In the United States, aortic aneurysms are the 13th leading cause of deathsee 1, 2 About 15,000 individuals die every year due to the rupture of aortic aneurysms. Based on autopsy studies it has been estimated that 1-2% of the population harbor aneurysms in their aorta with up to 10% prevalence in older age groups.1, 2 Most aortic aneurysms go undetected until rupture and the mortality from ruptured aneurysms is as high as 90%.1, 2
Along the length of the aorta, significant heterogeneity occurs in the distribution of aneurysm disease. The prevalence of abdominal aortic aneurysms (AAAs) located in the infrarenal section of the aorta is at least three times higher than that of thoracic aortic aneurysms and dissections (TAAD).1, 2 In TAAs, about 50% involve the ascending aorta, 10% the arch and 40% the descending thoracic aorta.1, 2 Only about 25% of patients with TAAD have a concomitant AAA, and multisegmental disease is found in only about 10% of cases.1, 2
There are also other differences between TAAD and AAA: 1) age-at-onset for TAAD (65 years) is approximately 10 years earlier than for AAA (75 years); and 2) AAAs are predominantly a disease of Caucacian males with 6:1 male:female ratio, whereas TAADs occur only slightly more frequently in males (1.7:1). Additional differences can be found in the pathobiology of these aneurysms. AAAs are characterized by signs of local chronic inflammation of the aortic wall, decrease in the number of smooth muscle cells in the aortic media layer and fragmentation of the extracellular matrix of the aorta at the site of the aneurysm see 3. Increased local expression of proinflammatory cytokines and matrix metalloproteinases (MMPs) have also been demonstrated.3 Furthermore, AAAs can be induced in a surgical experimental model in which elastases are infused into rodent aorta.4 TAADs are characterized by medial necrosis also known as “Erdheim’s cystic medial necrosis” and more recently referred to as “medial degeneration”, mucoid infiltration, and cyst formation with elastin degradation and vascular smooth muscle cell apoptosis.1
Both TAAD and AAA are silent diseases often without symptoms.2 They can, however, be readily identified through imaging techniques. TAADs can be detected by echocardiography or CT, and family members of TAAD-patients will benefit from imaging by identifying their TAADs before catastrophic consequences. Presently, there are no recommendations about large scale screening of populations for TAAD. On the other hand, for AAA ultrasonography screening studies have demonstrated cost-effectiveness of population-based ultrasonography screening programs and a decrease in the number of aneurysm-related deaths.see5 Several recommendations have been made including a recent consensus statement, in which Kent et al.6 recommended ultrasonography screening for AAA for all individuals over 60 years of age and for those over 50 years of age with family history for AAA and the recommendation by the US Preventive Service Task Force7 to screen for AAA in men aged 65 to 75 years who have ever smoked.
Aneurysms are a Complex Disease
Aortic aneurysms are a complex multifactorial disease with genetic and environmental risk factors. Genetic factors have been shown to play a role in the etiology of TAAD and AAA even when they are not associated with the Marfan syndrome (MFS), Ehlers-Danlos syndrome (EDS), the Loeys-Dietz syndrome (LDS), or other rare aortic syndromes.
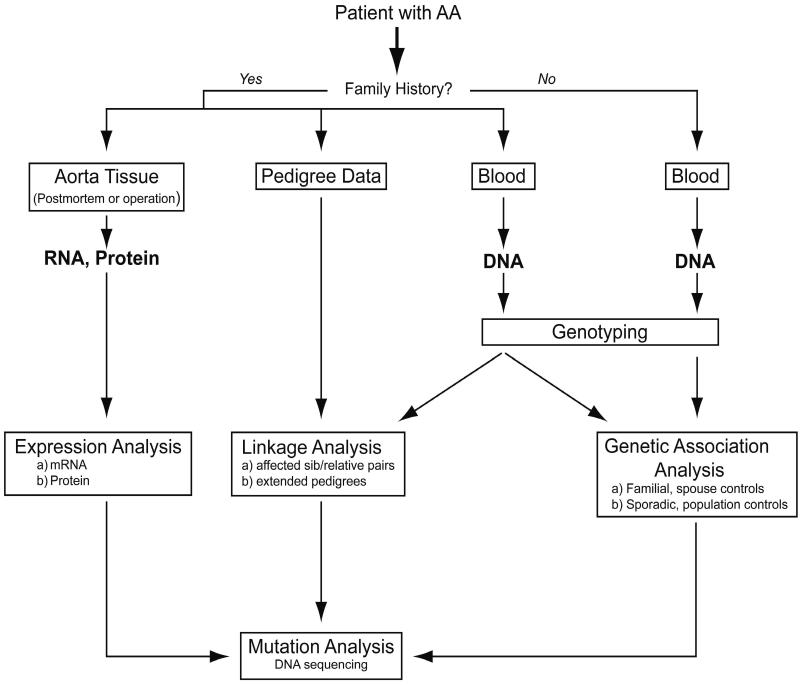
As shown in Table 1, the TAAD and AAA susceptibility loci identified so far do not overlap, suggesting that different genetic risk factors contribute to these two forms of aneurysmal disease. Furthermore, both TAAD and AAA demonstrate genetic heterogeneity as has been shown to be the case for yet another form of aneurysms, the intracranial aneurysms (see8). Elucidation of the genetic risk factors for aneurysmal diseases will require multidisciplinary approaches9 (Figure 1), in which animal studies4, although not discussed here, will play a key role.
Table 1.
Chromosomal Loci Harboring Genes for Syndromic and Non-Syndromic Aortic Aneurysms
| Chromosomal Region | Disease | Inheritance | OMIM ID | OMIM Locus Symbol | Gene |
|---|---|---|---|---|---|
| 2q31 | Ehlers-Danlos syndrome type IV | AD | 130050 | EDS4 | COL3A1 |
| 3p22 | TAAD | AD | 608967 | AAT3 | TGFBR2 |
| 3p22 | Loeys-Dietz Syndrome | AD | 609192 | LDS | TGFBR2 |
| 4q31 | AAA | 609782 | AAA2 | ||
| 5q13-q14 | TAAD | AD | 607087 | AAT2 | |
| 9q33-q34 | TAAD | AD | 610380 | AAT5 | TGFBR1 |
| 9q33-q34 | Loeys-Dietz syndrome | AD | 609192 | LDS | TGFBR1 |
| 11q23.3-q24 | TAAD | AD | 607086 | AAT1 | |
| 15q21.1 | Marfan syndrome | AD | 154700 | MFS | FBN1 |
| 15q24-26 | TAAD | AD | AAT6 | ||
| 16p13.13-p13.12 | TAAD with PAD | AD | 132900 | AAT4 | MYH11 |
| 19q13 | AAA | 609781 | AAA1 |
All loci except the EDS4 and AAT5 were identified by DNA linkage studies using either family-based or “affected relative pair” approaches. For a complete list of the original studies, please see http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=OMIM. No genes harboring mutations in aneurysm patients in the AAA1, AAA2, AAT1, AAT2, or AAT6 loci have yet been identified. PAD, patent ductus arteriosus; AD, autosomal dominant; COL3A1, gene symbol for type III procollagen; TGFBR1 and 2, gene symbols for transforming growth factor β receptors 1 and 2; MYH11, gene symbol for smooth muscle myosin heavy chain. Official approved gene symbols were obtained from www.gene.ucl.ac.uk/nomenclature.
Figure 1.
