Abstract
Carcinogen-DNA adducts could lead to mutations in critical genes, eventually resulting in cancer. Many studies have shown that retinoic acid (RA) plays an important role in inducing cell apoptosis. In this study we have tested the hypothesis that levels of carcinogen-DNA adducts can be diminished by DNA repair or/and by eliminating damaged cells through apoptosis. Our results showed that the levels of total DNA adducts in HepG2 cells treated with benzo(a)pyrene (BP, 2 μM) + RA (1 μM) were significantly reduced compared to those treated with BP only (P=0.038). In order to understand the mechanism of attenuation of DNA adducts, further experiments were performed. Cells were treated with BP (4 μM) for 24 h to initiate DNA adduct formation, following which the medium containing BP was removed, and fresh medium containing 1 μM RA was added. The cells were harvested 24 h after RA treatment. Interestingly, the levels of total DNA adducts were lower in the BP/RA group (390.46 ± 34.19) than those in the BP/DMSO group (543.70 ± 32.82), P=0.032. Analysis of cell apoptosis showed an increase in BP+RA group, compared to BP or RA only group. Our results also indicated that attenuation of BP-DNA adducts by RA was not primarily due to its effects on CYP1A1 expression. In conclusion, our results suggest a mechanistic link between cellular apoptosis and DNA adduct formation, phenomena that play important roles in BP-mediated carcinogenesis. Furthermore, these results help in the understanding of the mechanisms of carcinogenesis, especially in relation to the chemopreventive properties of nutritional apoptosis inducers.
Keywords: DNA adducts, HepG2, Benzo(a)pyrene, 32P-Postlabeling assay, DNA repair, Apoptosis
Introduction
Complex polycyclic aromatic hydrocarbons (PAH) mixtures may contain hundreds to thousands of compounds with different aromatic ring numbers. Numerous PAHs have been classified by the U.S. Environmental Protection Agency (EPA) as probable human carcinogens including model carcinogen benzo(a)pyrene (BP) (U.S., 2003). BP exhibits its biological effects through metabolic activation by cytochrome P450 enzymes to electrophilic species that are capable of reacting with nucleophilic sites of DNA to form adducts. DNA adducts represent premutagenic lesions, which play an essential role in the initiation phase of carcinogenesis through mutations of essential genes, ultimately leading to cancer. Covalent DNA adducts (modifications) are very sensitive and useful biomarkers for carcinogen exposure (Randerath et al., 1986; Randerath et al., 1989; Randerath et al., 1996; Randerath et al., 1997b; Randerath et al., 1999) and oxidative stress (Randerath et al., 1997a; Zhou et al., 2000; Zhou et al., 2001; Zhou et al., 2004). DNA adducts are formed directly by the covalent binding of carcinogens and mutagens or their metabolites to nitrogen bases in DNA, representing the initiation stage of carcinogenesis. The induction of mutations is due primarily to chemical alterations in the structure of DNA that results in inaccurate replication of a particular region of the genome (Parkinson, 2001). DNA adducts can be removed by DNA repair processes (Hang, 2004) and damaged cells can be eliminated by cell death including apoptosis (Green and Martin, 1995; Vermeulen et al., 2005), but during chronic exposures or high dose exposure to carcinogens such as smoking they often reach steady-state levels in carcinogen target tissues. Therefore, reduction in DNA adduct quantities by chemopreventive agents reduces tumor yield and it is very important measurement in cancer prevention.
Apoptosis is a physiological process characteristic of pluricellular organisms leading to self-destruction of the cell (Mohamad et al., 2005). Cell death is an essential strategy for the control of the dynamic balance in living system. Apoptosis involves the activation of an-energy-requiring intracellular machinery, which is tightly regulated and conserved throughout evolution (Yuan, 1996). Single cell undergo apoptosis independent of the fates of their neighbors (Josephy, 2006). Undesirable cells are eliminated without leakage of the intracellular contents to the surrounding medium and no immune response is therefore triggered. As a defense system, apoptosis exists in living organisms. Infected cells or cells with damaged DNA are removed by apoptosis (Roos and Kaina, 2006). Apoptosis is an important response to toxicity, but also a pivotal process in differentiation and organogenesis in multicellular organisms. Many chemical compounds such as retinoids (Lu et al., 1997; Sun et al., 2002; Arce et al., 2005), ω-3 fatty acids (Thompson, 1995; Chang et al., 1998; Hong et al., 2000; Hong et al., 2002), and polychlorinated biphenyls (PCBs) (Lee et al., 2003; Sanchez-Alonso et al., 2004) induce apoptosis in cells and are therefore known as apoptosis inducers. DNA repair, especially, the nucleotide excision repair (NER), is a major cellular response to damage induced by environmental carcinogens (Braithwaite et al., 1998). Benzo(a)pyrene diol epoxide (BPDE), which is converted from BP by phase I enzyme, can irreversibly damage DNA by the formation of DNA adducts. NER plays an important role to remove such adducts and restore DNA to normal structure (Sturgis and Wei, 2002).
Chemoprevention is defined as the use of specific natural and/or synthetic chemical agents to reverse or suppress carcinogenesis and prevent the development of invasive cancer (Hong and Lippman, 1995). The chemopreventive approach depends on the ability of certain chemical agents to block mutagenesis and control cellular differentiation and proliferation in epithelial tissues (Hong and Lippman, 1995). Retinoids are a group of compounds that regulate cell growth and differentiation. Retinoids have been used as chemopreventive and anticancer agents because of their pleiotropic regulator function in cell differentiation, growth, proliferation, DNA repair and apoptosis (Noy,; Mrass et al., 2004; Bogos et al., 2008; Gottschling et al., 2008). The effects of retinoids are thought to be mediated through the metabolite retinoic acid, which binds to nuclear retinoic acid receptors that then interact with specific retinoic acid response elements (Sankaranarayanan R, 1996). However, chronic toxicities from long term therapy with retinoids may result in some side-effects such as skeletal abnormalities and growth inhibition if used in children (David et al., 1988).
HepG2 cells, a metabolically competent human hepatoma cells, retain activities of phase I and phase II enzymes that are involved in the bioactivation (toxication) and detoxication of chemicals (Natarajan and Darroudi, 1991; Knasmuller et al., 1998). BP is a pro-carcinogen that needs to be biotransformed by cytochrom P450 1A/1B1 and epoxide hydroxylase to benzo(a)pyrene diol epoxide (BPDE), which is capable of forming DNA adducts. Therefore, this cell line is suitable to study DNA adduct formation induced by BP (Zhou et al., 2007). Here we have tested the hypothesis that levels of carcinogen-DNA adducts induced by BP can be diminished by eliminating damaged cells via augmentation of apoptosis.
