1. INTRODUCTION
Retinal prosthetics are designed to restore vision in patients with photoreceptor degenerative diseases, such as retinitis pigmentosa (RP) and macular degeneration. Subretinal microphotodiode arrays (MPAs), which response to incident light in a gradient fashion, have been designed to replace degenerating photoreceptors. Such devices have been implanted into rats (Ball et al., 2001), cats (Chow et al., 2001) and humans (Chow et al., 2004). These studies have revealed that implantation of a MPA device is capable of restoring some visual function in patients (Chow et al., 2004) and eliciting a superior colliculus response in normal and degenerating rats (DeMarco et al., 2007). Furthermore, the low level electrical stimulation produced by the MPA device has been shown to have neuroprotective properties (Pardue et al., 2005).
When RCS rats are implanted with an MPA device at the beginning of the degenerative process, photoreceptor function and morphology are preserved (Pardue et al., 2005). Subretinal electrical stimulation may provide protection to the photoreceptors by stimulating the selective expression of FGF-2 in the RCS rat (Ciavatta et al., 2006). While these studies show promise for subretinal electrical stimulation as a treatment of RP, implantation of MPA devices in S334ter rats does not preserve photoreceptors (Walker et al., 2005). We hypothesize that this may be due to the underlying mutations between RCS and S334ter rats. RCS rats have a recessive mutation in a tyrosine kinase gene, Mertk, which results in failed phagocytosis of shed outer segments by the retinal pigment epithelium (Mullen et al., 1976; D’Cruz et al., 2000); while S334ter rats have a rhodopsin mutation which leads to photoreceptor death (Lee et al., 2003).
Rat models with photoreceptor degeneration are few while there are numerous mouse models of RP that have been described (Chang et al., 2002; Dalke and Graw, 2005). Thus, to further elucidate whether the neuroprotective effect of subretinal electrical stimulation is generalized to all types of photoreceptor degeneration, implantation of mouse models of RP would be advantageous. This study describes the development of surgical techniques and the success of implanting a small mouse eye with a subretinal MPA device.
2. METHODS
2.1. Experimental and Implant Design
Adult wild-type C57Bl/6J mice (n=6) were obtained from an in-house breeding colony originating from mice purchased from Jackson Laboratories (Bar Harbor, ME). Mice were implanted at 21–28 days of age and retinal function was measured every two weeks until 8 weeks post-implantation. After the final measurement, mice were euthanized and the eyes enucleated for histological assessment. All procedures were approved by the local Institutional Animal Care and Use Committee and carried out in accordance with the Association for Research in Vision and Ophthalmology statement concerning the use of animals in ophthalmic and vision research.
The MPA device was identical in electrical properties to devices described previously (Chow et al., 2001). Briefly, each device consisted of a silicon disk covered on the top side with a microphotodiode array. However, the devices were manufactured in a smaller size to accommodate the small mouse eye, measuring 23µm thick and 0.5mm in diameter.