How to study the pathogenesis and risk factors of AAA in humans? Schematic drawing of the approaches used to identify genetic risk factors of aortic aneurysms (AA), and the genomic and proteomic approaches used to study biological processes involved in AA. Information from one approach can facilitate another approach. Modified with permission from Kuivaniemi & Tromp, 2000.89
Genetics of TAADs
First reports on familial occurrence of “Erdheim’s cystic medial necrosis” date back more than 60 years, although it is not possible to establish if these cases were syndromic or non-syndromic TAADs.10 In 1967 Hanley and Jones11 reported on TAAD in two sisters and the son of one of them and noted that the patients did not fit the diagnostic criteria of MFS. Many reports have been published since then and systematic studies have established that approximately 20% of non-syndromic TAAD patients have a positive family history for aneurysms.12 Most TAAD families appear to be consistent with autosomal dominant inheritance pattern.
To date five susceptibility loci for TAAD have been identified in DNA linkage studies with family-based approaches13 and a sixth locus was found by candidate gene approach.14 The loci have been designated as AAT1 through AAT6 (Table 1) and include 3p24-25,13 5q13-14,13 9q33-q34,14 11q23-24, 15q24-26,13 and 16p13.13-p13.12.15 Two of the loci (3p24-25 and 9q33-q34) are exactly the same as the genetic loci for the LDS, a rare autosomal dominant disease characterized with hypertelorism, craniosynostosis, structural brain abnormalities, mental retardation, congenital heart disease, bifid uvula with or without cleft palate, and generalized arterial tortuosity with ascending aortic aneurysm and dissection. Also, the family used for the identification of the AAT4 locus on 16p13.13-p13.12 was a large 178-member French family with TAAD and patent ductus arteriosus (PAD). These findings suggest that there is some overlap between the syndromic and non-syndromic forms of TAAD on the molecular level. Based on the six TAAD loci identified so far, genetic heterogeneity of TAAD is already obvious. Yet, they do not explain the familial aggregation of TAAD in all the families that have been studied, suggesting that additional loci will be found.13
Mutations in the Positional Candidate Genes in Patients with Syndromic and Non-Syndromic Forms of TAAD
Three genes have been found to harbor mutations in patients with TAAD (Table 1). The AAT3 locus contains the gene for transforming growth factor β receptor 2 (TGFBR2) and mutations in it were identified in 4/80 TAAD families in one study,16 although another study failed to identify mutations in 70 aneurysm patients.17 The gene for transforming growth factor β receptor 1 (TGFBR1) is located on the AAT5 locus and a mutation in this gene was found in one patient with TAAD.14 Again, another research group found no mutations in this gene in 70 aneurysm patients.17 These findings support the hypothesis that dysregulation of TGF signaling is one of the potential mechanisms leading to TAAD,13 but it is too early to estimate what fraction of TAAD patients have a mutation in the genes affecting this molecular pathway.
In the study by Loeys et al.17 mutations in the TGFBR1 and TGFBR2 genes were also found in 52/52 and 12/40 patients with LDS and EDS, respectively, both of which are rare, syndromic forms of TAAD. Other studies have also reported mutations in patients with LDS and MFS-related disorders in these two genes.14, 18 The clinical and genetics communities are debating the interpretation of these results. Should molecular diagnosis be adopted, where all individuals with a mutation in the same gene are classified as having the same disease or should the clinical manifestations be the basis for diagnosis? The case for molecular diagnosis is that the spectrum of clinical manifestations of rare genetic diseases can be wide, and therefore overlap with those seen in more common diseases; consequently, presence of a mutation in the same gene would classify the disease. For example, one could argue that all patients with mutations in TGFBR2 gene should be classified as having LDS. The case for diagnosis based on clinical manifestations is that genetic analyses are useful for explaining the underlying pathogenesis. Thus mutations in the same gene could lead to different but overlapping phenotypes, e.g., non-syndromic TAAD vs. LDS for mutations in TGFBR2
The AAT4 locus contains the gene for smooth muscle myosin heavy chain 11 (MYH11) and mutations in it have been identified in two families with TAAD and PAD.19
In summary, further studies are needed to establish estimates on the effect size on the population level for the three genes (TGFBR1, TGFBR2 and MYH11) harboring mutations in TAAD patients in some families and to identify additional susceptibility genes for TAAD in the other three mapped and undetermined number of unmapped genetic loci.
Genetics of AAA: from Familial Aggregation to Susceptibility Loci
M. A. Clifton, who first reported clustering of AAAs in a single family in 1977,20 speculated that there might be a genetic basis for some cases of ‘atherosclerotic AAA’, as the disease was known at that time. During the 1980’s and 1990’s, there were too many publications to quote in a brief review. However, four groups merit notation for unique conclusions or methods during the early years of AAA genetics research. Tilson and Seashore reported an initial 16 families, followed by an accumulated series of 50 families.21 A more recent study on 233 AAA families found that most (72%) families appeared to show autosomal recessive inheritance pattern.22
Johansen and Koepsel wrote the second noteworthy early communication.23 In addition to the merit of its large scale (250 AAA probands), this paper included control subjects (250 probands) with atherosclerotic occlusive disease (AOD). Approximately 19% of AAA probands had an affected first degree relative by history; while only 2% of the AOD patients had a relative with AAA. After adjustments for age and sex, there was a 12-fold increase in risk for the relatives of AAA probands. These authors were the first to suggest that screening might be advisable for relatives, to detect AAA disease in time to save life.
Darling and coauthors24 wrote the third paper on this short list, and it is included because it described a situation, for which there is still no molecular explanation. In a study of 542 patients undergoing AAA repair, 15% were known to have a first degree relative with an aneurysm, versus 2% of a control group of 500 patients of similar age and sex with no aneurysmal disease (p < 0.001). An analysis was carried out to determine whether there were unique features among the cases known to be familial (FAAA) by comparison to the ones that appeared to be sporadic. The ratio of women to men in the FAAA cases was 2.5-fold higher than in the sporadic cases, in which men consistently outnumber women. In addition, the male FAAA cases tended to be approximately 5 years younger than the non-FAAA male patients. Finally, there was an unusually high incidence of rupture (41%) in the FAAA cases. The authors inferred that women in the FAAA cases increased the likelihood of unfortunate outcomes and used the term “Black Widow” to caption this situation. While this terminology is perhaps overly emotive, one author of this review (mdt) has reached a similar conclusion on the basis of cases that have come to his attention. He is aware of one family where 9/10 offspring of a woman, who died with rupture, developed aneurysmal disease.
The fourth and final paper in this short list was by Majumder et al.25 to address one of the inherent difficulties of doing clinical genetics in diseases of late-age-at-onset, such as Alzheimer’s disease and AAA. Specifically, there is the obvious difficulty of ascertainment of the status of other first-degree relatives. The parents are usually deceased, and the children have years to live before passing through the window of peak risk (approximately 80 years of age in males with AAA). In addition, some siblings may have died young and been presumed to have died with myocardial infarction. Sixteen percent of 43 probands in the study by Majumder et al.25 were already known to have an affected first degree relative by history. Indeterminate siblings were invited to undergo ultrasound screening, and 51% agreed. Six AAAs were discovered in families that had previously been considered negative for clustering, raising the percentage of multiplex families to almost 30%. This rather remarkable finding anticipates the conclusion of Verloes and coworkers26 when analyzing the families of over 300 Belgian AAA patients that one of the common AAA susceptibility genes operates as an autosomal dominant manner with surprisingly high, yet age-related penetrance.
The findings described above support the hypothesis that AAAs are a complex genetic disease (Table 2). Additional supportive data exist. Female AAA patients have been found to have an increased operative mortality for intact and ruptured AAAs as compared to male patients.see27 This might be due to such factors as later referral of female patients to treatment or the size standards for AAAs being the same in females and males (although females are generally smaller and have smaller aorta; thus a 5 cm aneurysm in a female patient is in a more advanced stage than a 5 cm aneurysm in a male patient). It is, however, possible that the differences in the outcomes are due to genetic factors. Since the prevalence of AAA is about six times higher in men than in women, it would be expected that when the disease does occur in females, it is due to the presence of a larger number of liabilities, a phenomenon characteristic of multifactorial diseases, and well-documented in such diseases as pyloric stenosis and many congenital heart defects.see 28 Female AAA-patients would then represent the more severe spectrum of the aneurysmal disease, and their offspring expected to have higher risk of developing AAAs.