Materials and methods
Chemicals
Benzo(a)pyrene (BP) and retinoic acid were obtained from Sigma-Aldrich (St. Louis, MO).
Cell Culture and Treatments
HepG2, an adherent type human hepatoma cell line (Knasmuller et al., 1998), was obtained from American Type Culture Collection (Manassas, VA, USA). HepG2 cells were maintained in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal calf serum, vitamins, essential and non-essential amino acids, 100 units/ml penicillin, and 100 μg/ml streptomycin. The stock culture was grown at 37°C in an atmosphere of 5% CO2 and 95% air under high humidity conditions. All chemicals were dissolved in DMSO. Cells were treated in duplicate or triplicate for all groups. Experiment 1: Cells incubated with 2 μM BP only or BP + 1 μM retinoic acid (RA) for 24 h. Then the medium was removed and the cells were washed twice with fresh medium. The cells were harvested by trypsinization and centrifugation (3,000 rpm for 5 min) and the pellets kept at -80°C until DNA extraction. Experiment 2: Cells were incubated with 4 μM BP and BP+RA for 24 h or 48 h. Experiment 3: HepG2 cells were challenged with 4 μM BP for 24 h. After which the medium containing BP was removed and washed twice. The cells were treated with RA for 24 h, and then harvested and processed as described above.
Protein Isolation and CYP1A1 Analysis
Ethoxyresorufin-O-deethylase (EROD), which predominantly reflects CYP1A1 activity was assayed in whole cell lysates essentially as described previously (Moorthy, 2000). Each experiment was run in triplicate.
Apoptosis Detection Assay
The cells were harvested and staining was carried out using the annexin V-FITC apoptosis detection kit (BioVision, Mountain View, CA). Briefly, 2 × 105 cells were resuspended in 1 × binding buffer and incubated with annexin V-FITC and propidium iodide (PI) for 5 min in darkness at room temperature. Annexin V FITC binding was analyzed by FACS Vantage SE DiVa flow cytometer (BD Biosciences, Franklin Lakes, NJ) equipped with FL1 detector (530/30 bandpass filter). Propidium iodide staining was detected on FL3 detector (610/20 bandpass filter). The results are based on a percentage of apoptotic cells of total gated cells (104 cells).
Western blot analysis
Cell lysates were obtained and equal amounts of protein from each sample were diluted with loading buffer, denatured, and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by protein transfer to polyvinylidene fluoride membranes (Amersham, Little Chalfont, UK). Proteins were detected by incubation with corresponding antibodies followed by blotting with secondary antibody. The blots were then exposed to chemiluminescent substrate (Amersham) for detection. This analysis was performed three times.
DNA Adduct Analyses
DNA was isolated by solvent extraction combined with enzymatic digestion of protein and RNA (Gupta, 1984) and stored at -80°C until use. The nuclease P1-enhanced bisphosphate version of the 32P-postlabeling assay was performed as described by Reddy (Reddy and Randerath, 1986) and Randerath (Randerath et al., 1999). Briefly, DNA (10 μg) was enzymatically degraded to normal (Np) and modified (Xp) deoxyribonucleoside 3′-monophosphates and their base-modified derivatives with a mixture of micrococcal endonuclease and spleen phosphodiesterase. After 3′-dephosphorylation of the normal nucleotides with nuclease P1, the enriched nuclease P1-resistant modified 3′-nucleotides were converted to 5′-32P-labeled deoxyribonucleoside 3′,5′-bisphosphate derivatives by incubation with carrier-free (γ-32P)ATP and T4 polynucleotide kinase. The radioactively labeled modified nucleotides were separated by multidirectional anion-exchange thin-layer chromatography (TLC) on polyethyleneimine (PEI)-cellulose sheets (Mabon et al., 1996). After removal of orthophosphate and traces of radioactive impurities by one-dimensional development (D1) with solvent 1 (Table 1), bulky labeled DNA adducts retained in the lower (2.8 × 1.0 cm) part of D1 chromatogram. The cut-out containing adducts were excised from the D1 chromatogram, then were contact-transferred to fresh thin-layer sheets and resolved by two-dimensional TLC with solvents 2 and 3 as outlined in Table 1. Radioactive spots were located by imaging using an Instant Imager from Packard Instrument Co. (800 Research Parkway, Meriden, CT 06450) and by screen-enhanced autoradiography on Kodak XAR-5 X-ray film at -80°C for ∼5 h. Radioactivities of TLC fractions from individual cell culture sample were determined with the aid of an Instant Imager (Zhou et al., 1999). Appropriate blank rates were automatically subtracted by the instrument from sample values. The extent of DNA adducts was calculated from corrected sample count rates (Reddy and Randerath, 1986). Quantitative data represented minimum estimates because 100% recovery presumably was not achieved. Statistical analysis of the data was performed by using the unpaired Student's t-test (Zar, 2009).
Table 1.
Solvents for thin-layer chromatography
| Solvent | Composition |
|---|---|
| 1 | 2.3 M sodium phosphate, pH 5.75 |
| 2 | 3.82 M lithium formate, 6.75 M urea, pH 3.35 |
| 3 | 0.72 M sodium phosphate, 0.40 M Tris-HCl, 7.65 M urea, pH 8.2 |
Results
HepG2 cells treated with BP or BP+RA exhibited qualitatively similar profiles of DNA adducts (Fig. 1). BP is a pro-carcinogen that is metabolically activated by CYP1A/1B1 enzymes and epoxide hydrolase to BPDE which binds to N2 of guanosine, and represents the major DNA adduct, BPDE-deoxyguanosine (BPDE-dG) dduct (Jeffrey et al., 1977; Randerath et al., 1998) (Fig. 1). In addition, BP also induces the formation of other minor adducts (Fig. 1).
Fig. 1.
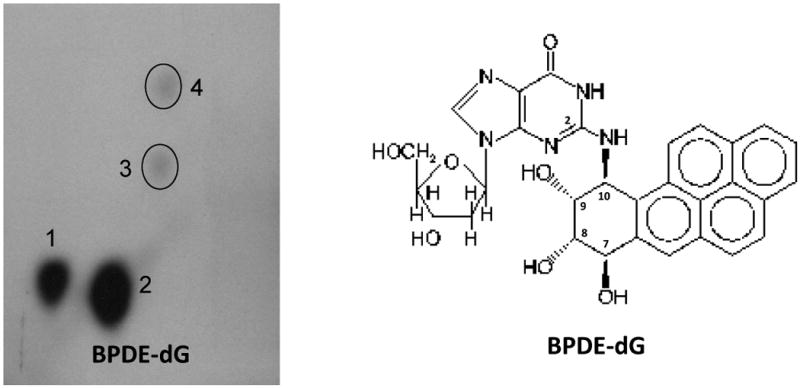
Typical pattern of 32P-postlabeled benzo(a)pyrene DNA adducts with a major adduct benzo(a)pyrene-7,8-dihyrodiol-9,10-epoxide deoxy-guanosine (BPDE-dG) in HepG2 cells. Cells were treated with BP (2 μM) for 24 hours. Screen-enhanced film exposure was performed with Kodak XAR-5 film at room temperature for 5 hours.
