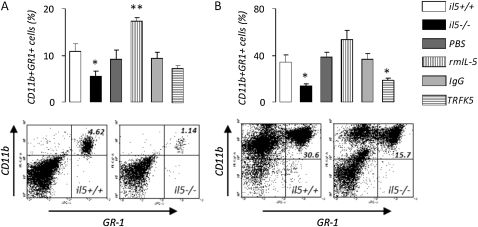
We also investigated inflammatory cells in MPEs from il5+/+ and il5−/−, and PBS-, rmIL-5–, IgG-, and TRFK5-treated mice. We found no differences in the absolute or relative abundance of macrophages, lymphocytes, and neutrophils (Figures 6A and 6B). However, we found significantly decreased numbers of eosinophils in MPE fluid from il5−/− mice and significantly lower numbers of these cells in MPEs from mice treated with TRFK5 (Figure 6C). These findings were recapitulated when pleural tumor tissue was examined for eosinophils using the Luna technique (39): significantly decreased numbers of these cells were observed in pleural tumor tissue from il5−/− mice compared with il5+/+ mice (Figure 6D). Mice treated with rmIL-5 had significantly higher numbers of eosinophils in pleural tumors and mice treated with TRFK5 had significantly lower numbers of eosinophils in pleural tumors, compared with the respective controls. Based on previous reports of experimental tumor promotion by CD11b+GR1+ myeloid suppressor cells (41), we examined the numbers of these cells in blood and MPE from our experimental mice (Figure 7). We found significantly decreased numbers of myeloid suppressor cells in MPE and blood from il5−/− compared with il5+/+ mice. In addition, mice treated with rmIL-5 showed increased circulating suppressor cells in the blood, and mice treated with TRFK5 had significantly lower numbers of these cells in MPE fluid, compared with respective controls. Collectively, these results suggested that enhanced eosinophil and myeloid suppressor cell recruitment may contribute to the MPE-promoting effects of host-derived IL-5.
Figure 6.
IL-5 promotes eosinophil accumulation in malignant pleural effusion (MPE) and pleural tumors. Total nucleated cells (A), differential cell counts (B), and eosinophil number (C) in MPEs and eosinophil counts in pleural tumor tissue (D) obtained from wild-type (il5+/+), IL-5 knock-out (il5−/−), and phosphate-buffered saline–, rmIL-5–, IgG-, and TRFK5-treated il5+/+ C57BL/6 mice (n = 17, 13, 7, 7, 7, and 11, respectively) 14 days after intrapleural delivery of LLC cells. IL-5 deficiency ablates, exogenous IL-5 enhances, and IL-5 neutralization inhibits eosinophil recruitment to MPE. Microphotographs show representative images of MPE (left, May-Gruenwald-Giemsa stain; bar = 50 μm; original magnification ×400; arrows, eosinophils) and tissue (right, Biebrich scarlet stain; bar = 50 μm; original magnification ×400; arrows, eosinophils) eosinophil staining from il5+/+ and il5−/− mice. Columns = mean; bars = SE. *P < 0.05, **P < 0.01, and ***P < 0.001, respectively, for comparison with appropriate control. Ig = immunoglobulin; LLC = Lewis lung cancer; Lym = lymphocytes; MΦ = macrophages; MPE = malignant pleural effusion; Neu = neutrophils; PBS = phosphate-buffered saline; rm = recombinant mouse; TRFK5 = anti–IL-5 neutralizing antibody.
Figure 7.
IL-5 promotes myeloid suppressor cell recruitment to malignant pleural effusion (MPE). CD11b+GR-1+ myeloid suppressor cells in (A) blood and (B) MPEs obtained from wild-type (il5+/+), IL-5 knock-out (il5−/−), and phosphate-buffered saline–, rmIL-5–, IgG-, and TRFK5-treated il5+/+ C57BL/6 mice (A: n = 8, 8, 5, 3, 5, and 4, respectively; B: n = 8, 3, 5, 3, 5, and 4, respectively) 14 days after intrapleural delivery of LLC cells. IL-5 deficiency decreases, exogenous IL-5 enhances, and IL-5 neutralization inhibits recruitment of these cells in response to MPE. Images show representative flow cytometry results of blood (left; numbers, percentage of cells in quadrant) and MPE (right; numbers, percentage of cells in quadrant) from il5+/+ and il5−/− mice. Columns = mean; bars = SE. *P < 0.05 and **P < 0.01, respectively, for comparison with appropriate control. Ig = immunoglobulin; LLC = Lewis lung cancer; MPE = malignant pleural effusion; PBS = phosphate-buffered saline; rm = recombinant mouse; TRFK5 = anti–IL-5 neutralizing antibody.