Table 2.
Evidence that AAA is a Genetic Disease
| Study Type | Observation | Year | Literature |
|---|---|---|---|
| Case report | Three brothers had AAA | 1977 | 20 |
| Interview AAA patients for family history of AAA | 13% of AAA patients report positive family history | 1984-2001 | Summarized in 90 |
| Ultrasonography examination for 1o relatives of AAA patients | 17% of brothers and 4% of sisters have AAA | 1989-2005 | Summarized in 90 |
| Formal segregation analysis | Genetic model: best explanation for familial aggregation of AAA | 1991, 1995 | 25, 26 |
| Clinical comparison of FAAA with SAAA | Differences: 1) Age at diagnosis: SAAA>FAAA 2) Age at rupture: SAAA>FAAA 3) Incidence of rupture: SAAA<FAAA 4) Male:female-ratio: SAAA>FAAA | 1989-2006 | 24, 27, 90 |
| Demographics of AAA population | 1) Prevalence of AAA higher in whites 2) AAA in males vs. females: operative mortality: F>M rupture rate: F>M 3) Relative risk for 1o family members: 12–18 | 1986-2006 | 23, 24, 90 |
| DNA linkage study | Identification of 2 genetic loci: AAA1 and AAA2 | 2004 | 29 |
FAAA, familial abdominal aortic aneurysm; SAAA, sporadic abdominal aortic aneurysm; F, female; M, male; 1o, first degree
A whole-genome DNA linkage study using affected relative pair approach has been carried out for AAA.29 The study identified two genetic loci for AAA, designated as AAA1 on chromosome 19q13 and AAA2 on chromosome 4q31 (Table 1). van Vlijmen-van Keulen et al.30 re-analysed three of the AAA families with a different statistical method and also found linkage on chromosome 19q13 providing further validity to the Shibamura et al.29 study. Both positional candidate intervals contain a large number of biologically and physiologically feasible candidate genes for AAA and studies to identify the specific mutations are in progress.
Candidate Gene Analysis for AAAs
A large number of studies have been published on analyzing potentially biologically relevant candidate genes for AAA in a case-control-based genetic association studies or by re-sequencing such candidate genes in AAA patients. These studies have been summarized in a recent review31 and will not be discussed in detail here. The candidate gene studies for AAA have focused mostly on three classes of genes: 1) genes for the structural components of the aortic wall (collagens, proteoglycans, elastin etc.); 2) genes for enzymes responsible to degrade the structural molecules (MMPs, their inhibitors etc.); and 3) genes for proteins involved in the immune response. The underlying hypothesis in genetic association studies is that a sequence variation in a gene can confer a higher risk to an individual to develop an AAA by influencing the activity or expression of that gene. It is important to note that such sequence changes are not usually the cause of the disease, but rather contribute to the susceptibility for developing an AAA and additional factors, genetic and environmental, are needed to manifest the disease. Although significant associations have been obtained in individual studies, efforts to replicate them in follow-up studies have failed. For example, one study found an association to a promoter polymorphism in the MMP9 gene,32 whereas another study with a slightly larger sample set did not detect any association with the same variant.33 The discrepancy could be due to several reasons: 1) the identified genetic risk factors have small effect sizes, 2) there are population-based differences in the genetic risk factors for AAA, or 3) the initial findings were false positives due to small sample sizes or bias in selecting control groups. Detailed recommendations on how to design, conduct, interpret and report on genetic association studies have been published recently.34
Aneurysms as a Disease of the Immune System
Early Discovery Work Leads to Autoantigen Hypothesis for AAAs
Before the introduction of biologically inert prosthetic materials as aortic replacements, the potential importance of the immune system as a mediator of the inflammatory response in the adventitia and outer media of the typical AAA in man was recognized. Charles Rob35, who had extensive experience with the use of human aortic homografts, pointedly observed that the disintegration of some grafted human aortas had features of a severe immune rejection.36 Similar suggestions were made for the failure of bovine carotid heterographs, when they were once used for femoropopliteal bypass grafts in man.37
The theory of immune involvement in AAA was strengthened by animal studies. Peterson et al.38 performed aortic autografts in spontaneously hypertensive (SHR) and normotensive Wistar Kyoto (WKY) rats. No aneurysms were observed, so the surgical procedure of transplantation alone did not initiate AAA disease. We infer that this fact rules out one of the older theories of the pathogenesis of AAA, specifically, that AAA results from deprivation of the outer media from oxygen provided by vasa vasorum, at least in the rat. When the authors transplanted SHR rat aortas to the WKY rats, aneurysms did occur, with infiltration of inflammatory cells and SMC apoptosis. However, when WKY aortas were transplanted to SHR hosts, no aneurysms were seen. The authors concluded that “immunologic rejection but not abnormal hemodynamics is necessary for development of allograft aneurysm in this model.” Since the SHR mutation is on the WKY strain, the transplants were not allografts (in a strict sense). If the SHR and WKY strains were of different evolutionary lineage, it would be tempting to speculate that the use of different rat histocompatibility antigens might explain the results; but that was not the case here. Instead, it may be more reasonable to hypothesize that there has been a stable phenotypic alteration in the aortas that have been exposed to hypertension in the SHR donors, perhaps unmasking an epitope of a extracellular matrix protein detected as ‘non-self’ by the WKY recipient.
Another important discovery was through the work of Anidjar and Dobrin39 who developed the widely used elastase-infusion model. One of the most interesting aspects of this model is that 24 hours after injury with elastase, aortic dilation is minimal. Aneurysms develop about a week later, during which time a major influx of inflammatory cells has occurred. Halpern et al.40 studied this model in more detail to characterize the histopathological and enzymatic events that were detectable during the latency period, confirming that there was a dramatic increase in all subsets of immune/inflammatory cells by day 7. Immunoglobulins colocalizing with extracellular matrix were also evident by day 7. The authors concluded that autoimmune processes played a role in the pathogenesis of the Anidjar-Dobrin model, perhaps because the initial injury by elastase unmasked reactive epitopes in the aortic matrix. This and other models have been used extensively for the study of AAA and the results have been reviewed recently.4
Interest in the role of autoimmunity in human AAA began independently by two research groups. One of them was initially interested in the possibility of mutations that might lead to instability of collagen crosslinking in load-bearing fibrils of the aortic wall, and the crosslink deoxypyridinoline (d-Pyr) was assayed.41 However, instead of being deficient as expected, d-Pyr was significantly elevated. Since d-Pyr is a hallmark of immature collagen, the question arose whether it was elevated in a reparative adaptation to collagen destruction by an inflammatory process. This led to an analysis of differences in the site and characteristics of the inflammatory infiltrate in AAA versus AOD.42 Russell bodies, aggregates of immunoglobulins that are hallmarks of autoimmune diseases such as Hashimoto’s thyroiditis, were noted in the adventitia and outer media of AAA specimens. Immunoglobulins from AAA specimens were purified and showed to react with the adventitial microfibril associated with elastin and collagen43. Purification of an immunoreactive protein led to partial characterization of the first putative autoantigen, named Aneurysm-Associated Antigenic Protein – 40 kDa (AAAP-40).44 Another research group found extensive inflammatory cell infiltration in AAA tissue samples.45, 46
AAA is an Immunological Disease
The early work described above introduced the concept that autoimmunity may be responsible for the pathogenesis of AAA.42-44, 46 Such a mechanism assumes the breakdown of the immunoregulatory mechanisms and, of tolerance in general, which will permit the generation of an autoimmune response against self antigens. Molecular mimicry is another mechanism that may be responsible for the pathogenesis of AAA and the generation of autoimmunity.see47 Molecular mimicry is defined as the sharing of common antigenic epitope(s) between a microorganism (such as bacteria and viruses) and self (host) antigens (reviewed in48). An immune response against a bacterial or viral antigenic epitope may recognize, by molecular mimicry, as nonself a self epitope, which is homologous (crossreacting) with the antigenic epitope of the microorganism that initiated the T-cell response. This T-cell response may be propagated by the self crossreacting epitope, long after the clearance of the virus or of the microorganism. It appears that molecular mimicry is responsible for several autoimmune diseases (reviewed in 48). The immune response to the antigenic epitope(s) of the microorganism may be critical for breaking tolerance and would permit the development of an immune response against self antigens. Therefore, these T-cells will recognize, by molecular mimicry, both the cross-reacting antigenic epitopes, the one of the microorganism and the other of the host antigen, and may play a critical role in the pathogenesis of the disease.
