Abstract
Subtle alterations in synaptic function contribute to the pathophysiology associated with several neuropsychiatric diseases. Modifications in synaptic vesicle trafficking can cause frequency-dependent changes in neurotransmission, alter information coding in neural circuits, and affect long-term plasticity. Rett syndrome, a neurodevelopmental disorder that arises from mutations in the methyl-CpG-binding protein-2 (MeCP2) gene, is a salient example for such a disease state in which synaptic transmission—in particular, spontaneous neurotransmission and short-term synaptic plasticity, have been altered. MeCP2 is widely believed to be a transcriptional repressor that silences methylated genes. Recent studies have identified synaptic deficits associated with the loss of MeCP2 in several brain regions, including the hippocampus. These findings suggest a synaptic basis for neurological symptoms associated with Rett syndrome and suggest an important role for transcriptional repression in the regulation of neurotransmission. These studies also highlight the importance of histone deacetylation and DNA methylation, two key epigenetic mechanisms in controlling synaptic function. These mechanisms are essential for chromatin remodeling in neurons as well as for repression of gene activation by MeCP2 and related methyl-binding proteins. Future work focusing on the regulation of DNA methylation and histone deacetylation by synaptic activity and how these epigenetic alterations affect neurotransmission will be critical to elucidate the mechanisms underlying Rett syndrome. In addition, this work will also help delineate a key pathway that regulates properties of neurotransmission in the central nervous system that may underlie additional neuropsychiatric disorders.
Rett syndrome (RTT) is an X-linked neurodevelopmental disorder that has an incidence of 1:10000 live female births and is one of the leading causes of mental retardation and autistic behavior in females (1). Individuals affected with RTT experience normal development up to the age of 6–18 months, at which time they fail to acquire new skills and enter a period of motor skill regression. With time, RTT symptoms become more pronounced and include a wide range of neurological defects including mental retardation, autism-like behavior, seizures, sleep disturbances, problems with gait, decelerated head growth, and stereotypical hand movements. In addition, most children afflicted with RTT show a loss of social and cognitive abilities.
Mutations in the coding region of the methyl-CpG-binding protein 2 (MECP2) gene account for > 96% of classic RTT cases (2–7). These mutations are predicted to result in loss of MeCP2 function (8,9). In addition to classic RTT, mutations in the MECP2 gene have been identified in other patient populations including Angelman syndrome, autism, learning disabilities, and mental retardation syndromes (10–15). The cyclin-dependent kinase-like 5 (CDKL5) gene encodes for serine/threonine kinase 9, which has been demonstrated to phosphorylate MeCP2. Mutations in this gene have been identified in some atypical RTT individuals, particularly the early-onset seizures variant, suggesting that impairing MeCP2 function may also be sufficient to trigger a RTT-like phenotype (16–18). However, the majority of congenital variants of RTT do not have mutations in either MECP2 or CDKL5 (19,20). A recent study has identified disruption of FOXG1, a transcriptional repressor involved in neuronal progenitor proliferation, in some patients with the congenital variant of RTT as well as a patient with severe mental retardation and RTT-like features, although a direct link between FOXG1 and MeCP2 remains unknown (21,22). Duplication of the MECP2 gene has also been detected in some male patients with mental retardation and progressive neurological symptoms (23). Collectively, these findings strongly suggest that alterations in MeCP2 expression or function may contribute to disease progression with strong neurological phenotypes and furthermore that the levels of MeCP2 expression must be tightly controlled under normal circumstances.
The MECP2 gene encodes a DNA binding protein that interacts with methylated cytosines in genomes. However, compared with other methyl-CpG binding proteins, MeCP2 binding requires an enrichment of A/T bases adjacent to methyl-CpGs (24). Normally, MeCP2 is believed to act as a transcriptional repressor by binding to target gene promoters and silencing their transcription in many tissues. However, a recent study suggests that MeCP2 may act as either a transcriptional activator or repressor in the hypothalamus (25). Another group using ChIP-chip analysis of a human neuroblastoma cell line, SH-SY5Y, found that MeCP2 interacts closely with promoters that are actively expressed (26). These recent studies suggest that MeCP2 may have a function independent of transcriptional repression, perhaps in transcriptional activation, but they do not rule out that MeCP2 may be repressing a repressor that triggers the transcriptional activation.
MeCP2 is expressed in many tissues; however, it is interesting that the majority of the RTT deficits are pronounced in the central nervous system (CNS). MeCP2 is expressed at high levels in the mammalian brain—in particular, in neurons, not glia. In immature neurons, MeCP2 expression is low but increases during neuronal maturation and reaches its highest level of expression in postmitotic neurons (27,28). This expression profile of MeCP2 during development may suggest that MeCP2 is involved in neuronal maturation and dendritic arborization (29). Interestingly, this high level of MeCP2 expression in postmitotic neurons continues throughout adulthood, suggesting that MeCP2 may be a necessary factor for proper neuronal function in mature neurons. Therefore, it is possible that alterations in MeCP2 expression in specific brain regions is responsible for the behavioral abnormalities observed in individuals afflicted with RTT and related mental retardation syndromes.