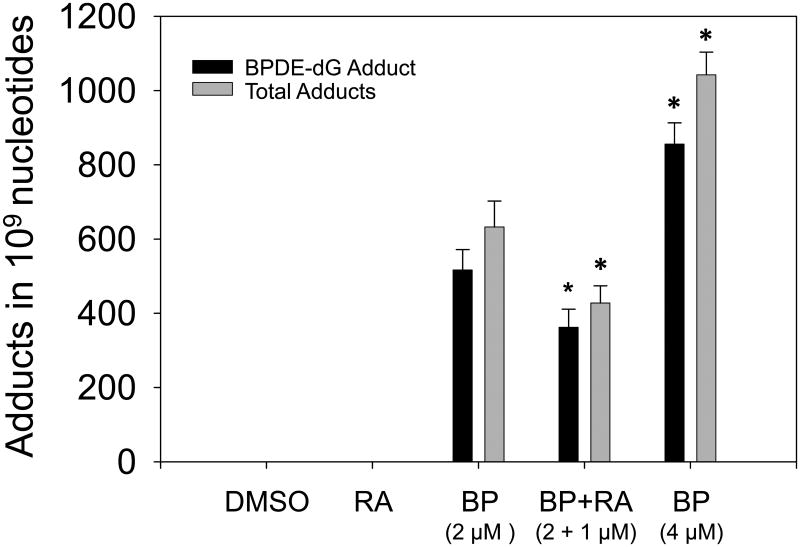
Treatment of HepG2 cells with BP (2 μM) for 24 h led to marked induction of DNA adducts, the levels of BPDE-dG and total DNA adducts (spots 1 to 4) being 516.63 ± 55.08 and 632.48 ± 69.77 adducts in 109 nucleotides, respectively (Fig. 2). Co-treatment of RA (1 μM) with BP (2 μM) significantly decreased BPDE-dG and total adducts after a 24 h incubation (Fig. 2). RAL values of BPDE-dG and total adducts in BP + RA group were 362.06 ± 49.00 and 427.70 ± 46.26 adducts in 109 nucleotides, respectively. Comparisons of levels of BPDE-dG or total adducts between BP only and BP+RA groups obtained P values 0.046 and 0.038, respectively. (Fig. 2).
Fig. 2.
Comparison of the levels of BPDE-dG and total DNA adducts in HepG2 cells. Cells were treated with BP (2 μM) or BP + RA (1 μM). Cells were harvested 24 h after treatment. RA decreased the levels of BPDE-dG and total adducts significantly, * P=0.046; ** P=0.038. RAL, Relative Adduct Labeling (adducted nucleotides/total nucleotides).
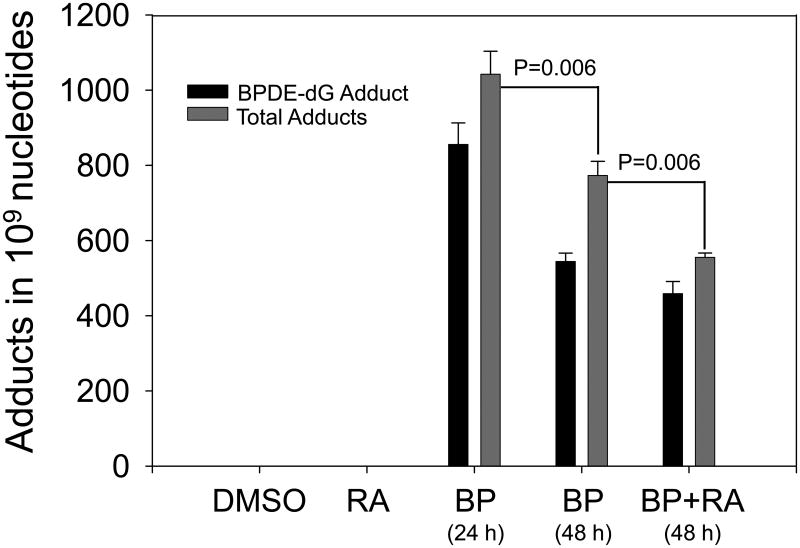
Dose-response was observed when cells treated with 4 μM BP for 24 h (Fig. 3) compared to 2 μM BP group (Fig. 2). Total adduct levels of 4 μM BP group were 1042.29 ± 61.36 in 109 nucleotides (Fig. 3). Adduct levels in 4 μM BP group was significant higher than those in 2 μM BP group (P=0.02). Time-response results displayed that 24 h cell incubation with BP obtained higher yields of DNA adducts compared to 48 h incubation. After 48 h incubation, adduct levels (773.05 ± 37.64) were diminished by about 26% compared to the 24 h time point (P=0.06). At 48 h, RA (1 μM) also diminished the levels of DNA adducts by about 30%. The difference of total DNA adduct levels between BP only (773.05 ± 37.64) and BP + RA groups (555.38 ± 11.47) was very significant (P=0.006).
Fig. 3.
Levels of BPDE-dG and total DNA adducts in HepG2 cells treated with 4 μM BP or 4 μM BP+1 μM RA for 24 h or 48 h. Compared to 24 h, total adduct levels in 48 h incubation were lower (*p=0.06). However, after 48 h incubation, BP+RA significantly decreased levels of total adducts compared to BP only (** P=0.006).