2.2 Surgical Procedures
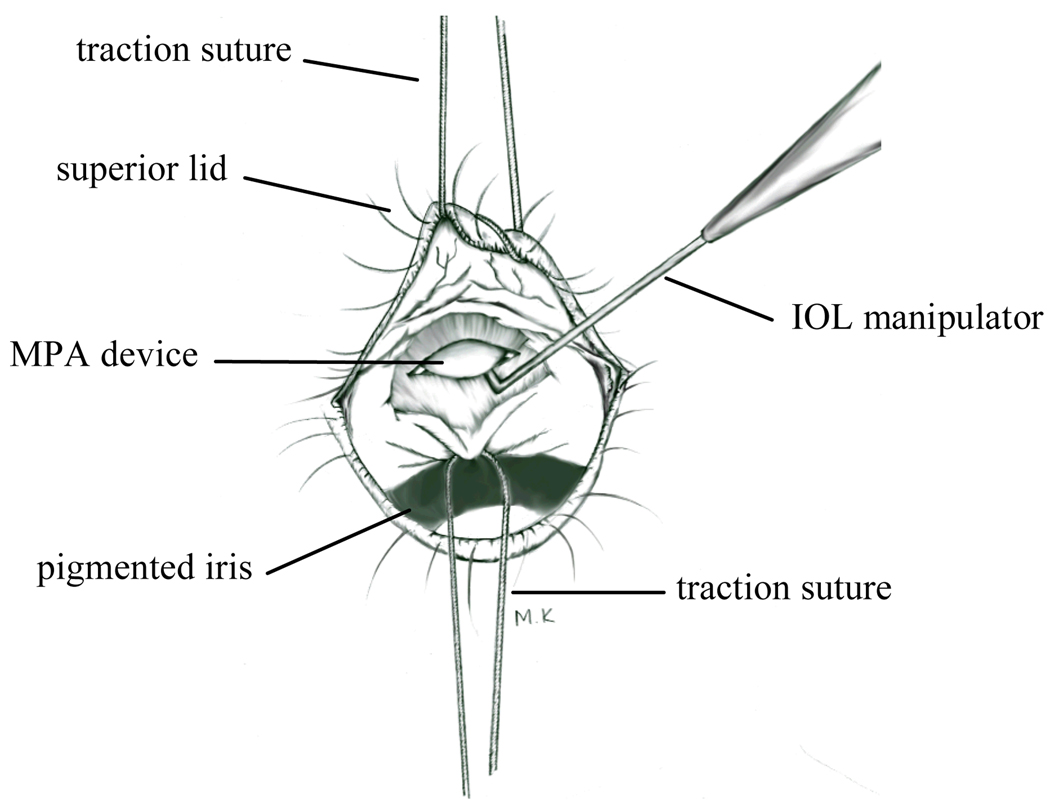
Surgical procedures were similar to that described for the rat eye (Ball et al., 2001). After dilation of the pupils (1% mydriacyl, 2.5% phenylephrine) and anaesthetization of the cornea (0.5% tetracine HCl), the anaesthetized (ketamine 80 mg/kg; xylazine 16 mg/kg) mouse was placed on a heating pad. A traction suture (8-0) placed in the superior lid was used to retract the upper lid while a second traction suture placed in the superior limbus was used to rotate the eye inferiorly (Figure 1). The conjunctiva and underlying tenon capsule were opened using iris spring scissors to reveal the superior limbus. The tip of a 16 gauge stiletto blade was used to make a 0.6 mm long incision through the sclera, choroids, RPE, and retina, about 1 mm posterior to the limbus (Figure 1). The eye was wet with saline (0.9% NaCl) and the retina was allowed to detach naturally along the incision over a period of 10 minutes, after which the implant was gently manipulated into the subretinal space using the tips of two IOL manipulators. The eye was then rotated back to primary position and the fundus was examined to confirm subretinal placement of the device. A drop of antibiotic solution was applied to the eye (Neosporin Ophthalmic Solution) and the mouse was given yohimbine (2.1 mg/kg) to reverse the effects of the xylazine and speed recovery from anesthesia (Turner and Albassam, 2005).
Figure 1.
Sketch of the mouse eye during surgery of the subretinal MPA device. The eye is rotated inferiorly and the superior sclera is exposed. See text for details of surgery.
2.3 Electroretinography (ERG)
Retinal function was monitored by recording electroretinograms (ERGs) with an automated system (LKC system; Gaithersburg, MD). After overnight dark-adaptation, mice were anesthetized (ketamine 80 mg/kg; xylazine 16 mg/kg) and their pupils dilated (1% mydriacyl, 2.5% phenylephrine) under dim red light. Mice were placed on a homeothermic heating pad inside a Faraday cage. ERGs were recorded from both eyes simultaneously with a nylon fiber electrode contacting the center of the cornea through a layer of 1% methylcellulose. Platinum needle electrodes placed in the cheek and tail served as reference and ground, respectively.
Dark-adapted responses to stimuli from a Ganzfeld xenon flash lamp were recorded in a series presented in order of increasing strength, ranging from 0.001 to 137 cd s/m2. The interstimulus intervals were increased from 15 to 60 seconds with increasing flash strenth. To record cone-mediated responses, mice were light-adapted for 10 minutes (30 cd/m2) and then presented with a series of flashes at 2.1 Hz, ranging from 0.15 to 75 cd s/m2.