Attempts to model the disease by generating constitutive Mecp2 knockout (KO) mice results in the recapitulation of many of the neurological symptoms of RTT, although these mice die early in postnatal development (30–32). The early postnatal lethality of these constitutive Mecp2 KOs prevents their use in behavioral characterization studies. To circumvent these potential problems, conditional KO mice, in which floxed Mecp2 mice were crossed with calcium-calmodulin-dependent protein kinase II (CaMKII)-Cre transgenic mice to delete Mecp2 selectively in the forebrain, have been generated (30). This CaMKII-Cre mouse line expresses Cre recombinase in forebrain regions during early postnatal (P14) development. These mice have recently been characterized in a wide array of behavioral tests. The conditional Mecp2 KO mice have many of the behavioral abnormalities that are reminiscent of the symptoms seen in RTT patients, including impaired motor coordination, increased anxiety, and abnormal social interaction with other mice (33). These data suggest that expression of MeCP2 in postnatal neurons is crucial for normal development and that the postnatal loss of MeCP2 in broad forebrain areas is sufficient to recapitulate many features of RTT.
To date, there have been two lines of MECP2 overexpression mice generated. One study used a targeting approach in which MECP2 from a P1-derived artificial chromosome was expressed under the endogenous human promoter (34). These mice showed enhanced motor coordination on the rotarod, less anxiety-related behavior, and increased context-dependent fear conditioning compared with wildtype littermate control mice (34). The second line of transgenic mice used a targeting approach in which MECP2 cDNA was inserted into the Tau locus to express MeCP2 specifically in postmitotic neurons. These mice express two- to fourfold more MeCP2 in the brain than wild type mice and display some neurological deficits including motor dysfunction, ataxia, and tremors, although an extensive behavioral analysis of these mice has not been reported (35). Nevertheless, it is intriguing that the overexpression of MeCP2 produces behavioral phenotypes opposite to those observed in the Mecp2 conditional KOs (33,35). Collectively, data from the conditional KO mice and the overexpression mice suggest slight alterations in the expression levels of MeCP2 can exert profound effects on behavior.
Although the conditional Mecp2 KOs were able to recapitulate many of the behavioral phenotypes associated with RTT patients, no major neuroanatomic abnormalities or neuronal loss were observed in these mice (30). Indeed, studies examining the brains of RTT patients as well as other recent mouse models of the disease have not found major neuropathologic abnormalities or neuronal loss but rather only subtle changes in neuronal morphology (27,36,37). Furthermore, the transgenic MECP2 overexpression mice also do not display any major neuropathologic abnormalities (34,35).
One of the most consistent changes in neuronal morphology observed in postmortem brains of RTT patients, as well as animal models of the disorder, are alterations in the structure of neuronal dendrites and dendritic spines, varying from increases in dendritic complexity to immature synaptic spine morphology. Similar modifications in neuronal morphology are seen in several neurodevelopmental disorders such as Fragile X and are typically interpreted as indicators of a malfunction of synaptic development and plasticity (36,38). This finding is particularly intriguing because individuals affected with RTT experience normal development up to the age of 6–18 months, the period of pronounced synaptogenesis in the brain, at which time they then fail to acquire new skills and undergo motor skill regression. Recent work has demonstrated that alterations in dendritic structure can result from changes in synaptic activity (39). Therefore, the morphological changes in dendritic structure in RTT patients and in mouse models of the disease may suggest an underlying synaptic deficit in neurotransmission in the CNS. Thus, it is plausible that changes in MeCP2 expression may contribute to functional alterations in synaptic transmission ultimately resulting in disease phenotypes.
The lack of major anatomic deficits in RTT patients and in the Mecp2-null knockout mice suggest the possibility that the behavioral deficits observed may be able to be reversed. Accordingly, two recent studies suggest that MECP2 activation in the brain of null Mecp2 mice can reverse some of the behavioral phenotypes (40,41). The reversibility of behavioral impairments in a disease model represents an intriguing and important advancement. However, the presence of MECP2 on the X-chromosome makes RTT symptoms vary with the degree of X-inactivation, making it unclear whether reintroduction of MECP2 in patients will be sufficient to alleviate the behavioral impairments.