Substantial evidence has been accumulated suggesting that AAA is a specific antigen-driven T-cell disease and that T-cells may be responsible for the initiation and/or the propagation of the disease (Table 3):
Table 3.
Evidence for AAA Being a Specific Antigen-Driven T-Cell Disease
| Observation* | Literature |
|---|---|
| Mononuclear cell infiltrates with CD3+ T-cells present in AAA | 46, 49-52 |
| Early-, intermediate- and late-activation antigens expressed by mononuclear cells infiltrating AAA | 47 |
| Association of HLA alleles with AAA | 58-60, 91 |
| APCs present in AAA | 46, 49-52, 61, 62 |
| Mononuclear cells infiltrating human AAA contain oligoclonal αβ TCR+ T-cells and γδ TCR+ T-cells | see47 |
| Putative self and non-self antigens identified in AAA | 47 |
| Autoantibodies present in AAA patients | 43, 44 |
| Cytokines play an important role in the pathogenesis of AAA | 51, 76-81 |
| Immunosuppressive drugs reduce the rate of aneurysm expansion in AAA models | 82, 83 |
| Increased levels of C-reactive protein in AAA patients | 79 |
| Enrichment of genes related to immune function seen in AAA microarray studies | 84 |
See text for details. APCs, antigen presenting cells; TCR, T-cell receptor
1. Mononuclear cell infiltrates containing CD3+ T-cells are present in AAAs
Extensive mononuclear cell infiltrates have been documented in the adventitia and to, a lesser but substantial degree, the media of AAAs in humans.46, 49-52 These infiltrates comprise of T and B lymphocytes, plasma cells, monocytes and NK/NKT cells.46, 49-52 CD3+ T-cells account for approximately 50% of the hemopoietic cells (CD45+) that have been recovered from AAA tissues, B-cells account for approximately 40%, NK-cells for 7% and macrophages for 2%.46, 49-52 These infiltrates are suggestive of activation of vascular-associated lymphoid tissue (known as VALT).49 Inflammatory cell infiltrates are absent from normal aorta, which at most may contain only few cells of the immune system.46 Mononuclear cell inflammatory infiltrates are found also in atherosclerotic plaques of patients with AOD,53 a common feature of AAA patient. There are, however, substantial differences in the inflammatory response found in AAA versus those seen in AOD.54
2. Early-, intermediate- and late-activation antigens are expressed by mononuclear cells infiltrating AAAs
T-lymphocytes and other mononuclear cells infiltrating AAAs express early-(CD69)-, intermediate-(CD25, CD38) and late-(CD45 RO, HLA class II) activation antigens.47 The expression of these activation antigens on the infiltrating inflammatory cells, suggests the presence of active on-going inflammation in these lesions. In particular, the presence of CD69+ T-cells in these AAA inflammatory sites, reveals that T-cell activation is occurring in situ in AAAs. The CD69 antigen is expressed on T-cells very early upon activation and it is responsible for T-cell interactions with cells of the monocyte/macrophage lineage leading to the production of interleukin 1 (IL1) (reviewed in 55, 56).
3. Association of HLA alleles with AAA
An association of HLA-DRB1 alleles (HLA-DR2 (*15 and *16), *12 and *13) was described by Tilson et al.57 and Rasmussen et al. (HLA-DRB1*04 and 15*).58 The presence of the DRβGln (70) residue was identified as a risk factor for AAA.58 These results are somewhat controversial, since other larger studies have not been able to replicate these findings.see 59, 60
4. Antigen-presenting cells (APC) are present in AAAs
Both professional and non-professional APC are present in AAAs, and include: (i) infiltrating cells of the monocyte/macrophage lineage;46, 49-52 (ii) vascular dendritic cells which have been reported to be in contact with T and B lymphocytes in human AAAs61 and are associated with formation of lymphoid follicles and lymph-node-like structures (VALT), primarily in the adventitia of these patients, suggesting that these APC may be responsible, at least in part, for the induction of both cellular and humoral immune responses;49, 61 (iii) activated endothelial cells may act as APC in AAAs;62 (iv) vascular smooth muscle cells expressing HLA class II are present in AAAs and may act as APC.62
5. Mononuclear cells infiltrating human AAAs contain oligoclonal αβ T-cell receptor (TCR)+ T-cells and γδ TCR+ T-cells47
a. Clonal expansions of αβ TCR+ T-cells
To determine whether human T-cells infiltrating AAAs contain clonally expanded populations of αβ TCR+ T-cells, α- or β-chain TCR transcripts were amplified from these AAAs by the nonpalindromic adaptor-polymerase chain reaction (NPA–PCR)/Vα- or Vβ-specific PCR.47, 63, 64 The NPA-PCR method was specifically developed for the amplification of transcripts with unknown or variable 5' ends, and such as the TCRs and the immunoglobulins.47, 63, 65 The amplified TCR transcripts were cloned and sequenced. Sequence analysis revealed the presence of substantial proportions of identical β-chain TCR transcripts in 9/10 patients examined. These clonal expansions were very strong. In certain patients the proportions of identical β-chain TCR transcripts in the AAAs were as high as 60% of the transcripts sequenced.
Amplification of α-chain TCR transcripts from human AAAs by NPA-PCR followed by cloning and sequencing revealed strong clonal expansions in 4/5 patients examined.47 β-chain TCR transcripts were clonally expanded in all four patients.47
Clonal expansions that were identified in AAAs using NPA-PCR amplification were subsequently confirmed by Vα- or Vβ-specific PCR amplification. Identical clonal expansions of TCR transcripts were identified by these two different amplification approaches, followed by cloning and sequencing. Peripheral blood mononuclear cells (PBMC) from normal donors were used as methodological controls in these experiments.47, 63 Amplification of α- or β-chain TCR transcripts from these PBMC by NPA-PCR/V-specific PCR followed by cloning and sequencing revealed unique α- or β-chain TCR transcripts when compared to each other typical of polyclonal populations of T lymphocytes.47, 63
Oligoclonal T-cell expansions were also found in the aortas of patients with two diseases that are related to AAA: giant cell arteritis66 and Takayasu arteritis.67, 68
b. Clonal expansions of γδ TCR+ T-cells
The presence of clonally expanded populations of γδ TCR+ T-cells was demonstrated in AAAs.47 Sequencing analysis revealed the presence of substantial proportions of identical copies of VγI, VγII, Vδ1 and Vδ2 TCR transcripts in human AAAs in all patients examined.see47
PBMC from normal donors were used as a methodological control for these studies and were found to have almost entirely unique γ- or δ-chain TCR transcripts, in a manner typical of polyclonal populations of T-cells.see47 However, sequence analysis of Vδ1 TCR transcripts from PBMC from normal donors revealed strong clonal expansions which were significant by the binomial distribution, in agreement with the reports of others.69, 70 The role of these Vδ1 clonal expansions demonstrated in PBMC from normal donors is poorly understood.