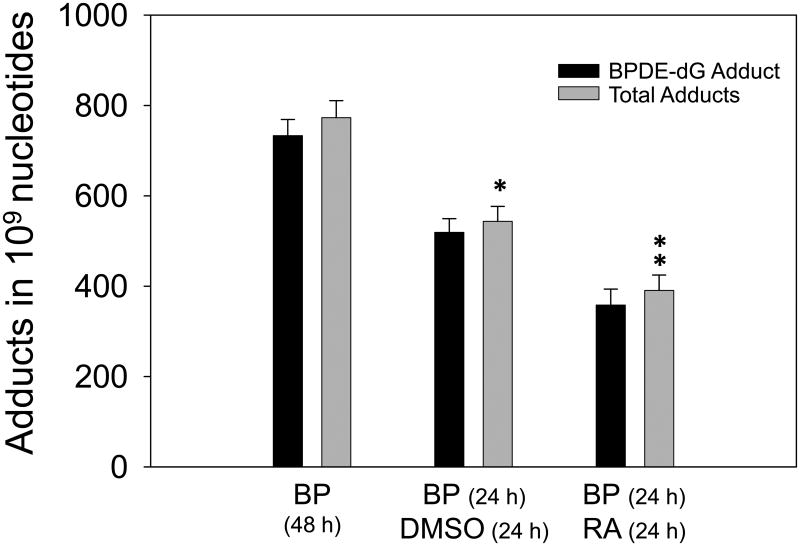
In order to study the mechanisms of modulation of BP-DNA adducts by RA, we carried out the following experiment. Since adduct levels were decreased with incubation time by about 30%, high dose of BP (4 μM) was selected in this experiment. Cells were treated with 4 μM BP for 24h. Then the medium with BP was removed and cells were washed twice with fresh medium. Then cells were treated with DMSO (control) or 1 μM RA for additional 24 h. Interestingly, the results showed that RA decreased carcinogen-DNA adduct levels by 28% through removal of damaged cells or DNA repair (Fig. 4). The levels of total DNA adducts in cells treated with BP (24 h), followed by DMSO (24 h), and treated with BP (24 h), followed by RA (24 h) were 543.70 ± 32.82 and 390.46 ± 34.19 in 109 nucleotides, respectively. P value was 0.032. These results indicated that RA mainly affected on the adducted nucleotides or damaged cells through inducing DNA repair and apoptosis, but not BP through detoxification.
Fig. 4.
Effect of RA treatment on BPDE adduct levels in HepG2 cells. Levels of BPDE-dG and total DNA adducts in cells treated with 4 μM BP for 24 h then incubated another 24 h with DMSO were significantly lower compared to 48 h incubated with 4 μM BP (*P=0.02). After 24 h incubation with 4 μM BaP, the fresh medium was replaced with medium containing 1 μM RA for another 24 h. Total adduct levels were diminished significantly by RA compared to the medium with DMSO in second 24 h incubation (** p=0.032).
Moreover, our results also showed that BP elicited significant induction of CYP1A1 enzyme activities after 24 h incubation (Table 2). However, co-treatment of the cells with BP and RA did not significantly alter the inducibility of CYP1A1 compared with BP treatment only. These results suggested that attenuation of BP-DNA adducts by RA was not primarily due to its effects on CYP1A1 expression. Expression of glutathione S-transferase Ya (GST Ya) was analyzed by Western blot for DMSO, BP. RA, and BP+RA groups. There was no induction of GST Ya activity among these groups.
Table 2.
Effect of BP and RA on EROD (CYP 1A1) activities
| Group | EROD Activities (pmol/mg/min) | P | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | DMSO | RA | BP | |
| DMSO | 3 | 4.08 | 0.26 | - | - | - |
| RA (1 μM) | 3 | 3.71 | 0.49 | 0.3178 | - | - |
| BP (2 μM) | 3 | 8.65 | 1.97 | 0.0161 | - | - |
| BP + RA | 3 | 8.49 | 0.43 | 0.0001 | 0.0002 | 0.8950 |
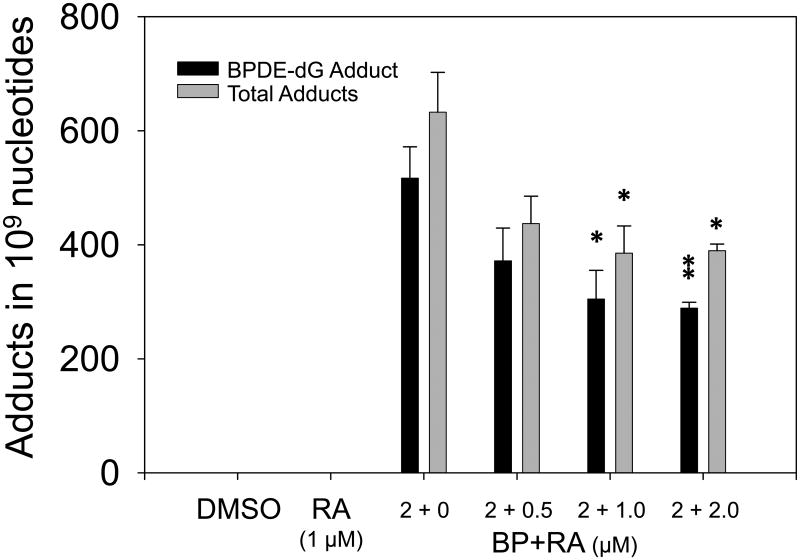
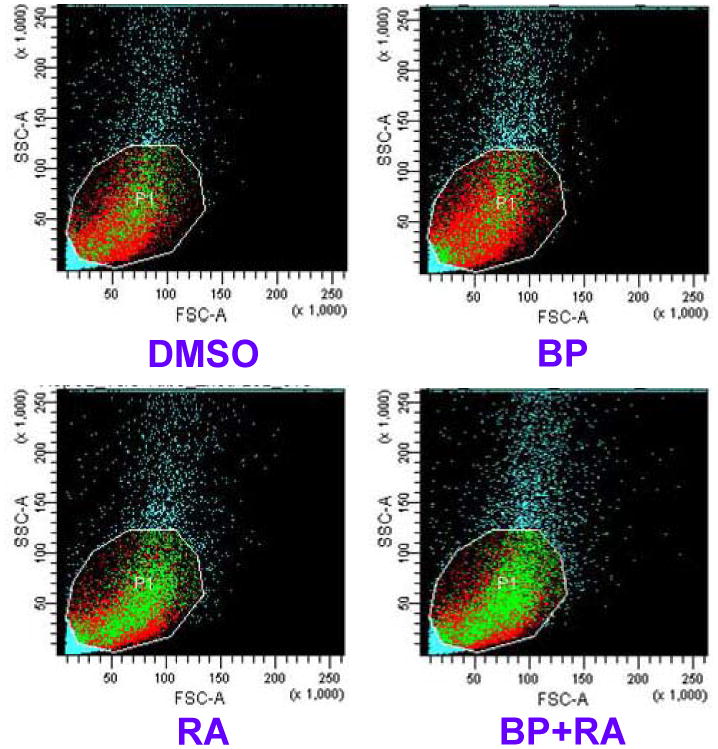
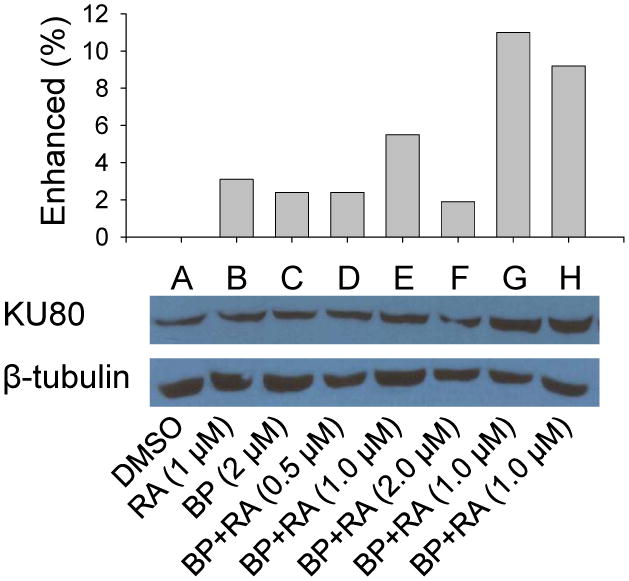
To address the issues whether cell apoptosis and DNA repair play important roles in attenuation of levels of BP-DNA adducts, apoptosis detection by flow cytometer and gene expression of DNA repair were analyzed. The samples were obtained from the first experiment (BP, 2 μM; RA, 1 μM; 24 h incubation). The results showed that apoptosis activity existed spontaneously in HepG2 cells. However, RA enhanced apoptosis in HepG2 cells. The rates of apoptosis were 3.2%, 3.3%, 6.6%, and 9.1% respectively for DMSO, BP, RA, and BP+RA groups. When DNA were injured by adducts, apoptosis was extensively enhanced by RA, resulting in loss of damaged cells. Fig. 5 demonstrated that BP+RA group increased cell apoptosis compared to BP or RA only groups. Two typical DNA repair genes, XRCC1 and KU80, were determined by Western blotting. Our results showed that the expression of XRCC1 in HepG2 cells was not affected by RA at doses of 0.5, 1.0, and 2.0 μM. However, the expression of KU80 was slightly enhanced by 1 μM RA after 24 h incubation compared to DMSO, BP, and RA groups (Fig. 6). Further enhancement of KU80 expression was observed at 48 h incubation (Fig. 6). These results indicate that DNA repair induced by RA may be also involved in the decrease of DNA adducts in HepG2 cells treated with BP.
Fig. 5.
Detection of apoptosis in HepG2 cells by flow cytometry. The apoptosis was determined by Annexin V Apoptosis Detection Kit.
Fig. 6.