MeCP2 and Synaptic Transmission
Recently, researchers have discovered a number of defects in synaptic function in several mouse models of RTT. It appears that the loss of MeCP2 function can lead to changes in spontaneous synaptic transmission as well as in short- and long-term synaptic plasticity. Two studies, one using a Mecp2-null mouse (42) and another using a mouse expressing a truncated form of MECP2 (43), found deficits in both long-term potentiation (LTP) and long-term depression (LTD) in hippocampal slices from these mice compared with control littermates. Interestingly, whereas the first study saw these changes only in older, symptomatic mice (42), the second found them also in younger, asymptomatic mice, suggesting the possibility that these synaptic deficits may be occurring before the manifestation of RTT-like behaviors (43). Additional defects in basal synaptic transmission were seen in these as well as two other studies. In cortical pyramidal neurons, the propensity of spontaneous miniature excitatory postsynaptic currents (mEPSCs) was reduced in Mecp2 knockout mice, whereas a small increase was seen in the overall synaptic charge of spontaneous inhibitory postsynaptic currents (mIPSCs) (44). In dissociated hippocampal cultures, a significant decrease in the frequency of spontaneous mEPSCs in Mecp2 knockout versus control neurons was observed, whereas no change was seen in mIPSC properties (45). These changes in synaptic transmission—in particular, short-term plasticity—may be quite important in underlying mechanisms of neurological dysfunction observed in RTT patients. In addition, each of these studies investigating MeCP2 and synaptic transmission points to a possible imbalance between excitatory and inhibitory activity in the brains of Mecp2 mutant mice, perhaps toward less excitation and therefore more inhibition, but this needs to be explored further. There are a number of studies suggesting an abnormal ratio of excitation/inhibition in the brain activity of autistic patients (46–48), and because RTT is considered an autism-spectrum disorder, it is not unreasonable to hypothesize that something similar may be occurring in RTT patients.
Conversely, synaptic deficits have also been reported in transgenic overexpressing MECP2 mice (34). In a mouse model expressing MECP2 under the endogenous human promoter in wildtype animals, enhanced LTP and paired-pulse facilitation have been observed (34). Although these are the only synaptic measurements that have been reported following MECP2 overexpression, it is intriguing that the findings are opposite to the results observed with Mecp2 KO mice. Interestingly, a recent study has reported directly correlated changes in the number of glutamatergic synapses in autapse cultures in response to loss or increase in MeCP2 levels, suggesting that MeCP2 is a critical factor in regulating glutamatergic synapse formation (49). However, these changes in synapse numbers were only observed at an early developmental time period not at later stages of development (49). Collectively, these data demonstrate that alterations of MeCP2 expression impact synaptic function, as well as potentially synapse numbers, indicating that MeCP2-expression is critical for normal neuronal function.
Impact of Transcriptional Repression Inhibitors on Synaptic Transmission
The regulation of gene expression is important for all aspects of a neuron including maturation, survival, and responses to exogenous stimuli. Mutations that result in loss of function of a transcriptional repressor, such as MeCP2, would be expected to produce alterations in gene expression and ultimately neuronal function (42–45). However, microarray studies have not yielded consistent large-scale gene changes in RTT patients or in mouse models of the disease (25,50–52). A recent study suggests that MeCP2 may function independent of a transcription factor and instead play a role in RNA splicing (53). Yet another study found MeCP2 localized to the postsynaptic compartment suggesting some additional role outside of the nucleus (54).
Previous studies have shown that MeCP2 is a prototypical member of the methyl-CpG-binding domain protein family linked with transcriptional repression. MECP2 has two functional domains, a methyl-CpG binding domain (MBD) and a transcription repressor domain (TRD). The majority of the RTT disease–causing mutations occurs within these functional domains (2–5,7). MeCP2, through its MBD, binds to methylated-CpG sites and, through its TRD, interacts with a multiprotein corepressor complex and is believed to silence gene expression (55). This multiprotein corepressor complex contains corepressor proteins histone deacetylases 1 and 2 (HDAC1 and HDAC2) as well as Sin3A. HDACs are a family of enzymes that modulate chromatin structure, facilitating protein-DNA interactions and transcriptional control by catalyzing the removal of the acetyl group from acetylated lysines of histone proteins to repress gene expression. Importantly, several HDAC inhibitor drugs are under development as possible therapeutics for diseases such as cancer. A very broad-acting HDAC inhibitor, trichostatin A (TSA), has been demonstrated to relieve transcriptional repression by MeCP2 (55,56).