All these clonal expansions identified in AAAs were statistically significant, as determined by the binomial distribution. Comparison of the nucleic acid and the deduced amino acid sequences of the TCR transcripts sequenced in these studies to those in the GENBANK/ EMBL/SWISS PROT databases revealed that all sequences identified were novel, i.e. were not described previously, and typical of the corresponding TCR transcripts.
The majority of the γδ TCR+ T-cells recognize whole proteins, in a manner independent of the major histocompatibility complex (MHC). In contrast, the vast majority of αβ TCR+ T-cells recognize peptides in association with MHC. Other γδ TCR+ T-cells recognize lipids, glycolipids, carbohydrates, bacterial phosphoantigens and other ligands in an MHC independent manner. It has been suggested that γδ TCR+ T-cells use their TCR as a pattern recognition receptor71. This may be important in the event that the microorganisms which have been proposed as putative antigens in AAA (see below) indeed play a role in the pathogenesis of the disease. Gamma/delta T-cells have been proposed to be a bridge between the innate and the adaptive immune system.71
The only possible explanation for the presence of substantial proportions of identical copies of α-, β-, γ- or δ-chain TCR transcripts in AAAs, is that the T-cell clones utilizing these TCR transcripts have undergone proliferation and clonal expansion in vivo in response to specific, as yet unidentified antigen(s), self or non-self.47 T-cells are comprised of many different T-cell clones. Each T-cell clone recognizes antigen (antigenic epitope) through its TCR. TCR are highly polymorphic molecules, expressed only on cells of T-cell lineage (reviewed in 72). Each clone of T-cells expresses a different TCR molecule, which is acting as a fingerprint of that particular T-cell clone. The αβ TCR and the γδ TCR are expressed on different T-cell clones and their expression is mutually exclusive. The maximum theoretical number of the αβ TCR heterodimers has been calculated to be 1018 and of the γδ TCR heterodimers 1019 (reviewed in 72). Elimination of more than 90% of the thymocytes by thymic selection reduces the size of the T-cell repertoire to the order of 106 different β-chain TCR polypeptide chains in the peripheral blood, each one pairing with 25 or more different α-chain TCR polypeptides.73 These estimates allow for the presence in the peripheral blood of approximately 2.5x107 different T-cell clones, a number which is still very large.73 Similar estimates can be obtained for the γδ TCR. Therefore, the probability of finding by chance substantial proportions of an individual TCR transcript, either α-, or β-, or γ- or δ -chain, in an independent sample of T-cells, is negligible. The appearance of these multiple identical copies of TCR transcripts must be the result of specific antigen-driven proliferation and clonal expansion of individual T-cell clones responding to the antigenic epitopes that they recognize. This is the only possible mechanism to explain the presence of substantial proportions of identical copies of TCR transcripts found in human AAAs.
6. Autoantibodies and AAA
The presence of autoantibodies in AAAs was well documented in early studies by Tilson and associates43, 44 and has been described in a previous section of this review. A critical observation was that purified IgG from AAA lesions identified a host protein expressed in normal aortic tissue, demonstrating an autoimmune antibody response in AAA.43, 44 B cells and plasma cells are present in AAA lesions and express the CD69 and CD80 activation markers.74 Also, significantly higher proportions of IgA-positive and IgG-positive B cells are present in AAA lesions in comparison to those found in the peripheral blood from the same patient.74 Little attention has been paid to the clonality of B cells infiltrating AAA lesions. One report, using a genomic PCR approach suggested that there are no restrictions in the usage of VH gene segments by B cells infiltrating atherosclerotic abdominal aortic aneurysms.75 Additional studies, however, are needed to clarify this issue.
7. Putative self and non-self antigens that may elicit cellular and antibody responses in AAA
Although many questions on the pathogenesis of AAA remain to be answered in order to provide a definite proof that AAA is a specific antigen-driven autoimmune disease, several putative self and non-self antigens have been identified (Table 4). These antigens elicit cellular and humoral immune responses in AAA. Among the non-self antigens, perhaps the most studied is Chlamydia pneumoniae, which may be involved in the initiation or the acceleration of AAA.see47C. pneumoniae is frequently found in the vessel walls of AAA patients by immunohistochemical analysis, transmission electron microscopy and tissue culture approaches. C. pneumoniae specific T lymphocytes were found in the mononuclear cell infiltrates of AAAs.see47 In addition, non-self antigens may be responsible for initiating AAA by molecular mimicry (see above). These microorganisms may initiate an immune response, which is then propagated by the host crossreacting determinants leading to clinical disease, long after the microorganism is cleared.
Table 4.
Putative Antigens that may Elicit Cellular and Humoral Responses in AAA
| Antigen | Literature |
|---|---|
| A. Self antigens | |
| Elastin and elastin fragments | see47 |
| Collagen type I | see47 |
| Collagen type III | see47 |
| Aortic aneurysm antigenic protein-40 (AAA-P)* | 43, 44 |
| Oxidized low density lipoprotein | see47 |
| B. Non-self antigens | |
| Chlamydia pneumoniae | see47 |
| Cytomegalovirus | see47 |
| Salmonella | see47 |
| Treponema palladium | see47 |
Also known as human microbial-associated glycoprotein-36 (MAGP-36).
8. The role of cytokines in the pathogenesis of AAA
Substantial production of mostly proinflammatory cytokines in AAAs is well documented.51, 76-78 They are produced by activated T-cells, monocytes and other infiltrating mononuclear cells, as well as by several types of cells of the aorta. Studies with a mouse AAA model developed using a CD4-/- knockout mouse have shown that CD4+ T-cells producing IFNγ play a critical role in matrix remodeling in AAA and the pathogenesis of the disease.78 These CD4-/- knockout mice were resistant to the induction of aneurysms.78 However, intraperitoneal application of IFN-γ partially reconstituted the development of aneurysms in these CD4-/- mice.78 Targeted deletion of IFN-γ resulted in inhibition of the development of aneurysmal disease78 and in attenuation of MMP expression. The development of aneurysms in IFNγ-/- knockout mice can be restored by infusing competent splenocytes from the wild-type mice from where the knockout was generated.78 In human AAA high levels of IFNγ transcripts, but not of IL4, have been reported.51, 76, 77 Increased proportions of IFNγ producing CD4+CD28- T-cells (lacking the costimulatory molecule CD28) have been reported both in AAA tissue and in peripheral blood of patients with AAA.76 These studies demonstrate the critical role of T-cells and IFNγ in the pathogenesis of AAA. The overexpression of the transcription factor T-bet taken in connection with the absence of significant expression of the GATA-3 factor, suggests that a Th1 type response predominates in AAAs.77 In addition to the increased expression of proinflammatory cytokines in AAAs, increased levels of C-reactive protein have been documented in aneurysmal disease,79 although this appears to reflect rather a universal response to vascular injury, than a response specific to AAA.79 However, it should be mentioned that studies showing that Th2 predominant immune responses prevail in human AAA have also been reported.80 AAAs lacked IFNγ receptor expression according to this report, although IFNγ was expressed in these lesions.80 The same research group reported that Th2-predominant inflammation and blockage of IFNγ signaling resulted in extensive AAA formation and substantially increased the levels of MMPs in murine allografted aortas.81 The studies76-83 cited above and many other reports in the literature paint a controversial picture for the type of cytokine responses, Th1 or Th2, that predominate in AAA lesions. Although many studies have reported the predominance of a Th1 response in human AAA specimens and experimental models of AAA, other studies have reported the predominance of a Th2 cytokine response.