DISCUSSION
In these studies, we identified both IL-5 and eosinophils in MPEs from humans and immunocompetent mice, and showed that IL-5 plays an important role in formation and progression of experimental MPE. Importantly, host-derived rather than tumor-derived IL-5 seems to regulate MPE through alterations on the pleural microenvironment. Moreover, our results indicate that host-derived IL-5 does not exert direct effects on cancer cells, but rather on other host cells. In this connection, we observed enhanced eosinophil and myeloid suppressor cell recruitment to experimental MPE in an IL-5–dependent manner. Although IL-5 is well known as a chemotactic agent for eosinophil recruitment, it is uncertain whether IL-5 also exerts direct effects to recruit myeloid suppressor cells. The presence of these leukocytes in pleural tumors and MPEs was associated with reduced tumor cell apoptosis in vivo. Collectively, our data support a model in which pleural invasion by adenocarcinoma cells induces IL-5 expression by the host, leading to recruitment of eosinophils and myeloid suppressor cells (and possibly other immune and inflammatory cells) that facilitate tumor cell survival in the pleural space and enhance vascular permeability, leading to MPE.
Although these studies support the involvement of IL-5 in the pathogenesis of experimental adenocarcinoma-induced MPE, the relevance of our findings to human MPE remains to be fully clarified. We identified significant numbers (>10%) of eosinophils in 13% and levels of IL-5 in 41% of human MPEs, suggesting that IL-5 could impact tumor progression in a substantial percentage of human MPEs. Indeed, the low concentration of IL-5 and relatively small number of eosinophils in our experimental MPE models support the idea that IL-5 protumorigenic pathways may be broadly relevant to human MPEs and not limited to the fraction of MPEs clinically defined as eosinophilic.
Our results are consistent with recent evidence that supports a protumorigenic role for inflammatory cells and pathways in epithelial carcinogenesis, tumor progression, and metastasis (16–19). However, the precise role of the various inflammatory cells and mediators in each step of tumor progression is largely unknown. This is especially true for MPE, because of the lack, until recently, of immunocompetent animal models (12, 13, 38, 42). Experimental MPE progression is intimately associated with an inflammatory response, and several tumor-specific MPE-promoting pathways are proinflammatory, including VEGF/VEGFR1, IL-6/signal transducer and activator of transcription-3, and NF-κB/TNF-α/MCP-1 (13–15, 22–24). Available data suggest that: (1) the inflammatory response that accompanies MPE may actually promote it (13–15, 22–24); (2) this effect of inflammation may be greater with MPE compared with solid tumors, a difference possibly explained by the pleural microenvironment, which is a privileged niche for inflammation and vascular hyperpermeability (42); and (3) tumor and host components contribute to the inflammatory cascade in MPE, with each playing distinct roles (42, 43). Although prior studies have identified several tumor cell–specific biologic pathways that promote experimental MPE by modifying the host immune and inflammatory response, to our knowledge this is the first example of a host factor (IL-5) that enhances experimental MPE progression by modulating the inflammatory response.
IL-5 is important in allergic inflammatory responses, eosinophil recruitment, and regulation of eosinophil lifespan (25). Prior information suggesting a possible role for the IL-5/eosinophil axis in progression of malignancies includes (1) the presence of eosinophils in MPE and other tumors (30, 44); (2) the role of IL-5 in tumor-associated eosinophilia (26–29); (3) the oncogenic effects of IL-5Rβ (or βc) signaling in hematopoietic cells (45); and (4) the observation that IL-5–dependent eosinophil recruitment in response to eukaryotic microorganisms is not always protective (46, 47). Indeed, our results using genetic and cytokine/antibody-based approaches all converged toward a marked MPE-promoting effect of IL-5. Although most studies have focused on the impact of IL-5 on eosinophil differentiation, trafficking, and tissue survival (25), IL-5 has also been shown to be involved in mononuclear cell recruitment to inflammatory sites (46). It is important to determine in future studies whether the important role for IL-5 in experimental MPE formation is mediated primarily through eosinophil recruitment and activation; recruitment of other immune inflammatory cells (like the myeloid suppressor cells); or other effects of IL-5 signaling.