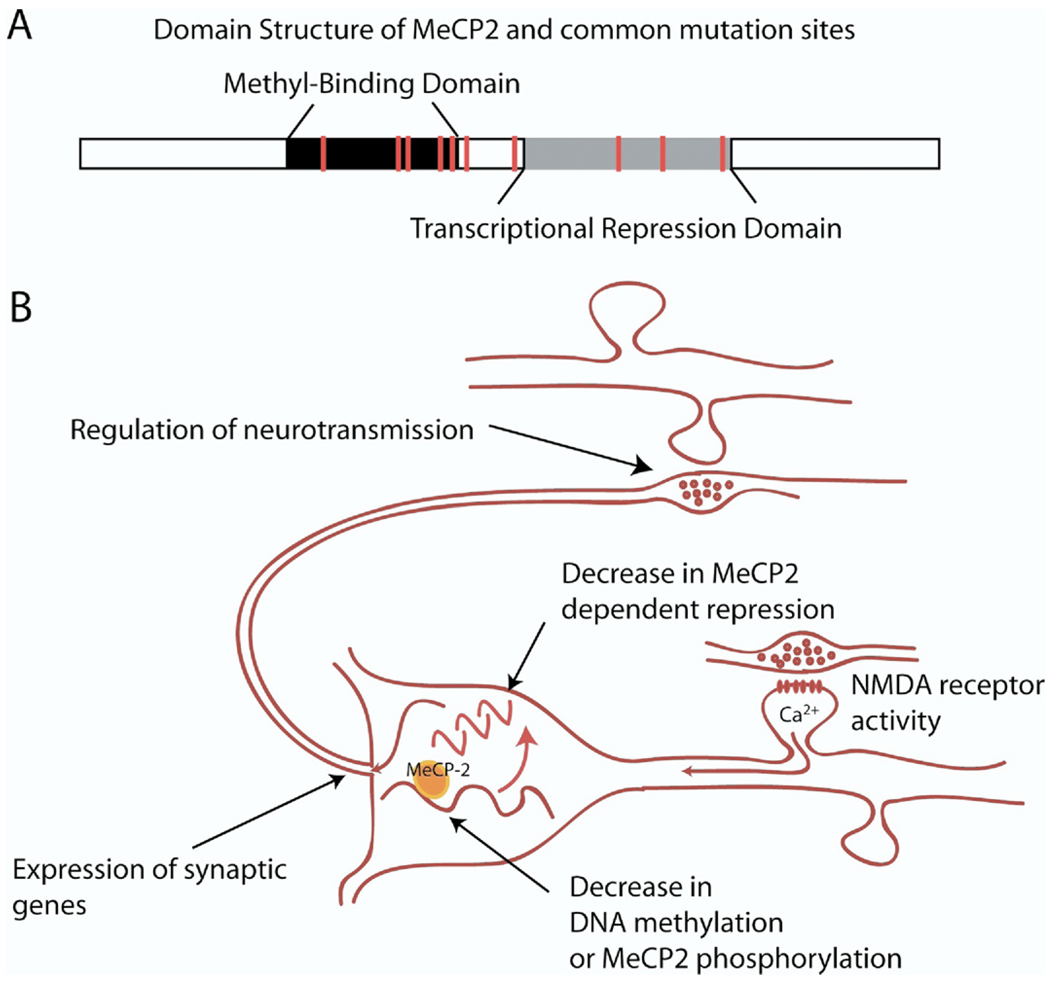
To examine whether the synaptic deficits observed in various RTT animal models were due to MeCP2’s role as a transcriptional repressor, wildtype hippocampal cultures from C57BL/6 mice have been treated with HDAC inhibitors, including TSA, and spontaneous neurotransmission was examined (45). Inhibition of HDAC activity resulted in a similar decrease in mEPSC frequency to that observed in MeCP2 KO neurons. Interestingly, treatment with actinomycin D, an inhibitor of transcriptional activation, at this time point did not affect synaptic transmission, suggesting that the synaptic deficits were selectively due to impairments in transcriptional repression and not transcriptional activation. This decrease in mEPSC frequency produced by HDAC inhibitors was reversed when combined with actinomycin D, suggesting that newly transcribed genes are involved in the suppression of synaptic function. Importantly, this alteration in spontaneous synaptic transmission was occluded in MeCP2 KO neurons treated with HDAC inhibitors, suggesting that altered synaptic transmission by HDAC inhibition requires MeCP2 and may be mediated through transcriptional repression by MeCP2. If these deficits in synaptic transmission are mediated through MeCP2’s role as a transcriptional repressor, then these data suggest that specific synaptic proteins may be MeCP2 target genes that are altered when MeCP2 function is impaired, as with the disease-causing mutations, and thus contribute to the alterations in synaptic transmission (Figure 1). One intriguing aspect to this hypothesis is that the expression of synaptic genes are tightly controlled, and even slight changes in their expression can profoundly influence synaptic transmission, which could be easily missed by a broad-scale microarray approach searching for large changes in gene expression.
Figure 1.
(A) Cartoon depicts the domain structure of MeCP2 as well as sites of common mutations. Mutations associated with Rett syndrome (denoted by red vertical bars) can be found both in methyl-CpG-binding and transcriptional repression domains of MeCP2 as well as the region in between. These mutants typically result in loss of MeCP2 function. (B) A model summarizing current notions on the relationship between MeCP2 function and synaptic activity. According to this model, synaptic activation of N-methyl-D-aspartate (NMDA) receptors leads to the demethylation of specific gene promoters or phosphorylation leading to unbinding of methyl-binding transcriptional repressors such as MeCP2. Removal of transcriptional repression results in an increase in transcription of target genes including brain-derived neurotrophic factor, which in turn regulate neurotransmission. This model suggests a homeostatic mechanism by which neuronal nuclei can monitor alterations in activity levels and adjust neurotransmitter output via altering gene expression.