Proteins isolated from HepG2 cells in different groups were probed with KU80 antibody by Western blot. A, DMSO; B, RA (1 μM); C, BP (2 μM); D, BP (2 μM) + RA (0.5 μM); E, BP (2 μM) + RA (1.0 μM); F, BP (2 μM) + RA (2.0 μM). Groups from A to F were incubated for 24 h. G, BP (2 μM) + RA (1.0 μM), incubated 48 h; H, BP (2 μM) + RA (1.0 μM) for 24 h, then RA (1.0 μM) only for additional 24 h.
Discussion
In this study we have tested the hypothesis that levels of carcinogen-DNA adducts will be diminished by eliminating damaged cells through apoptotic pathways or/and by DNA repair. DNA is the main target of environmental genotoxins such as PAHs. DNA adducts, induced by chemical carcinogens, are very sensitive biomarkers in chemical carcinogenesis. The formation of covalent DNA adducts is a key event in the initiation of neoplasia and the adducts also contribute to the progression of tumors (McCarty et al., 2009). BPDE-dG adducts can be eliminated by apoptosis and DNA repair. If the DNA adducts are repaired or adducted cells are removed, DNA and cells are returned to their normal state. But if the adducts persist during DNA replication, miscoding can occur, which leads to a permanent mutation in the DNA sequence (Hecht, 2003). Reduction in quantities of DNA adducts by chemopreventive agents could significantly reduce tumor incidence (Tang et al., 2001; Wang et al., 2010). Our results demonstrate that DNA adducts are useful biomarkers in cancer prevention studies, especially during the initiation phase of carcinogenesis.
DNA repair system, especially NER which is the primary repair system for most bulky DNA adducts (Reardon and Sancar, 2005), plays an important role in the removal of mutated nucleotides including DNA adducts. KU80 protein, which is encoded by the XRCC5 gene, may play a role in attenuation of DNA adducts (Guggenheim et al., 2009). Our results displayed that KU80 was enhanced by RA at 24 h. It got further enhanced at 48 h. These results indicated that DNA repair may contribute to the reduction of carcinogen DNA adducts in HepG2 cells. However, repair is inevitably less than perfect, and carcinogen-DNA adducts accumulate over time, especially during exposure to high concentrations of environmental carcinogens or chronic exposure to carcinogens such as cigarette smoking. Therefore, apoptosis (programmed cell death) may also play important roles as a subsequent defense tool in removing damaged cells with bulky DNA adducts or mutated DNA (Hecht, 2003).
In fact, the elimination of unwanted cells by apoptosis is an important developmental and homeostatic process in multicellular organisms (Ellis et al., 1991; Metzstein et al., 1998). Excessive DNA damage causes relatively insufficient amount of apoptosis that results in uncontrolled cell proliferation, which could lead to cancer. Induction of apoptosis by natural chemopreventive agents may increase elimination of more damaged cells. Our studies showed that RA enhanced cell apoptosis which may contribute to decrease of carcinogen-DNA adducts. Interestingly, bulky DNA adducts induced by BP after 24 h incubation in HepG2 cells were further diminished by incubation with RA only for additional 24 h incubation (Fig. 4), suggesting that RA eliminated DNA adducts through apoptosis or/and DNA repair pathways, but not through detoxification of BP. Selection of chemopreventive agents, time and dose regimens may be very important for the modulation of apoptosis. In this study, we selected RA, as an apoptosis inducer, to determine if it could diminish BP-DNA adducts in vitro in a human cell line.
The formation of DNA adducts is considered as a crucial step in the initiation phase of carcinogenesis (Lippman and Hawk, 2009). Recently, our laboratory observed a significant positive linear regression between levels of DNA adducts and tumor incidence in animal experiments (data not shown). Animals treated with different concentrations of reconstituted mixtures of PAHs (mimic actual concentrations of environmental PAHs). Levels of DNA adducts detected at 1, 3, and 7days after treatment were significantly correlated with tumor incidence examined pathologically at 10 months. These results suggest if the formation of DNA adducts are suppressed in the phase of tumor initiation, the risk of tumors may be significantly diminished.