9. Treatment with immunosuppressive drugs significantly reduces the rate of aneurysm expansion in experimental AAA models
Immunosuppressive regiments have been shown to suppress the growth of experimental aortic aneurysms in support of the notion that aneurysmal disease is a T-cell dependent disease. Aneurysms in animals treated with rapamycin were significantly smaller in diameter versus controls, and they exhibited lower levels of NFκB and MMP9.82 Similarly, treatment with methylprednisone and cyclosporin significantly suppressed the growth of rat aortic aneurysms induced by elastase perfusion.83
10. Global gene expression profiles of AAA tissue reveal a significant enrichment of genes related to immune function
Microarray-based gene expression studies provide an unbiased way of obtaining a global gene expression signature for disease tissues. Analyzing the results of such profiles generated for AAA tissues showed an overrepresentation of biological pathways involved in immune response84 providing further evidence that AAA is an immunological disease.
Evidence for Involvement of the Immune System in TAAD
The immune system is likely to play a significant role also in TAADs, although the number of studies carried out in TAADs compared to those on AAAs is much lower. Mononuclear cell infiltrates containing high proportions of CD3+ T-cells and CD68+ monocytes have been identified in TAAD.85 CD3+ T-cells were localized primarily in the media and in the adventitia (surrounding the vasa vasorum). Both CD3+ T-cells and monocytes coexisted with vascular cell death apoptotic markers and they may be responsible, at least in part, for the elimination of smooth muscle cells in these aneurysms.85
In TAADs approximately half of the patients exhibited transmural inflammation and increased expression of IFN-γ in aneurysm tissue, whereas, Th2 cytokines were undetectable.86 In this group of patients with transmural inflammation the inner media was devoid of mononuclear cell infiltrates.86 However, specimens with inner media lymphocytic infiltration also exhibited increased IFNγ production and induction of the IFNγ inducible chemokines IP-10 and Mig.86 Transmural inflammation and production of IFNγ were associated with increased aortic diameter, intimal thickening, decreased amount of extracellular matrix proteins and preserved density of vascular smooth muscle cells.86 Intimal expansion and outward vascular remodeling of these aneurysms correlated positively with Th1, but not with Th2 immune responses.86 Expression studies of 1,185 genes revealed distinct patterns of expression between TAAD and infrarenal AAAs, TAADs and normal aorta from the same site, and infrarenal AAAs and normal aorta from the same site.54 This high degree of molecular heterogeneity of degenerative aneurysms may reflect the involvement of different mechanisms in the pathophysiology of these disorders.54 Genomic and proteomic studies revealed that the levels of expression of 138 genes in peripheral blood leukocytes of patients undergoing thoracoabdominal aortic aneurysm repair and the concentrations of seven plasma proteins discriminated between patients who developed multiorgan dysfunction syndrome and those who did not.87
Conclusion
Aortic aneurysms are an important cardiovascular disease particularly in the aging population of industrialized countries. They are a complex disease with both genetic and environmental factors contributing to the disease process, which involves formation, growth and rupture. There are even regional differences along the length of the aorta, AAAs being much more common than TAADs, and based on the pathophysiology and other features it is reasonable to hypothesize that TAADs and AAAs are separate disease entities. Aneurysms are often silent without symptoms until rupture occurs, but they can be detected effectively via imaging techniques. First-degree relatives of aneurysm patients have an increased risk of the disease and it is, therefore, important to offer appropriate advise to these individuals and counsel them to seek screening options. Although current surgical treatments give excellent results, there is a need to develop non-surgical approaches to manage small aneurysms. A targeted drug development will require detailed information about the pathogenesis of aneurysms, which at the present time is still limited regardless of major discoveries involving the role of immune system and genetic factors in the development of aneurysms. Unfortunately, the words of Sir William Osler “There is no disease more conducive to clinical humility than aneurysm of the aorta” are still true and only increased efforts towards understanding the pathogenesis and associated risk factors will change the outcome of this disease.88 To ensure that progress in the field continues, new innovative approaches as well as resources are needed. In this review article we have discussed the fundamental research discoveries related to immunology and genetics of aneurysms, but urge the readers to get more information from the proceedings of a recent meeting on “The Abdominal Aortic Aneurysm: Genetics, Pathophysiology, and Molecular Biology published as the Annals of the New York Academy of Sciences (Vol. 1085, 2006).
Acknowledgements
The authors thank Dr. Gerard Tromp for preparing the figure.
Funding Sources The original work carried out in the authors’ laboratories was funded in part by the National Heart, Lung, and Blood Institute of NIH (HL045996 and HL064310 to H.K, HL064340 to C.D.P. and HL064334 to M.D.T.).
Footnotes
Disclosures None.
References
- 1.Beckman JA.Aortic aneurysms: Pathophysiology, epidemiology, and prognosis. In: Creager MA, Dzau VJ, Loscalzo J, eds. Vascular Medicine Philadelphia: Saunders Elsevier Inc.2006 [Google Scholar]
- 2.Beckman JA, Creager MA.Aortic aneurysms: Clinical evaluation. In: Creager MA, Dzau VJ, Loscalzo J, eds. Vascular Medicine Philadelphia: Saunders Elsevier Inc; 2006 [Google Scholar]
- 3.Thompson RW.Reflections on the pathogenesis of abdominal aortic aneurysms Cardiovasc Surg 200210389-394 [DOI] [PubMed] [Google Scholar]
- 4.Golledge J, Muller J, Daugherty A, Norman P.Abdominal aortic aneurysm: pathogenesis and implications for management Arterioscler Thromb Vasc Biol 2006262605-2613 [DOI] [PubMed] [Google Scholar]
- 5.Ogata T, MacKean GL, Cole CW, Arthur C, Andreou P, Tromp G, Kuivaniemi H.The lifetime prevalence of abdominal aortic aneurysms among siblings of aneurysm patients is eightfold higher than among siblings of spouses: an analysis of 187 aneurysm families in Nova Scotia, Canada J Vasc Surg 200542891-897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kent KC, Zwolak RM, Jaff MR, Hollenbeck ST, Thompson RW, Schermerhorn ML, Sicard GA, Riles TS, Cronenwett JL.Screening for abdominal aortic aneurysm: a consensus statement J Vasc Surg 200439267-269 [DOI] [PubMed] [Google Scholar]
- 7.Fleming C, Whitlock EP, Beil TL, Lederle FA.Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force Ann Intern Med 2005142203-211 [DOI] [PubMed] [Google Scholar]