DNA Methylation as an Epigenetic Mechanism That Regulates Synaptic Transmission
As stated earlier, the MECP2 gene encodes a DNA binding protein that binds to methylated cytosines, which is widely believed to enable MeCP2 to act as a transcriptional repressor at target gene promoters, silencing their transcription. DNA methylation is a prominent epigenetic mechanism that regulates gene expression independent of changes in the DNA sequence. Dysregulation of DNA methylation may therefore provide a more general mechanism whereby altered gene expression in neurons give rise to several neuropsychiatric disorders including those within the autism spectrum. DNA methylation typically leads to repression of gene expression and promotes genome stability in various species. In mammals, DNA methylation plays roles in many processes, such as X chromosome inactivation, genomic imprinting, and chromosome stability. DNA methyl-transferases (DNMTs) are the enzymes responsible for adding methyl groups at the 5-position of cytosine residues within CpG dinucleotides. During development, widespread methylation changes occur in primordial germ cells and preimplantation embryos (57,58). Following cellular differentiation, DNA methylation changes are less numerous and are thought to control the tissue-specific gene expression required to maintain the identity of cells. Studies of DNA demethylation in differentiated cells suggests a passive mechanism by which methylation patterns are lost during the DNA replication that occurs with each cellular division (59). The idea that an active mechanism of demethylation can occur in a replication-independent manner is rather controversial. However, evidence for this type of demethylation does exist. For example, demethylation of transfected DNA into nonreplicating cells has been shown to occur (60). More recently, the growth arrest and DNA damage protein Gadd45a was discovered to play a role in active demethylation of DNA in proliferating as well as in nondividing cells by promoting DNA repair and thereby erasing methylation marks (61). In addition, recent studies also suggest that DNMTs may also act as DNA demethylases leading to cyclical DNA methylation (62,63).
The importance for DNA methylation in the brain is becoming apparent because of its association with a number of neurodevelopmental disorders. Both Fragile X and ICF (Immunodeficiency, Centromeric region instability, Facial anomalies) syndromes, arise from malfunctions in the establishment of normal DNA methylation patterns (64,65). Rett syndrome is caused by mutations in the DNA methyl-binding protein MeCP2, a protein important for interpreting DNA methylation and controlling the repression of gene transcription (2). Patients of all three syndromes manifest levels of mental retardation, suggesting the importance for proper DNA methylation in the regulation of normal brain function.
DNMTs are highly expressed in neurons in the adult brain, suggesting that they may have a functional role in postmitotic neurons (66–69). Methylcytosine analogs, including 5-azacytidine, can inhibit DNA methylation in many cell types (70). Recent studies suggest that inhibiting DNA methylation in hippocampal slices blocks long-term memory and synaptic plasticity (71,72). Treatment with methylcytosine analogs has also been shown to block LTP and memory formation following contextual fear conditioning, a hippocampal-dependent associative learning and memory task (71,72). In other studies, prolonged depolarization of cultured cortical neurons has been reported to result in a decrease in methylation in the promoter region of brain-derived neurotrophic factor (BDNF), a neurotrophin important for synaptic plasticity (73). These studies suggest that there may be a relationship between synaptic activity and DNA methylation in mature neurons. However, the mechanisms behind these changes in DNA methylation and synaptic function are unknown.
In a recent study, spontaneous synaptic transmission between postmitotic neurons was demonstrated to be regulated by alterations in DNA methylation that occur in response to synaptic activity (74). Treatment of hippocampal neurons with DNMT inhibitors resulted in a significant decrease in the frequency of mEPSCs as well as in the rate of spontaneous synaptic vesicle fusion, which correlated with a decrease in BDNF promoter I methylation and an increase in BDNF expression but were blocked with inhibition of synaptic activity. These results demonstrate that effects on both synaptic transmission and BDNF promoter methylation are dependent on the background activity occurring in the neuronal cultures. This activity-dependent regulation is partially due to the calcium influx through N-methyl-D-aspartate receptors and presumably leads to the demethylation of specific gene promoters. This demethylation of specific genes may be mediated by an active demethylating enzyme in neurons; however, the existence of such an enzymatic activity remains an open question. Alternatively, activity may drive demethylation through a process that takes advantage of the DNA damage and repair machinery (66).
Regardless of the underlying mechanism(s), there is increasing support for activity-driven changes in DNA methylation in postmitotic neurons. In addition to regulation of BDNF expression, both increases and decreases in the DNA methylation patterns of two genes involved in synaptic plasticity, protein phosphatase 1 and reelin, respectively, were found in the hippocampus following fear conditioning (72).
MeCP2 Function and the Synaptic Basis of Rett Syndrome
Recent results demonstrate an intimate relationship between DNA methylation in neurons and the function of MeCP2. The deficit seen in spontaneous synaptic transmission following treatment of hippocampal neurons with DNMT inhibitors was occluded in the absence of MeCP2 (74). These findings suggest a role for DNA methylation in the control of synaptic function, which shares a common pathway with the methyl-binding protein MeCP2. Furthermore, these results suggest that neuronal activity can drive the transcription of genes important for controlling neurotransmitter release by regulating their methylation status. In addition to regulation of DNA methylation, activity-dependent phosphorylation of MeCP2 has recently been shown to cause its dissociation from target genes and relieve its repression of transcription (75). Furthermore, the same group showed that Ca2+-calmodulin-dependent kinase II mediated phosphorylation of MeCP2 can be driven by Ca2+ influx as well as excitatory synaptic activity, leading to the expression of several activity-induced genes including BDNF (76). The same study also showed that phosphorylation-dependent regulation of MeCP2 function was required for normal dendritic patterning and dendritic spine development. Taken together with MeCP2 phosphorylation, activity-dependent loss of DNA methylation can be powerful mechanism by which MeCP2 is released from the promoters of target genes. These findings on activity-dependent regulation of MeCP2 function and their impact on neurotransmission as well as neuronal morphology suggest a homeostatic mechanism by which neuronal nuclei can monitor alterations in activity levels and adjust neurotransmitter output via altering gene expression and thus affect network excitability.