In experimental MPE, recruitment of eosinophils and CD11b+GR-1+ myeloid suppressor cells is associated with IL-5 production. Eosinophils are seminal players in allergic inflammation and host immune responses against eukaryotic microorganisms (25). Recent experimental work and older clinical observation have linked these cells with enhanced progression of solid and hematologic malignancies (26–30, 48), although the nature of this relationship is not well defined. Similarly, CD11b+GR-1+ myeloid suppressor cells are tumor-promoting bone marrow–derived inflammatory cells produced in response to tumors (41). These cells are important mediators of tumor-induced immune dysfunction via suppressing effector T cells (49). Based on the absence of direct effects of IL-5 on tumor cells, we examined the abundance of these cells in our experimental animals. We found consistent increases in the relative abundance of myeloid suppressor cells in MPE and blood from mice with increased IL-5 bioactivity. On the contrary, genetic and antibody-mediated interventions to reduce IL-5 bioactivity resulted in decreased numbers of these cells. We speculate that IL-5 may be involved in the differentiation of myeloid suppressor cells, because its receptor shares a common β-subunit with the granulocyte-macrophage colony–stimulating factor, the primary stimulus for their development in the bone marrow of patients with cancer (49). Hence, recruitment of specific inflammatory cell populations by IL-5 is likely to be critical for mediating the intrapleural fluid accumulation and tumor dissemination in experimental MPE.
Conclusions
Herein we describe an unprecedented biologic effect of host-derived IL-5 in malignant progression to form MPE. Because this cytokine is present in a significant proportion of human MPEs, it is possible that IL-5 may function to promote the human condition. Because anti–IL-5 directed therapies are currently being tested for benign human diseases, the present work may serve, pending further validation, to set a rational framework for future trials of IL-5 neutralization against malignant pleural disease.
Supplementary Material
Originally Published in Press as DOI: 10.1164/rccm.201001-0001OC on July 1, 2010
Supported by National Institutes of Health grant HL61419, a Lung SPORE Discovery grant, and the Department of Veterans Affairs (T.S.B.), and the Greek State Scholarship Foundation (I.K.Y.) and the Thorax Foundation, Athens, Greece (G.T.S.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author Disclosure: G.T.S. received industry-sponsored grants from Wyeth Hellas SA and Novartis Hellas SA for past funding of research projects for $10,001–$50,000 each. T.P.S. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.P.K. received industry-sponsored grants from Wyeth Hellas SA and Novartis Hellas SA for past funding of research projects for $10,001–$50,000 each. K.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. I.K. received lecture fees from Schering-Plough for $1001–$5000 and industry-sponsored grants from Wyeth Hellas SA for $50,001–$100,000, and from Novartis Hellas SA and GlaxoSmithKline for $10,001–$50,000 each. C.R. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.F. received a sponsored grant from the Department of Defense for $50,001–$100,000 and her spouse/life partner from the National Institutes of Health for more than $100,001. F.E.Y. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.S.P. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.S.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Light RW. Clinical practice: pleural effusion. N Engl J Med 2002;346:1971–1977. [DOI] [PubMed] [Google Scholar]
- 2.Antony VB, Loddenkemper R, Astoul P, Boutin C, Goldstraw P, Hott J, Rodriguez Panadero F, Sahn SA. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987–2001. [DOI] [PubMed] [Google Scholar]
- 3.Light RW, Lee YCG. Textbook of pleural diseases, 2nd ed. London: Hodder and Arnold; 2008.
- 4.Antunes G, Neville E, Duffy J, Ali N, on behalf of the BTS Pleural Disease Group. BTS guidelines for the management of malignant pleural effusions. Thorax 2003;58:ii29–ii38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugiura S, Ando Y, Minami H, Ando M, Sakai S, Shimokata K. Prognostic value of pleural effusion in patients with non-small cell lung cancer. Clin Cancer Res 1997;3:47–50. [PubMed] [Google Scholar]