2.4 Histology
After eight weeks of implantation, mice were euthanized and their eyes enucleated. Each eye was marked on the corneal surface to indicate its orientation and was immersion fixed overnight in 2% paraformaldehyde/ 2.5% glutaraldehyde. Eyes were rinsed in phosphate buffer, dehydrated through a graded ethanol series and embedded in plastic resin (Embed 812/Der736; Electron Microscopy Science, Hatfield, PA). Retinal sections were cut on an ultramicrotome (Reichert; 0.5 µm) and stained with toluidine blue. Morphological analysis was made by examining retinal layers adjacent and overlying the MPA device.
3. RESULTS
3.1 Retinal Function
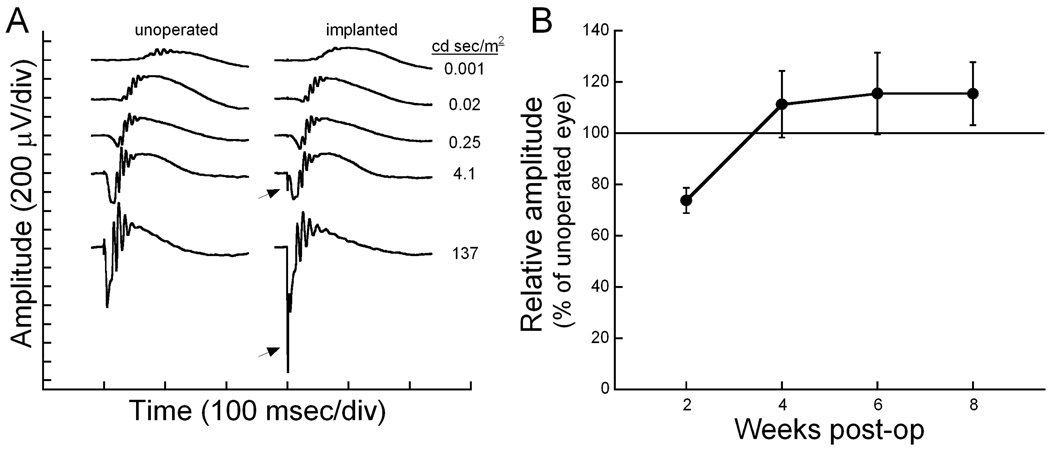
Implantation of a subretinal MPA device produced no significant differences in retinal function between the implanted and opposite (unoperated) eye. Figure 2A shows the dark-adapted ERG waves recorded from a mouse in which one eye was implanted with the MPA device while the other eye served as an unoperated control. Amplitude and implicit time of both the a- and b-waves are similar between the two eyes. Note that the electrical activity of the implant is seen as a fast negative spike in the ERG trace. As expected, the microphotodiodes respond in a gradient fashion to the incident light, producing an implant spike that becomes larger with increasing intensities. At the brightest flash intensities, the implant spike amplitude was 180 µV (4.1 cd sec/m2) and 1300 µV (137 cd sec/m2).
Figure 2.
A. Dark-adapted ERG waves to a series of flash intensities, as indicated on the right. The electrical activity of the implant (arrow) can be seen in the two brightest flash intensities as a fast (0.5 msec) spike. B. Plot of the dark-adapted ERG b-wave response to a bright stimulus (137 cd sec/m2) across the follow-up period. The average amplitude of the implanted eye is plotted in proportion to the opposite control eye. Error bars represent standard error of the mean.
Figure 2B shows the maximal dark-adapted b-wave amplitude across time. At two weeks after implantation, retinal function of the implanted eyes is reduced. Similar reductions in ERG amplitudes 1–2 weeks after subretinal surgery were reported previously for rats (Pardue et al., 2005) and cats (Chow et al., 2002). However, by 4 weeks after surgery, retinal function is equal to or greater than the opposite, unimplanted eye. Cone-mediated response also showed no differences between implanted and unoperated eyes.