In summary, we are now only beginning to unravel the complex network of epigenetic interactions that control neuronal function underlying behavioral adaptations. Identification of mutations in the MeCP2 gene as the causative factor for the Rett syndrome triggered a string of detailed studies elucidating the role of MeCP2 in regulation of synaptic activity. The availability of excellent tools to study the exact function of MeCP2 and associated proteins in neurotransmission makes Rett Syndrome and MeCP2 an ideal Rosetta Stone for deciphering epigenetic regulation of neuronal function. This effort not only will lead to potential therapeutic interventions for a devastating neurological disorder but also will shine light onto cellular mechanisms that underlie autism spectrum disorders in general.
Acknowledgments
This work was supported by Grant Nos. MH077944 and MH081060 from the National Institute of Mental Health (LMM). ETK is an established investigator of the American Heart Association.
Footnotes
Drs. Monteggia and Kavalali report no biomedical financial interests or potential conflicts of interest.
References
- 1.Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: Report of 35 cases. Ann Neurol. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- 2.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 3.Amir RE, Zoghbi HY. Rett syndrome: Methyl-CpG-binding protein 2 mutations and phenotype-genotype correlations. Am J Med Genet. 2000;97:147–152. doi: 10.1002/1096-8628(200022)97:2<147::aid-ajmg6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Bienvenu T, Carrié A, de Roux N, Vinet MC, Jonveaux P, Couvert P, et al. MECP2 mutations account for most cases of typical forms of Rett syndrome. Hum Mol Genet. 2000;9:1377–1384. doi: 10.1093/hmg/9.9.1377. [DOI] [PubMed] [Google Scholar]
- 5.Huppke P, Laccone F, Kramer N, Engel W, Hanefeld F. Rett syndrome: Analysis of MECP2 and clinical characterization of 31 patients. Hum Mol Genet. 2000;9:1369–1375. doi: 10.1093/hmg/9.9.1369. [DOI] [PubMed] [Google Scholar]
- 6.Ravn K, Nielsen JB, Schwartz M. Mutations found within exon 1 of MECP2 in Danish patients with Rett syndrome. Clin Genet. 2005;67:532–533. doi: 10.1111/j.1399-0004.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 7.Wan M, Lee SS, Zhang X, Houwink-Manville I, Song HR, Amir RE, et al. Rett syndrome and beyond: Recurrent spontaneous and familial MECP2 mutations at CpG hotspots. Am J Hum Genet. 1999;65:1520–1529. doi: 10.1086/302690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballestar E, Yusufzai TM, Wolffe AP. Effects of Rett syndrome mutations of the methyl-CpG binding domain of the transcriptional repressor MeCP2 on selectivity for association with methylated DNA. Biochemistry. 2000;39:7100–7106. doi: 10.1021/bi0001271. [DOI] [PubMed] [Google Scholar]
- 9.Yusufzai TM, Wolffe AP. Functional consequences of Rett syndrome mutations on human MeCP2. Nucleic Acids Res. 2000;28:4172–4179. doi: 10.1093/nar/28.21.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ylisaukko-Oja T, Rehnström K, Vanhala R, Kempas E, von Koskull H, Tengström C, et al. MECP2 mutation analysis in patients with mental retardation. Am J Med Genet A. 2005;132:121–124. doi: 10.1002/ajmg.a.30416. [DOI] [PubMed] [Google Scholar]
- 11.Zoghbi HY. MeCP2 dysfunction in humans and mice. J Child Neurol. 2005;20:736–740. doi: 10.1177/08830738050200090701. [DOI] [PubMed] [Google Scholar]
- 12.Carney RM, Wolpert CM, Ravan SA, Shahbazian M, Ashley-Koch A, Cuccaro ML, et al. Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr Neurol. 2003;28:205–211. doi: 10.1016/s0887-8994(02)00624-0. [DOI] [PubMed] [Google Scholar]
- 13.Chahrour M, Zoghbi HY. The story of Rett syndrome: From clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Lam CW, Yeung WL, Ko CH, Poon PM, Tong SF, Chan KY, Lo IF, et al. Spectrum of mutations in the MECP2 gene in patients with infantile autism and Rett syndrome. J Med Genet. 2000;37:E41. doi: 10.1136/jmg.37.12.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson P, Black G, Ramsden S, Barrow M, Super M, Kerr B, Clayton-Smith J. Angelman syndrome phenotype associated with mutations in MECP2, a gene encoding a methyl CpG binding protein. J Med Genet. 2001;38:224–228. doi: 10.1136/jmg.38.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scala E, Ariani F, Mari F, Caselli R, Pescucci C, Longo I, et al. CDKL5/STK9 is mutated in Rett syndrome variant with infantile spasms. J Med Genet. 2005;42:103–107. doi: 10.1136/jmg.2004.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao J, Van Esch H, Hagedorn-Greiwe M, Hoffmann K, Moser B, Raynaud M, et al. Mutations in the X-linked cyclin-dependent kinase-like 5 (CDKL5/STK9) gene are associated with severe neurodevelopmental retardation. Am J Hum Genet. 2004;75:1149–1154. doi: 10.1086/426460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaving LS, Christodoulou J, Williamson SL, Friend KL, McKenzie OL, Archer H, et al. Mutations of CDKL5 cause a severe neurodevelopmental disorder with infantile spasms and mental retardation. Am J Hum Genet. 2004;75:1079–1093. doi: 10.1086/426462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erlandson A, Samuelsson L, Hagberg B, Kyllerman M, Vujic M, Wahlström J. Multiplex ligation-dependent probe amplification (MLPA) detects large deletions in the MECP2 gene of Swedish Rett syndrome patients. Genet Test. 2003;7:329–332. doi: 10.1089/109065703322783707. [DOI] [PubMed] [Google Scholar]