Chemopreventive agents decrease carcinogen-DNA adducts through several pathways: 1) inactivation of chemical carcinogens by detoxification enzymes, such as upregulated phase I and II enzymes, for example, CYP1A1 (Uno et al., 2004; Uno et al., 2006) and glutathione transferases; 2) enhancement of DNA repair systems; and 3) induction of cell apoptosis. Support for the chemopreventive approach is based on the biologic concepts of field cancerization and multistep carcinogenesis (Hong and Lippman, 1995). Currently, most of chemopreventive measurements (Dennis et al., 2009; Lippman and Hawk, 2009; Tsao et al., 2009) focus on reversing premalignant lesions and preventing second primary tumors, or slowing tumorigenesis.
In other words, these approaches affect cells in the last phase of carcinogenesis --- Progression. If the chemopreventive measurements can start from earlier phases of carcinogenesis such as initiation and promotion for high risk populations such as smokers and others exposed to high concentrations of environmental or occupational carcinogens, significant consequence of cancer prevention may be observed in long term.
Recent years, natural chemopreventive agents have received great attention for cancer prevention because of their various health benefits, lack of toxicity and less side effects (Manson et al., 2005). Natural agents and their derivatives such as vitamin A, selenium, green tea, fish oil, curcumin, resveratrol, deguelin, myoinositol, aspirin, and probiotics have potential benefits in chemoprevention (Hong et al., 2000; Dennis et al., 2009; Lippman and Hawk, 2009). In our current studies, RA, a natural chemopreventive agent, was used as an apoptosis inducer. The results displayed that significantly reduced BP-DNA adducts in HepG2 cells after adducts formed/cells damaged (Fig. 4).
As previously reported (Couroucli et al., 2006), RA can modulate CYP1A1 activity in animals. However, our results displayed that RA did not induce CYP1A1 activity in HepG2 cells by itself (Table 2). Co-treatment of the cells with BP and RA did not significantly alter the inducibility of CYP1A1 compared to BP only group (Table 2), suggesting that attenuation of BP-DNA adducts by RA was not primarily due to its effects on CYP1A expression.
In conclusion, our results showed that RA significantly diminished levels of bulky DNA adducts in HepG2 cells induced by BP. Cell apoptosis and DNA repair were probably involved to remove damaged cells and adducted nucleotides. Future animal studies should help our understanding the roles of chemopreventive agents in cancer prevention through carcinogen detoxication, cell apoptosis and DNA repair.
Fig. 7.
Proteins isolated from HepG2 cells in different groups were probed with KU80 antibody by Western blot. Samples A to F were incubated for 24 h. Sample G, BP (2 μM) + RA (1.0 μM), incubated for 48 h; H, BP (2 μM) + RA (1.0 μM) for 24 h, then incubated with RA (1.0 μM) only for additional 24 h.
Acknowledgments
This research work was supported by grants from the National Institutes of Health, numbers ES04917, ES009132, ES09106 (National Institute of Environmental Health Sciences), and numbers HL070921 and HL 087174 (National Heart, Lung, and Blood Institute). We grateful acknowledge A. Prejusa for his assistant in analysis of cell apoptosis.
Footnotes
Conflict of interest statement: No potential conflict of interests were disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arce F, Gatjens-Boniche O, Vargas E, Valverde B, Diaz C. Apoptotic events induced by naturally occurring retinoids ATRA and 13-cis retinoic acid on human hepatoma cell lines Hep3B and HepG2. Cancer Lett. 2005;229:271–281. doi: 10.1016/j.canlet.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Bogos K, Renyi-Vamos F, Kovacs G, Tovari J, Dome B. Role of retinoic receptors in lung carcinogenesis. J Exp Clin Cancer Res. 2008;27:18. doi: 10.1186/1756-9966-27-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite E, Wu X, Wang Z. Repair of DNA lesions induced by polycyclic aromatic hydrocarbons in human cell-free extracts: involvement of two excision repair mechanisms in vitro. Carcinogenesis. 1998;19:1239–1246. doi: 10.1093/carcin/19.7.1239. [DOI] [PubMed] [Google Scholar]
- Chang WL, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. The Journal of nutrition. 1998;128:491–497. doi: 10.1093/jn/128.3.491. [DOI] [PubMed] [Google Scholar]
- Couroucli XI, Liang YW, Jiang W, Barrios R, Moorthy B. Attenuation of oxygen-induced abnormal lung maturation in rats by retinoic acid: possible role of cytochrome P4501A enzymes. The Journal of pharmacology and experimental therapeutics. 2006;317:946–954. doi: 10.1124/jpet.105.100677. [DOI] [PubMed] [Google Scholar]
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Medical toxicology and adverse drug experience. 1988;3:273–288. doi: 10.1007/BF03259940. [DOI] [PubMed] [Google Scholar]
- Dennis T, Fanous M, Mousa S. Natural products for chemopreventive and adjunctive therapy in oncologic disease. Nutrition and cancer. 2009;61:587–597. doi: 10.1080/01635580902825530. [DOI] [PubMed] [Google Scholar]
- Ellis RE, Yuan JY, Horvitz HR. Mechanisms and functions of cell death. Annual review of cell biology. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Gottschling S, Reinhard H, Pagenstecher C, Kruger S, Raedle J, Plotz G, Henn W, Buettner R, Meyer S, Graf N. Hypothesis: Possible role of retinoic acid therapy in patients with biallelic mismatch repair gene defects. European journal of pediatrics. 2008;167:225–229. doi: 10.1007/s00431-007-0474-3. [DOI] [PubMed] [Google Scholar]
- Green DR, Martin SJ. The killer and the executioner: how apoptosis controls malignancy. Current opinion in immunology. 1995;7:694–703. doi: 10.1016/0952-7915(95)80079-4. [DOI] [PubMed] [Google Scholar]