3.2 Retinal Morphology
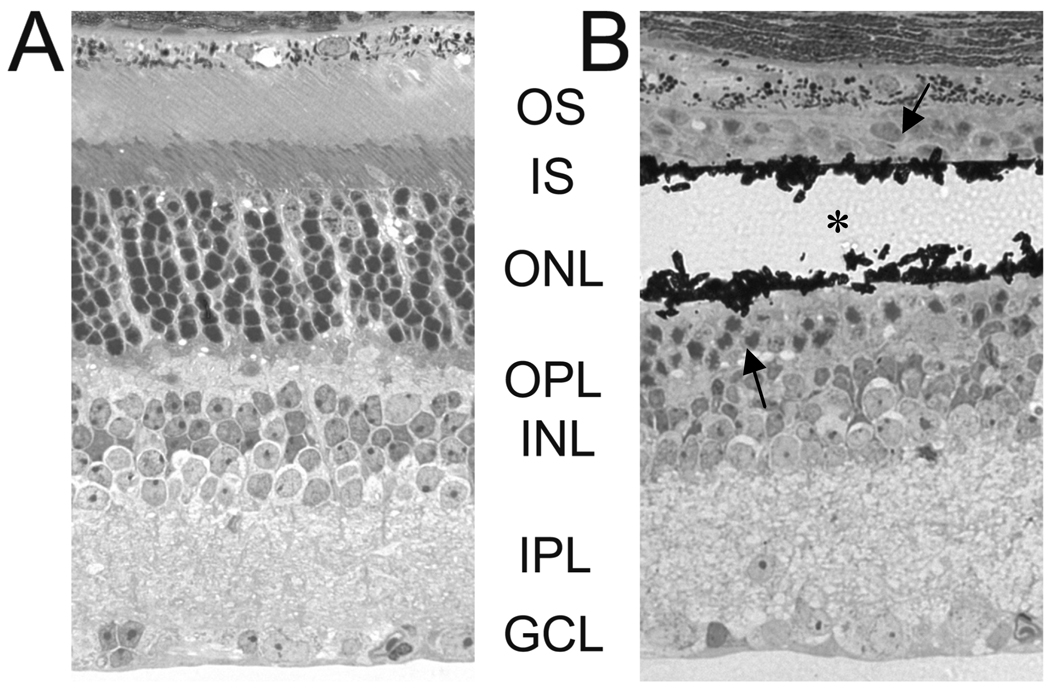
Figure 3 shows the histological appearance of an unoperated mouse retina (A) compared to retina directly overlying the MPA device (B). Areas adjacent to the implant had the same appearance as unoperated eyes, with all retinal layers intact. However, directly overlying the implant device, there is a localized loss of photoreceptors segments and decreased number of photoreceptor nuclei (Figure 3B). This same reaction has also been found in normal rats (Ball et al., 2001) and cats (Chow et al., 2001; Pardue et al., 2001) implanted with a subretinal MPA device. At 8 weeks post-op, photoreceptors are still present immediately adjacent to the implant. The inner retina has normal morphology and thickness in areas adjacent to and directly over the implant.
Figure 3.
Photomicrographs of mouse retina A) from an unoperated eye and B) directly overlying the implant. The black material in (B) is the remnants of the MPA device that shattered during sectioning. The asterisk indicates the position of the MPA device. The arrows indicate the remnants of photoreceptor nuclei around the implant.
4. DISCUSSION
These data demonstrate the feasibility of implanting a 0.5 mm subretinal MPA device into normal mouse eyes. The normal mouse retina reacts to a solid foreign object in the subretinal space in the same way as other mammalian species with dual retinal circulation: the photoreceptors nourished by the choroid degenerate. The loss of photoreceptors and subsequent inner retinal changes that have been documented in cats parallel the changes found in retinal detachment models (Pardue et al., 2001). Thus, we hypothesize that the solid nature of the MPA implant causes a localized retinal detachment. However, the presence of the MPA device and its electrical activity appear to be well tolerated by the retina as a whole, with no other indications of rejection, inflammation, or toxicity. With the demonstration of successful subretinal implantation of the normal mouse eye, various mouse models of retinal degeneration can now be tested to determine the generalized effect of subretinal electrical stimulation on photoreceptor preservation.