- 20.Scala E, Longo I, Ottimo F, Speciale C, Sampieri K, Katzaki E, et al. MECP2 deletions and genotype-phenotype correlation in Rett syndrome. Am J Med Genet A. 2007;143A:2775–2784. doi: 10.1002/ajmg.a.32002. [DOI] [PubMed] [Google Scholar]
- 21.Ariani F, Hayek G, Rondinella D, Artuso R, Mencarelli MA, Spanhol-Rosseto A, et al. FOXG1 is responsible for the congenital variant of Rett syndrome. Am J Hum Genet. 2008;83:89–93. doi: 10.1016/j.ajhg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papa FT, Mencarelli MA, Caselli R, Katzaki E, Sampieri K, Meloni I, et al. A 3Mb deletion in 14q12 causes severe mental retardation, mild facial dysmorphisms and Rett-like features. Am J Med Genet A. 2008;146A:1994–1998. doi: 10.1002/ajmg.a.32413. [DOI] [PubMed] [Google Scholar]
- 23.Van Esch H, Bauters M, Ignatius J, Jansen M, Raynaud M, Hollanders K, et al. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am J Hum Genet. 2005;77:442–453. doi: 10.1086/444549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klose RJ, Sarraf SA, Schmiedeberg L, McDermott SM, Stancheva I, Bird AP, et al. DNA binding selectivity of MeCP2 due to a requirement for A/T sequences adjacent to methyl-CpG. Mol Cell. 2005;19:667–678. doi: 10.1016/j.molcel.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasui DH, Peddada S, Bieda MC, Vallero RO, Hogart A, Nagarajan RP, et al. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc Natl Acad Sci U S A. 2007;104:19416–19421. doi: 10.1073/pnas.0707442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Matarazzo V, Cohen D, Palmer AM, Simpson PJ, Khokhar B, Pan SJ, Ronnett GV. The transcriptional repressor Mecp2 regulates terminal neuronal differentiation. Mol Cell Neurosci. 2004;27:44–58. doi: 10.1016/j.mcn.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Francke U. Mechanisms of disease: Neurogenetics of MeCP2 deficiency. Nat Clin Pract Neurol. 2006;2:212–221. doi: 10.1038/ncpneuro0148. [DOI] [PubMed] [Google Scholar]
- 30.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 31.Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 32.Tate P, Skarnes W, Bird A. The methyl-CpG binding protein MeCP2 is essential for embryonic development in the mouse. Nat Genet. 1996;12:205–208. doi: 10.1038/ng0296-205. [DOI] [PubMed] [Google Scholar]
- 33.Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol Psychiatry. 2006;59:468–476. doi: 10.1016/j.biopsych.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 34.Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet. 2004;13:2679–2689. doi: 10.1093/hmg/ddh282. [DOI] [PubMed] [Google Scholar]
- 35.Luikenhuis S, Giacometti E, Beard CF, Jaenisch R. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc Natl Acad Sci U S A. 2004;101:6033–6038. doi: 10.1073/pnas.0401626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- 37.Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 38.Jugloff DG, Jung BP, Purushotham D, Logan R, Eubanks JH. Increased dendritic complexity and axonal length in cultured mouse cortical neurons overexpressing methyl-CpG-binding protein MeCP2. Neurobiol Dis. 2005;19:18–27. doi: 10.1016/j.nbd.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Zito K, Svoboda K. Activity-dependent synaptogenesis in the adult Mammalian cortex. Neuron. 2002;35:1015–1017. doi: 10.1016/s0896-6273(02)00903-0. [DOI] [PubMed] [Google Scholar]
- 40.Giacometti E, Luikenhuis S, Beard C, Jaenisch R. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proc Natl Acad Sci U S A. 2007;104:1931–1936. doi: 10.1073/pnas.0610593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis. 2006;21:217–227. doi: 10.1016/j.nbd.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, et al. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson ED, Kavalali ET, Monteggia LM. MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr Biol. 2006;16:710–716. doi: 10.1016/j.cub.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 46.Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Post-mortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- 47.Rubenstein JL, Merzenich MM. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serajee FJ, Zhong H, Nabi R, Huq AH. The metabotropic glutamate receptor 8 gene at 7q31: Partial duplication and possible association with autism. J Med Genet. 2003;40:e42. doi: 10.1136/jmg.40.4.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colantuoni C, Jeon OH, Hyder K, Chenchik A, Khimani AH, Narayanan V, et al. Gene expression profiling in postmortem Rett Syndrome brain: Differential gene expression and patient classification. Neurobiol Dis. 2001;8:847–865. doi: 10.1006/nbdi.2001.0428. [DOI] [PubMed] [Google Scholar]