- 8.Weinsheimer S, Goddard K, Parrado A, Lu Q, Sinha M, Lebedeva E, Ronkainen A, Niemelä M, Khusnutdinova E, RI K, Helin K, Jääskeläinen J, Sakovich V, Land S, Kuivaniemi H, Tromp G.Association of kallikrein gene polymorphisms with intracranial aneurysms Stroke 2007382670-2676 [DOI] [PubMed] [Google Scholar]
- 9.Wassef M, Upchurch GR, Jr., Kuivaniemi H, Thompson RW, Tilson MD., 3rdChallenges and opportunities in abdominal aortic aneurysm research J Vasc Surg 200745192-198 [DOI] [PubMed] [Google Scholar]
- 10.von Meyenburg H.Ueber spontane Aortenruptur bei zwei Bruedern Schweiz. Med. Wschr 193920976-979 [Google Scholar]
- 11.Hanley WB, Jones NB.Familial dissecting aortic aneurysm. A report of three cases within two generations Br Heart J 196729852-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albornoz G, Coady MA, Roberts M, Davies RR, Tranquilli M, Rizzo JA, Elefteriades JA.Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns Ann Thorac Surg 2006821400-1405 [DOI] [PubMed] [Google Scholar]
- 13.Pannu H, Avidan N, Tran-Fadulu V, Milewicz DM.Genetic basis of thoracic aortic aneurysms and dissections: potential relevance to abdominal aortic aneurysms Ann N Y Acad Sci 20061085242-255 [DOI] [PubMed] [Google Scholar]
- 14.Matyas G, Arnold E, Carrel T, Baumgartner D, Boileau C, Berger W, Steinmann B.Identification and in silico analyses of novel TGFBR1 and TGFBR2 mutations in Marfan syndrome-related disorders Hum Mutat 200627760-769 [DOI] [PubMed] [Google Scholar]
- 15.Khau Van Kien P, Mathieu F, Zhu L, Lalande A, Betard C, Lathrop M, Brunotte F, Wolf JE, Jeunemaitre X.Mapping of familial thoracic aortic aneurysm/dissection with patent ductus arteriosus to 16p12.2-p13.13 Circulation 2005112200-206 [DOI] [PubMed] [Google Scholar]
- 16.Pannu H, Fadulu VT, Chang J, Lafont A, Hasham SN, Sparks E, Giampietro PF, Zaleski C, Estrera AL, Safi HJ, Shete S, Willing MC, Raman CS, Milewicz DM.Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections Circulation 2005112513-520 [DOI] [PubMed] [Google Scholar]
- 17.Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S, Roberts AE, Faravelli F, Greco MA, Pyeritz RE, Milewicz DM, Coucke PJ, Cameron DE, Braverman AC, Byers PH, De Paepe AM, Dietz HC.Aneurysm syndromes caused by mutations in the TGF-beta receptor N Engl J Med 2006355788-798 [DOI] [PubMed] [Google Scholar]
- 18.Singh KK, Rommel K, Mishra A, Karck M, Haverich A, Schmidtke J, Arslan-Kirchner M.TGFBR1 and TGFBR2 mutations in patients with features of Marfan syndrome and Loeys-Dietz syndrome Hum Mutat 200627770-777 [DOI] [PubMed] [Google Scholar]
- 19.Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, Bruneval P, Wolf JE, Michel JB, Jeunemaitre X.Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus Nat Genet 200638343-349 [DOI] [PubMed] [Google Scholar]
- 20.Clifton MA.Familial abdominal aortic aneurysms Br J Surg 197764765-766 [DOI] [PubMed] [Google Scholar]
- 21.Tilson MD, Seashore MR.Fifty families with abdominal aortic aneurysms in two or more first-order relatives Am J Surg 1984147551-553 [DOI] [PubMed] [Google Scholar]
- 22.Kuivaniemi H, Shibamura H, Arthur C, Berguer R, Cole CW, Juvonen T, Kline RA, Limet R, Mackean G, Norrgard O, Pals G, Powell JT, Rainio P, Sakalihasan N, van Vlijmen-van Keulen C, Verloes A, Tromp G.Familial abdominal aortic aneurysms: collection of 233 multiplex families J Vasc Surg 200337340-345 [DOI] [PubMed] [Google Scholar]
- 23.Johansen K, Koepsell T.Familial tendency for abdominal aortic aneurysms Jama 19862561934-1936 [PubMed] [Google Scholar]
- 24.Darling RC, 3rd, Brewster DC, Darling RC, LaMuraglia GM, Moncure AC, Cambria RP.Abbott WM.Are familial abdominal aortic aneurysms different? J Vasc Surg 19891039-43 [DOI] [PubMed] [Google Scholar]
- 25.Majumder PP, St Jean PL, Ferrell RE, Webster MW, Steed DL.On the inheritance of abdominal aortic aneurysm Am J Hum Genet 199148164-170 [PMC free article] [PubMed] [Google Scholar]
- 26.Verloes A, Sakalihasan N, Koulischer L, Limet R.Aneurysms of the abdominal aorta: familial and genetic aspects in three hundred thirteen pedigrees J Vasc Surg 199521646-655 [DOI] [PubMed] [Google Scholar]
- 27.Norman PE, Powell JT.Abdominal aortic aneurysm: the prognosis in women is worse than in men Circulation 20071152865-2869 [DOI] [PubMed] [Google Scholar]
- 28.Gelehrter T, Collins F. Principles of Medical Genetics. Baltimore: Williams & Wilkins; 1990. [Google Scholar]
- 29.Shibamura H, Olson JM, van Vlijmen-Van Keulen C, Buxbaum SG, Dudek DM, Tromp G, Ogata T, Skunca M, Sakalihasan N, Pals G, Limet R, MacKean GL, Defawe O, Verloes A, Arthur C, Lossing AG, Burnett M, Sueda T, Kuivaniemi H.Genome scan for familial abdominal aortic aneurysm using sex and family history as covariates suggests genetic heterogeneity and identifies linkage to chromosome 19q13 Circulation 20041092103-2108 [DOI] [PubMed] [Google Scholar]
- 30.Van Vlijmen-Van Keulen CJ, Rauwerda JA, Pals G.Genome-wide linkage in three Dutch families maps a locus for abdominal aortic aneurysms to chromosome 19q13.3 Eur J Vasc Endovasc Surg 20053029-35 [DOI] [PubMed] [Google Scholar]
- 31.Sandford RM, Bown MJ, London NJ, Sayers RD.The genetic basis of abdominal aortic aneurysms: a review Eur J Vasc Endovasc Surg 200733381-390 [DOI] [PubMed] [Google Scholar]
- 32.Jones GT, Phillips VL, Harris EL, Rossaak JI, van Rij AM.Functional matrix metalloproteinase-9 polymorphism (C-1562T) associated with abdominal aortic aneurysm J Vasc Surg 2003381363-1367 [DOI] [PubMed] [Google Scholar]
- 33.Ogata T, Shibamura H, Tromp G, Sinha M, Goddard KA, Sakalihasan N, Limet R, MacKean GL, Arthur C, Sueda T, Land S, Kuivaniemi H.Genetic analysis of polymorphisms in biologically relevant candidate genes in patients with abdominal aortic aneurysms J Vasc Surg 2005411036-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G, Hirschhorn JN, Abecasis G, Altshuler D, Bailey-Wilson JE, Brooks LD, Cardon LR, Daly M, Donnelly P, Fraumeni JF, Jr., Freimer NB, Gerhard DS, Gunter C, Guttmacher AE, Guyer MS, Harris EL, Hoh J, Hoover R, Kong CA, Merikangas KR, Morton CC, Palmer LJ, Phimister EG, Rice JP, Roberts J, Rotimi C, Tucker MA, Vogan KJ, Wacholder S, Wijsman EM, Winn DM, Collins FS.Replicating genotype-phenotype associations Nature 2007447655-660 [DOI] [PubMed] [Google Scholar]
- 35.May AG, DeWeese JA, Frank I, Mahoney EB, Rob CG.Surgical treatment of abdominal aortic aneurysms Surgery 196863711-721 [PubMed] [Google Scholar]
- 36.Tilson MD.Abdominal aortic aneurysm surgery: something old, something new Cardiovasc Surg 19942159-163 [PubMed] [Google Scholar]
- 37.Esquivel CO, Blaisdell FW.Why small caliber vascular grafts fail: a review of clinical and experimental experience and the significance of the interaction of blood at the interface J Surg Res 1986411-15 [DOI] [PubMed] [Google Scholar]
- 38.Petersen MJ, Abbott WM, H'Doubler PB, Jr., L'Italien GJ, Hoppel BE, Rosen BR, Fallon JT, Orkin RW.Hemodynamics and aneurysm development in vascular allografts J Vasc Surg 199318955-963 [PubMed] [Google Scholar]
- 39.Anidjar S, Dobrin PB, Chejfec G, Michel JB.Experimental study of determinants of aneurysmal expansion of the abdominal aorta Ann Vasc Surg 19948127-136 [DOI] [PubMed] [Google Scholar]