- 6.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710–1717. [DOI] [PubMed] [Google Scholar]
- 7.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L, International Association for the Study of Lung Cancer International Staging Committee. Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706–714. [DOI] [PubMed] [Google Scholar]
- 8.Jett JR, Scott WJ, Rivera MP, Sause WT. Guidelines on treatment of stage IIIB non-small cell lung cancer. Chest 2003;123:221S–225S. [DOI] [PubMed] [Google Scholar]
- 9.Light RW. Talc for pleurodesis? Chest 2002;122:1506–1508. [DOI] [PubMed] [Google Scholar]
- 10.Maskell NA, Lee YC, Gleeson FV, Hedley EL, Pengelly G, Davies RJ. Randomized trials describing lung inflammation after pleurodesis with talc of varying particle size. Am J Respir Crit Care Med 2004;170:377–382. [DOI] [PubMed] [Google Scholar]
- 11.Burgers JA, Kunst PW, Koolen MG, Willems LN, Burgers JS, van den Heuvel M. Pleural drainage and pleurodesis: implementation of guidelines in four hospitals. Eur Respir J 2008;32:1321–1327. [DOI] [PubMed] [Google Scholar]
- 12.Lee YC, Wilkosz S. Malignant pleural effusions: fixing the leaky faucet. Am J Respir Crit Care Med 2008;178:3–5. [DOI] [PubMed] [Google Scholar]
- 13.Stathopoulos GT, Zhu Z, Everhart MB, Kalomenidis I, Lawson WE, Bilaceroglu S, Peterson TE, Mitchell D, Yull FE, Light RW, et al. Nuclear factor-κB affects tumor progression in a mouse model of malignant pleural effusion. Am J Respir Cell Mol Biol 2006;34:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stathopoulos GT, Kollintza A, Moschos C, Psallidas I, Sherrill TP, Pitsinos EN, Vassiliou S, Karatza M, Papiris SA, Graf D, et al. Tumor necrosis factor-α promotes malignant pleural effusion. Cancer Res 2007;67:9825–9834. [DOI] [PubMed] [Google Scholar]
- 15.Stathopoulos GT, Psallidas I, Moschos C, Moustaki A, Kollintza A, Karabela S, Porfyridis I, Vassiliou S, Karatza M, Zhou Z, et al. A central role for tumor-derived monocyte chemoattractant protein-1 in malignant pleural effusion. J Natl Cancer Inst 2008;100:1464–1476. [DOI] [PubMed] [Google Scholar]
- 16.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell 2004;6:297–305. [DOI] [PubMed] [Google Scholar]
- 17.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461–466. [DOI] [PubMed] [Google Scholar]
- 18.Stathopoulos GT, Sherrill TP, Cheng DS, Scoggins RM, Han W, Polosukhin VV, Connelly L, Yull FE, Fingleton B, Blackwell TS. Epithelial nuclear factor-κB activation promotes urethane-induced lung carcinogenesis. Proc Natl Acad Sci USA 2007;104:18514–18519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stathopoulos GT, Sherrill TP, Han W, Sadikot RT, Yull FE, Blackwell TS, Fingleton B. Host nuclear factor kappaB activation potentiates lung cancer metastasis. Mol Cancer Res 2008;6:364–371. [DOI] [PubMed] [Google Scholar]
- 20.Weissflog D, Kroegel C, Luttmann W, Grahmann PR, Hasse J. Leukocyte infiltration and secretion of cytokines in pleural drainage fluid after thoracic surgery: impaired cytokine response in malignancy and postoperative complications. Chest 1999;115:1604–1610. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-García MA, Cases-Viedma E, Cordero-Rodríguez PJ, Hidalgo-Ramírez M, Perpiñá-Tordera M, Sanchis-Moret F, Sanchis-Aldás JL. Diagnostic utility of eosinophils in the pleural fluid. Eur Respir J 2000;15:166–169. [DOI] [PubMed] [Google Scholar]
- 22.Kalomenidis I, Light RW. Eosinophilic pleural effusions. Curr Opin Pulm Med 2003;9:254–260. [DOI] [PubMed] [Google Scholar]
- 23.Yano S, Shinohara H, Herbst RS, Kuniyasu H, Bucana CD, Ellis LM, Fidler IJ. Production of experimental malignant pleural effusions is dependent on invasion of the pleura and expression of vascular endothelial growth factor/vascular permeability factor by human lung cancer cells. Am J Pathol 2000;157:1893–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh H-H, Lai W-W, Chen HHW, Liu H-S, Su W-C. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene 2006;25:4300–4309. [DOI] [PubMed] [Google Scholar]
- 25.Wardlaw AJ, Kay AB. Eosinophils and their disorders. In: Beutler E, Lichtman MA, Coller BS, Kipps TJ, Seligsohn U, editors. Williams' hematology, 6th ed. New York: McGraw-Hill; 2001. p. 785.