- 51.Nagarajan RP, Hogart AR, Gwye Y, Martin MR, LaSalle JM. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1:e1–e11. doi: 10.4161/epi.1.4.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tudor M, Akbarian S, Chen RZ, Jaenisch R. Transcriptional profiling of a mouse model for Rett syndrome reveals subtle transcriptional changes in the brain. Proc Natl Acad Sci U S A. 2002;99:15536–15541. doi: 10.1073/pnas.242566899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young JI, Hong EP, Castle JC, Crespo-Barreto J, Bowman AB, Rose MF, et al. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc Natl Acad Sci U S A. 2005;102:17551–17558. doi: 10.1073/pnas.0507856102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aber KM, Nori P, MacDonald SM, Bibat G, Jarrar MH, Kaufmann WE, et al. Methyl-CpG-binding protein 2 is localized in the postsynaptic compartment: An immunochemical study of subcellular fractions. Neuroscience. 2003;116:77–80. doi: 10.1016/s0306-4522(02)00586-9. [DOI] [PubMed] [Google Scholar]
- 55.Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393 doi: 10.1038/30764. 386-289. [DOI] [PubMed] [Google Scholar]
- 56.Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 57.Jaenisch R, Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33 suppl:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 58.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 59.Ehrlich M. Expression of various genes is controlled by DNA methylation during mammalian development. J Cell Biochem. 2003;88:899–910. doi: 10.1002/jcb.10464. [DOI] [PubMed] [Google Scholar]
- 60.Paroush Z, Keshet I, Yisraeli J, Cedar H. Dynamics of demethylation and activation of the alpha-actin gene in myoblasts. Cell. 1990;63:1229–1237. doi: 10.1016/0092-8674(90)90418-e. [DOI] [PubMed] [Google Scholar]
- 61.Barreto G, Schäfer A, Marhold J, Stach D, Swaminathan SK, Handa V, et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445:671–675. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- 62.Métivier R, Gallais R, Tiffoche C, Le Péron C, Jurkowska RZ, Carmouche RP, et al. Cyclical DNA methylation of a transcriptionally active promoter. Nature. 2008;452:45–50. doi: 10.1038/nature06544. [DOI] [PubMed] [Google Scholar]
- 63.Kangaspeska S, Stride B, Métivier R, Polycarpou-Schwarz M, Ibberson D, Carmouche RP, et al. Transient cyclical methylation of promoter DNA. Nature. 2008;452:112–115. doi: 10.1038/nature06640. [DOI] [PubMed] [Google Scholar]
- 64.Hansen RS, Wijmenga C, Luo P, Stanek AM, Canfield TK, Weemaes CM, Gartler SM. The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1999;96:14412–14417. doi: 10.1073/pnas.96.25.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64:196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 66.Brooks PJ, Marietta C, Goldman D. DNA mismatch repair and DNA methylation in adult brain neurons. J Neurosci. 1996;16:939–945. doi: 10.1523/JNEUROSCI.16-03-00939.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng J, Chang H, Li E, Fan G. Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J Neurosci Res. 2005;79:734–746. doi: 10.1002/jnr.20404. [DOI] [PubMed] [Google Scholar]
- 68.Goto K, Numata M, Komura JI, Ono T, Bestor TH, Kondo H. Expression of DNA methyltransferase gene in mature and immature neurons as well as proliferating cells in mice. Differentiation. 1994;56:39–44. doi: 10.1046/j.1432-0436.1994.56120039.x. [DOI] [PubMed] [Google Scholar]
- 69.Inano K, Suetake I, Ueda T, Miyake Y, Nakamura M, Okada M, Tajima S. Maintenance-type DNA methyltransferase is highly expressed in post-mitotic neurons and localized in the cytoplasmic compartment. J Biochem. 2000;128:315–321. doi: 10.1093/oxfordjournals.jbchem.a022755. [DOI] [PubMed] [Google Scholar]
- 70.Robertson KD, Jones PA. DNA methylation: Past, present and future directions. Carcinogenesis. 2000;21:461–467. doi: 10.1093/carcin/21.3.461. [DOI] [PubMed] [Google Scholar]
- 71.Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, et al. Evidence that DNA(cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- 72.Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 73.Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 74.Nelson ED, Kavalali ET, Monteggia LM. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci. 2008;28:395–406. doi: 10.1523/JNEUROSCI.3796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 76.Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:2552–2569. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]