- Guggenheim ER, Xu D, Zhang CX, Chang PV, Lippard SJ. Photoaffinity isolation and identification of proteins in cancer cell extracts that bind to platinum-modified DNA. Chembiochem. 2009;10:141–157. doi: 10.1002/cbic.200800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RC. Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:6943–6947. doi: 10.1073/pnas.81.22.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang B. Repair of exocyclic DNA adducts: rings of complexity. Bioessays. 2004;26:1195–1208. doi: 10.1002/bies.20130. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nature reviews. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- Hong MY, Chapkin RS, Barhoumi R, Burghardt RC, Turner ND, Henderson CE, Sanders LM, Fan YY, Davidson LA, Murphy ME, Spinka CM, Carroll RJ, Lupton JR. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–1925. doi: 10.1093/carcin/23.11.1919. [DOI] [PubMed] [Google Scholar]
- Hong MY, Lupton JR, Morris JS, Wang N, Carroll RJ, Davidson LA, Elder RH, Chapkin RS. Dietary fish oil reduces O6-methylguanine DNA adduct levels in rat colon in part by increasing apoptosis during tumor initiation. Cancer Epidemiol Biomarkers Prev. 2000;9:819–826. [PubMed] [Google Scholar]
- Hong WK, Lippman SM. Cancer chemoprevention. Journal of the National Cancer Institute. 1995:49–53. [PubMed] [Google Scholar]
- Jeffrey AM, Weinstein IB, Jennette KW, Grzeskowiak K, Nakanishi K, Harvey RG, Autrup H, Harris C. Structures of benzo(a)pyrene--nucleic acid adducts formed in human and bovine bronchial explants. Nature. 1977;269:348–350. doi: 10.1038/269348a0. [DOI] [PubMed] [Google Scholar]
- Josephy P, Mannervik B. Oncogenes, tumor suppress genes, and mutations in cancer cells Molecular Toxicology. Second. Now York: Oxford University Press; 2006. pp. 155–190. [Google Scholar]
- Knasmuller S, Parzefall W, Sanyal R, Ecker S, Schwab C, Uhl M, Mersch-Sundermann V, Williamson G, Hietsch G, Langer T, Darroudi F, Natarajan AT. Use of metabolically competent human hepatoma cells for the detection of mutagens and antimutagens. Mutation research. 1998;402:185–202. doi: 10.1016/s0027-5107(97)00297-2. [DOI] [PubMed] [Google Scholar]
- Lee YW, Park HJ, Son KW, Hennig B, Robertson LW, Toborek M. 2,2′,4,6,6′-pentachlorobiphenyl (PCB 104) induces apoptosis of human microvascular endothelial cells through the caspase-dependent activation of CREB. Toxicology and applied pharmacology. 2003;189:1–10. doi: 10.1016/s0041-008x(03)00084-x. [DOI] [PubMed] [Google Scholar]
- Lippman SM, Hawk ET. Cancer prevention: from 1727 to milestones of the past 100 years. Cancer research. 2009;69:5269–5284. doi: 10.1158/0008-5472.CAN-09-1750. [DOI] [PubMed] [Google Scholar]
- Lu XP, Fanjul A, Picard N, Pfahl M, Rungta D, Nared-Hood K, Carter B, Piedrafita J, Tang S, Fabbrizio E, Pfahl M. Novel retinoid-related molecules as apoptosis inducers and effective inhibitors of human lung cancer cells in vivo. Nature medicine. 1997;3:686–690. doi: 10.1038/nm0697-686. [DOI] [PubMed] [Google Scholar]
- Mabon N, Moorthy B, Randerath E, Randerath K. Monophosphate 32P-postlabeling assay of DNA adducts from 1,2:3,4-diepoxybutane, the most genotoxic metabolite of 1,3-butadiene: in vitro methodological studies and in vivo dosimetry. Mutation research. 1996;371:87–104. doi: 10.1016/s0165-1218(96)90098-1. [DOI] [PubMed] [Google Scholar]
- Manson MM, Farmer PB, Gescher A, Steward WP. Innovative agents in cancer prevention. Recent results in cancer research. Fortschritte der Krebsforschung. 2005;166:257–275. doi: 10.1007/3-540-26980-0_17. [DOI] [PubMed] [Google Scholar]
- McCarty KM, Santella RM, Steck SE, Cleveland RJ, Ahn J, Ambrosone CB, North K, Sagiv SK, Eng SM, Teitelbaum SL, Neugut AI, Gammon MD. PAH-DNA adducts, cigarette smoking, GST polymorphisms, and breast cancer risk. Environmental health perspectives. 2009;117:552–558. doi: 10.1289/ehp.0800119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzstein MM, Stanfield GM, Horvitz HR. Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet. 1998;14:410–416. doi: 10.1016/s0168-9525(98)01573-x. [DOI] [PubMed] [Google Scholar]
- Mohamad N, Gutierrez A, Nunez M, Cocca C, Martin G, Cricco G, Medina V, Rivera E, Bergoc R. Mitochondrial apoptotic pathways. Biocell. 2005;29:149–161. [PubMed] [Google Scholar]
- Moorthy B. Persistent expression of 3-methylcholanthrene-inducible cytochromes P4501A in rat hepatic and extrahepatic tissues. The Journal of pharmacology and experimental therapeutics. 2000;294:313–322. [PubMed] [Google Scholar]
- Mrass P, Rendl M, Mildner M, Gruber F, Lengauer B, Ballaun C, Eckhart L, Tschachler E. Retinoic acid increases the expression of p53 and proapoptotic caspases and sensitizes keratinocytes to apoptosis: a possible explanation for tumor preventive action of retinoids. Cancer research. 2004;64:6542–6548. doi: 10.1158/0008-5472.CAN-04-1129. [DOI] [PubMed] [Google Scholar]
- Natarajan AT, Darroudi F. Use of human hepatoma cells for in vitro metabolic activation of chemical mutagens/carcinogens. Mutagenesis. 1991;6:399–403. doi: 10.1093/mutage/6.5.399. [DOI] [PubMed] [Google Scholar]
- Noy N. Between Death and Survival: Retinoic Acid in Regulation of Apoptosis. Annual review of nutrition. doi: 10.1146/annurev.nutr.28.061807.155509. [DOI] [PubMed] [Google Scholar]
- Parkinson A. Carsarett and Doull's Toxicology: The Basic Science of Poisons. In: Klaassen CD, editor. Biotransformation of xenobiotics. Vol. 133 2001. [Google Scholar]
- Randerath E, Avitts TA, Reddy MV, Miller RH, Everson RB, Randerath K. Comparative 32P-analysis of cigarette smoke-induced DNA damage in human tissues and mouse skin. Cancer research. 1986;46:5869–5877. [PubMed] [Google Scholar]
- Randerath E, Miller RH, Mittal D, Avitts TA, Dunsford HA, Randerath K. Covalent DNA damage in tissues of cigarette smokers as determined by 32P-postlabeling assay. J Natl Cancer Inst. 1989;81:341–347. doi: 10.1093/jnci/81.5.341. [DOI] [PubMed] [Google Scholar]
- Randerath E, Zhou GD, Donnelly KC, Safe SH, Randerath K. DNA damage induced in mouse tissues by organic wood preserving waste extracts as assayed by 32P-postlabeling. Archives of toxicology. 1996;70:683–695. doi: 10.1007/s002040050329. [DOI] [PubMed] [Google Scholar]