- 26.Isaacson NH, Rapoport P. Eosinophilia in malignant tumors: its significance. Ann Intern Med 1946;25:893. [DOI] [PubMed] [Google Scholar]
- 27.Samoszuk M. Eosinophils and human cancer. Histol Histopathol 1997;12:807–812. [PubMed] [Google Scholar]
- 28.Samoszuk M, Hansen L. Detection of interleukin 5 messenger RNA in Reed-Sternberg cells of Hodgkin's disease with eosinophilia. Blood 1990;75:13–16. [PubMed] [Google Scholar]
- 29.Walter R, Joller-Jemelka HI, Salomon F. Metastatic squamous cell carcinoma with marked blood eosinophilia and elevated serum interleukin-5 levels. Exp Hematol 2002;30:1–2. [DOI] [PubMed] [Google Scholar]
- 30.Wong DT, Bowen SM, Elovic A, Gallagher GT, Weller PF. Eosinophil ablation and tumor development. Oral Oncol 1999;35:496–501. [DOI] [PubMed] [Google Scholar]
- 31.Asquith KL, Ramshaw HS, Hansbro PM, Beagley KW, Lopez AF, Foster PS. The IL-3/IL-5/GM-CSF common receptor plays a pivotal role in the regulation of Th2 immunity and allergic airway inflammation. J Immunol 2008;180:1199–1206. [DOI] [PubMed] [Google Scholar]
- 32.Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest 2004;114:427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schumacher JH, O'Garra A, Shrader B, van Kimmenade A, Bond MW, Mosmann TR, Coffman RL. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol 1988;141:1576–1581. [PubMed] [Google Scholar]
- 34.Porcel JM. Establishing a diagnosis of pleural effusion due to heart failure. Respirology 2009;14:471–473. [DOI] [PubMed] [Google Scholar]
- 35.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Köhler G, Young IG, et al. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 1996;4:15–24. [DOI] [PubMed] [Google Scholar]
- 36.Schwarze J, Cieslewicz G, Hamelmann E, Joetham A, Shultz LD, Lamers MC, Gelfand EW. IL-5 and eosinophils are essential for the development of airway hyperresponsiveness following acute respiratory syncytial virus infection. J Immunol 1999;162:2997–3004. [PubMed] [Google Scholar]
- 37.Garlisi CG, Kung TT, Wang P, Minnicozzi M, Umland SP, Chapman RW, Stelts D, Crawley Y, Falcone A, Myers JG, et al. Effects of chronic anti-interleukin-5 monoclonal antibody treatment in a murine model of pulmonary inflammation. Am J Respir Cell Mol Biol 1999;20:248–255. [DOI] [PubMed] [Google Scholar]
- 38.Stathopoulos GT, Moschos C, Loutrari H, Kollintza A, Psallidas I, Karabela S, Magkouta S, Papiris SA, Roussos C, Kalomenidis I. Zoledronic acid is effective against experimental malignant pleural effusion. Am J Respir Crit Care Med 2008;178:50–59. [DOI] [PubMed] [Google Scholar]
- 39.Eversole RR, Mackenzie CD, Beuving LJ. A photoreactive fluorescent marker for identifying eosinophils and their cytoplasmic granules in tissues. J Histochem Cytochem 2003;51:253–257. [DOI] [PubMed] [Google Scholar]
- 40.Orlic D, Anderson S, Biesecker LG, Sorrentino BP, Bodine DM. Pluripotent hematopoietic stem cells contain high levels of mRNA for c-kit, GATA-2, p45 NF-E2, and c-myb and low levels or no mRNA for c-fms and the receptors for granulocyte colony-stimulating factor and interleukins 5 and 7. Proc Natl Acad Sci USA 1995;92:4601–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest 2006;116:2777–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stathopoulos GT, Lee YCG. Experimental models: pleural disease other than mesothelioma. In: Light RW, Lee YCG, editors. Textbook of pleural diseases, 2nd ed. London: Hodder and Arnold; 2008. p. 169–186.
- 43.Stathopoulos GT, Kalomenidis I. Animal models of malignant pleural effusion. Curr Opin Pulm Med 2009;15:343–352. [DOI] [PubMed] [Google Scholar]
- 44.Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur SI, O'Neill K, Colbert D, et al. Pivotal advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol 2006;79:1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCormack MP, Gonda TJ. Myeloproliferative disorder and leukaemia in mice induced by different classes of constitutive mutants of the human IL-3/IL-5/GM-CSF receptor common b subunit. Oncogene 1999;18:7190–7199. [DOI] [PubMed] [Google Scholar]
- 46.Huffnagle GB, Boyd MB, Street NE, Lipscomb MF. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J Immunol 1998;160:2393–2400. [PubMed] [Google Scholar]
- 47.Kirman J, Zakaria Z, McCoy K, Delahunt B, Le Gros G. Role of eosinophils in the pathogenesis of Mycobacterium bovis BCG infection in gamma interferon receptor-deficient mice. Infect Immun 2000;68:2976–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horiuchi H, Weller PF. Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am J Respir Cell Mol Biol 1997;17:70–77. [DOI] [PubMed] [Google Scholar]
- 49.Frey AB. Myeloid suppressor cells regulate the adaptive immune response to cancer. J Clin Invest 2006;116:2587–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.