REFERENCES
- Ball SL, Pardue MT, Chow AY, Chow VY, Peachey NS. Subretinal implantation of photodiodes in rodent models of photoreceptor degeneration. In: Anderson RE RE, LaVail MM MM, Hollyfield JG, editors. New Insights into Retinal Degenerative Diseases. New York: Kluwer/Plenum; 2001. pp. 175–182. [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- Chow AY, Pardue MT, Chow VY, Peyman GA, Liang C, Perlman JI, Peachey NS. Implantation of silicon chip microphotodiode arrays into the cat subretinal space. IEEE Trans. Neural Syst. Rehabil. Eng. 2001;9:86–95. doi: 10.1109/7333.918281. [DOI] [PubMed] [Google Scholar]
- Chow AY, Pardue MT, Perlman JI, Ball SL, Chow VY, Hetling JR, Peyman GA, Liang C, Stubbs EB, Jr, Peachey NS. Subretinal implantation of semiconductor-based photodiodes: durability of novel implant designs. J. Rehabil. Res. Dev. 2002;39:313–321. [PubMed] [Google Scholar]
- Chow AY, Chow VY, Peyman GA, Packo KH, Pollack JS, Schuchard R. The artificial silicon retina™ (ASR™) chip for the treatment of vision loss from retinitis pigmentosa. Arch. Ophthalmol. 2004;122:460–469. doi: 10.1001/archopht.122.4.460. [DOI] [PubMed] [Google Scholar]
- Ciavatta VT, Chrenek M, Wong P, Nickerson JM, Pardue MT. Growth factor expression following implantation of microphotodiode arrays in RCS rats. ARVO E-abstract. 2006:3177. [Google Scholar]
- Dalke C, Graw J. Mouse mutants as models for congenital retinal disorders. Exp. Eye Res. 2005;81:503–512. doi: 10.1016/j.exer.2005.06.004. [DOI] [PubMed] [Google Scholar]
- D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene MertK in the retina dystrophic RCS rat. Hum. Mol. Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- DeMarco PJ, Jr, Yarbrough GL, Yee CW, McLean GY, Sagdullaev BT, Ball SL, McCall MA. Stimulation via a subretinally placed prosthetic elicits central activity and induces a trophic effect on visual responses. Invest. Ophthalmol. Vis. Sci. 2007;48:916–926. doi: 10.1167/iovs.06-0811. [DOI] [PubMed] [Google Scholar]
- Lee D, Geller S, Walsh N, Valter K, Yasumura D, Matthes M, LaVail M, Stone J. Photoreceptor degeneration in Pro23His and S334ter transgenic rats. Adv. Exp. Med. Biol. 2003;533:297–302. doi: 10.1007/978-1-4615-0067-4_36. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, LaVail MM. Inherited retinal dystrophy: primary defect in pigment epithelium determined with experiment rat chimeras. Science. 1976;192:799–801. doi: 10.1126/science.1265483. [DOI] [PubMed] [Google Scholar]
- Pardue MT, Phillips MJ, Yin H, Sippy BD, Webb-wood S, Chow AY, Ball SL. Neuroprotective effect of subretinal implants in the RCS rat. Invest. Ophthalmol. Vis. Sci. 2005;46:674–682. doi: 10.1167/iovs.04-0515. [DOI] [PubMed] [Google Scholar]
- Pardue MT, Stubbs EB, Jr, Perlman JI, Narfstrom K, Chow AY, Peachey NS. Immunohistochemical studies of the retina following long-term implantation with subretinal microphotodiode arrays. Exp. Eye Res. 2001;73:333–343. doi: 10.1006/exer.2001.1041. [DOI] [PubMed] [Google Scholar]
- Turner PV, Albassam MA. Susceptiblity of rats to corneal lesions after injectable anesthesia. Comp. Med. 2005;55:175–182. [PubMed] [Google Scholar]
- Walker TA, Faulkner AE, Cheng Y, Yin H, Fernandes A, Phillips MJ, Ball SL, Chow AY, Pardue MT. Subretinal implantation induces photoreceptor preservation in RCS rat not seen in wild-type long evans or S334ter rats. ARVO E-Abstract. 2005:5267. [Google Scholar]