- Randerath E, Zhou GD, Randerath K. Organ-specific oxidative DNA damage associated with normal birth in rats. Carcinogenesis. 1997a;18:859–866. doi: 10.1093/carcin/18.4.859. [DOI] [PubMed] [Google Scholar]
- Randerath K, Randerath E, Zhou GD, Supunpong N, He LY, McDonald TJ, Donnelly KC. Genotoxicity of complex PAH mixtures recovered from contaminated lake sediments as assessed by three different methods. Environmental and molecular mutagenesis. 1999;33:303–312. doi: 10.1002/(sici)1098-2280(1999)33:4<303::aid-em7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Randerath K, Sriram P, Moorthy B, Aston JP, Baan RA, van den Berg PT, Booth ED, Watson WP. Comparison of immunoaffinity chromatography enrichment and nuclease P1 procedures for 32P-postlabelling analysis of PAH-DNA adducts. Chemico-biological interactions. 1998;110:85–102. doi: 10.1016/s0009-2797(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Randerath K, Zhou GD, Randerath E, Safe SH, Donnelly KC. Comparative 32P-postlabeling analysis of exogenous and endogenous DNA adducts in mouse skin exposed to a wood-preserving waste extract, a complex mixture of polycyclic and polychlorinated chemicals. Environmental and molecular mutagenesis. 1997b;29:372–378. [PubMed] [Google Scholar]
- Reardon JT, Sancar A. Nucleotide excision repair. Progress in nucleic acid research and molecular biology. 2005;79:183–235. doi: 10.1016/S0079-6603(04)79004-2. [DOI] [PubMed] [Google Scholar]
- Reddy MV, Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986;7:1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends in molecular medicine. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alonso JA, Lopez-Aparicio P, Recio MN, Perez-Albarsanz MA. Polychlorinated biphenyl mixtures (Aroclors) induce apoptosis via Bcl-2, Bax and caspase-3 proteins in neuronal cell cultures. Toxicology letters. 2004;153:311–326. doi: 10.1016/j.toxlet.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan RMB. Retinoids as cancer-preventive agents. IARC Sci Publ. 1996;139 [PubMed] [Google Scholar]
- Sturgis EM, Wei Q. Genetic susceptibility--molecular epidemiology of head and neck cancer. Current opinion in oncology. 2002;14:310–317. doi: 10.1097/00001622-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Sun SY, Yue P, Chen X, Hong WK, Lotan R. The synthetic retinoid CD437 selectively induces apoptosis in human lung cancer cells while sparing normal human lung epithelial cells. Cancer research. 2002;62:2430–2436. [PubMed] [Google Scholar]
- Tang D, Phillips DH, Stampfer M, Mooney LA, Hsu Y, Cho S, Tsai WY, Ma J, Cole KJ, She MN, Perera FP. Association between carcinogen-DNA adducts in white blood cells and lung cancer risk in the physicians health study. Cancer research. 2001;61:6708–6712. [PubMed] [Google Scholar]
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science (New York, NY. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Tsao AS, Liu D, Martin J, Tang XM, Lee JJ, El-Naggar AK, Wistuba I, Culotta KS, Mao L, Gillenwater A, Sagesaka YM, Hong WK, Papadimitrakopoulou V. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer prevention research (Philadelphia, Pa. 2009;2:931–941. doi: 10.1158/1940-6207.CAPR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S., E. P. A. EPA integrated risk information system (IRIS) Washington, DC: Environmental Protection Agency; 2003. [Google Scholar]
- Uno S, Dalton TP, Derkenne S, Curran CP, Miller ML, Shertzer HG, Nebert DW. Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Molecular pharmacology. 2004;65:1225–1237. doi: 10.1124/mol.65.5.1225. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, Shertzer HG, Gonzalez FJ, Nebert DW. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Molecular pharmacology. 2006;69:1103–1114. doi: 10.1124/mol.105.021501. [DOI] [PubMed] [Google Scholar]
- Vermeulen K, Van Bockstaele DR, Berneman ZN. Apoptosis: mechanisms and relevance in cancer. Annals of hematology. 2005;84:627–639. doi: 10.1007/s00277-005-1065-x. [DOI] [PubMed] [Google Scholar]
- Wang LE, Hu Z, Sturgis EM, Spitz MR, Strom SS, Amos CI, Guo Z, Qiao Y, Gillenwater AM, Myers JN, Clayman GL, Weber RS, El-Naggar AK, Mao L, Lippman SM, Hong WK, Wei Q. Reduced DNA repair capacity for removing tobacco carcinogen-induced DNA adducts contributes to risk of head and neck cancer but not tumor characteristics. Clin Cancer Res. 2010;16:764–774. doi: 10.1158/1078-0432.CCR-09-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. Evolutionary conservation of a genetic pathway of programmed cell death. Journal of cellular biochemistry. 1996;60:4–11. doi: 10.1002/(sici)1097-4644(19960101)60:1<4::aid-jcb2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Fifth. New Jersey: Prentice-Hall; 2009. [Google Scholar]
- Zhou G, Hernandez NS, Randerath E, Randerath K. Effects of different diets and dietary restriction on perinatal endogenous DNA adducts. Time dependence of oxidative and presumptive nonoxidative lesions. Mutation research. 2000;447:137–147. doi: 10.1016/s0027-5107(99)00211-0. [DOI] [PubMed] [Google Scholar]
- Zhou GD, Hernandez NS, Randerath E, Randerath K. Acute elevation by short-term dietary restriction or food deprivation of type I I-compound levels in rat liver DNA. Nutrition and cancer. 1999;35:87–95. doi: 10.1207/S1532791487-95. [DOI] [PubMed] [Google Scholar]
- Zhou GD, Moorthy B, Bi J, Donnelly KC, Randerath K. DNA adducts from alkoxyallylbenzene herb and spice constituents in cultured human (HepG2) cells. Environmental and molecular mutagenesis. 2007;48:715–721. doi: 10.1002/em.20348. [DOI] [PubMed] [Google Scholar]
- Zhou GD, Randerath E, Randerath K. Effects of dietary transition metals on oxidative DNA lesions in neonatal rats. Mutation research. 2001;479:71–79. doi: 10.1016/s0027-5107(01)00148-8. [DOI] [PubMed] [Google Scholar]
- Zhou GD, Randerath K, Donnelly KC, Jaiswal AK. Effects of NQO1 deficiency on levels of cyclopurines and other oxidative DNA lesions in liver and kidney of young mice. International journal of cancer. 2004;112:877–883. doi: 10.1002/ijc.20375. [DOI] [PubMed] [Google Scholar]