- 40.Halpern VJ, Nackman GB, Gandhi RH, Irizarry E, Scholes JV, Ramey WG, Tilson MD.The elastase infusion model of experimental aortic aneurysms: synchrony of induction of endogenous proteinases with matrix destruction and inflammatory cell response J Vasc Surg 19942051-60 [DOI] [PubMed] [Google Scholar]
- 41.Tilson MD.Further studies of a putative cross-linking amino acid (3-deoxypyridinoline) in skin from patients with abdominal aortic aneurysms Surgery 198598888-891 [PubMed] [Google Scholar]
- 42.Brophy CM, Reilly JM, Smith GJ, Tilson MD.The role of inflammation in nonspecific abdominal aortic aneurysm disease Ann Vasc Surg 19915229-233 [DOI] [PubMed] [Google Scholar]
- 43.Gregory AK, Yin NX, Capella J, Xia S, Newman KM, Tilson MD.Features of autoimmunity in the abdominal aortic aneurysm Arch Surg 199613185-88 [DOI] [PubMed] [Google Scholar]
- 44.Xia S, Ozsvath K, Hirose H, Tilson MD.Partial amino acid sequence of a novel 40-kDa human aortic protein, with vitronectin-like, fibrinogen-like, and calcium binding domains: aortic aneurysm-associated protein-40 (AAAP-40) [human MAGP-3, proposed] Biochem Biophys Res Commun 199621936-39 [DOI] [PubMed] [Google Scholar]
- 45.Rizzo RJ, McCarthy WJ, Dixit SN, Lilly MP, Shively VP, Flinn WR, Yao JS.Collagen types and matrix protein content in human abdominal aortic aneurysms J Vasc Surg 198910365-373 [DOI] [PubMed] [Google Scholar]
- 46.Koch AE, Haines GK, Rizzo RJ, Radosevich JA, Pope RM, Robinson PG, Pearce WH.Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response Am J Pathol 19901371199-1213 [PMC free article] [PubMed] [Google Scholar]
- 47.Platsoucas CD, Lu S, Nwaneshiudu I, Solomides C, Agelan A, Ntaoula N, Purev E, Li LP, Kratsios P, Mylonas E, Jung WJ, Evans K, Roberts S, Lu Y, Layvi R, Lin WL, Zhang X, Gaughan J, Monos DS, Oleszak EL, White JV.Abdominal aortic aneurysm is a specific antigen-driven T cell disease Ann N Y Acad Sci 20061085224-235 [DOI] [PubMed] [Google Scholar]
- 48.Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD.Theiler's virus infection: a model for multiple sclerosis Clin Microbiol Rev 200417174-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bobryshev YV, Lord RS.Vascular-associated lymphoid tissue (VALT) involvement in aortic aneurysm Atherosclerosis 200115415-21 [DOI] [PubMed] [Google Scholar]
- 50.Chan WL, Pejnovic N, Hamilton H, Liew TV, Popadic D, Poggi A, Khan SM.Atherosclerotic abdominal aortic aneurysm and the interaction between autologous human plaque-derived vascular smooth muscle cells, type 1 NKT, and helper T cells Circ Res200596675-683 [DOI] [PubMed] [Google Scholar]
- 51.Forester ND, Cruickshank SM, Scott DJ, Carding SR.Functional characterization of T cells in abdominal aortic aneurysms Immunology 2005115262-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearce WH, Koch AE.Cellular components and features of immune response in abdominal aortic aneurysms Ann N Y Acad Sci 1996800175-185 [DOI] [PubMed] [Google Scholar]
- 53.Hansson GK, Libby P.The immune response in atherosclerosis: a double-edged sword Nat Rev Immunol 20066508-519 [DOI] [PubMed] [Google Scholar]
- 54.Absi TS, Sundt TM, 3rd, Tung WS, Moon M, Lee JK, Damiano RR, Jr., Thompson RW.Altered patterns of gene expression distinguishing ascending aortic aneurysms from abdominal aortic aneurysms: complementary DNA expression profiling in the molecular characterization of aortic disease J Thorac Cardiovasc Surg 2003126344-357 [DOI] [PubMed] [Google Scholar]
- 55.Isler P, Vey E, Zhang JH, Dayer JM.Cell surface glycoproteins expressed on activated human T cells induce production of interleukin-1 beta by monocytic cells: a possible role of CD69 Eur Cytokine Netw 1993415-23 [PubMed] [Google Scholar]
- 56.Testi R, Phillips JH, Lanier LL.Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C J Immunol 19891421854-1860 [PubMed] [Google Scholar]
- 57.Hirose H, Takagi M, Miyagawa N, Hashiyada H, Noguchi M, Tada S, Kugimiya T, Tilson MD.Genetic risk factor for abdominal aortic aneurysm: HLA-DR2(15), a Japanese study J Vasc Surg 199827500-503 [DOI] [PubMed] [Google Scholar]
- 58.Rasmussen TE, Hallett JW, Jr., Metzger RL, Richardson DM, Harmsen WS, Goronzy JJ, Weyand CM.Genetic risk factors in inflammatory abdominal aortic aneurysms: polymorphic residue 70 in the HLA-DR B1 gene as a key genetic element J Vasc Surg 199725356-364 [DOI] [PubMed] [Google Scholar]
- 59.Badger SA, Soong CV, O'Donnell ME, Middleton D.The role of human leukocyte antigen genes in the formation of abdominal aortic aneurysms J Vasc Surg 200745475-480 [DOI] [PubMed] [Google Scholar]
- 60.Ogata T, Gregoire L, Goddard KA, Skunca M, Tromp G, Lancaster WD, Parrado AR, Lu Q, Shibamura H, Sakalihasan N, Limet R, MacKean GL, Arthur C, Sueda T, Kuivaniemi H. Evidence for association between the HLA-DQA locus and abdominal aortic aneurysms in the Belgian population: a case control study. BMC Med Genet. 2006;7:67. doi: 10.1186/1471-2350-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bobryshev YV, Lord RS, Parsson H.Immunophenotypic analysis of the aortic aneurysm wall suggests that vascular dendritic cells are involved in immune responses Cardiovasc Surg 19986240-249 [DOI] [PubMed] [Google Scholar]
- 62.Lindholt JS, Shi GP.Chronic inflammation, immune response, and infection in abdominal aortic aneurysms Eur J Vasc Endovasc Surg 200631453-463 [DOI] [PubMed] [Google Scholar]
- 63.Oleszak EL, Lin WL, Legido A, Melvin J, Hardison H, Hoffman BE, Katsetos CD, Platsoucas CD.Presence of oligoclonal T cells in cerebrospinal fluid of a child with multiphasic disseminated encephalomyelitis following hepatitis A virus infection Clin Diagn Lab Immunol 20018984-992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu B, Sakkas LI, Goldman BI, Jeevanandam V, Gaughan J, Oleszak EL, Platsoucas CD.Identical alpha-chain T-cell receptor transcripts are present on T cells infiltrating coronary arteries of human cardiac allografts with chronic rejection Cell Immunol 200322575-90 [DOI] [PubMed] [Google Scholar]
- 65.Chen PF, Freedman RS, Chernajovsky Y, Platsoucas CD.Amplification of immunoglobulin transcripts by the non-palindromic adaptor polymerase chain reaction (NPA-PCR). Nucleotide sequence analysis of two human monoclonal antibodies recognizing two cell surface antigens expressed in ovarian, cervix, breast, colon and other carcinomas Hum Antibodies Hybridomas 19945131-142 [PubMed] [Google Scholar]
- 66.Weyand CM, Schonberger J, Oppitz U, Hunder NN, Hicok KC, Goronzy JJ.Distinct vascular lesions in giant cell arteritis share identical T cell clonotypes J Exp Med 1994179951-960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nityanand S, Giscombe R, Srivastava S, Hjelmstrom P, Sanjeevi CB, Sinha N, Grunewald J, Lefvert AK.A bias in the alphabeta T cell receptor variable region gene usage in Takayasu's arteritis Clin Exp Immunol 1997107261-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seko Y, Sato O, Takagi A, Tada Y, Matsuo H, Yagita H, Okumura K, Yazaki Y.Restricted usage of T-cell receptor Valpha-Vbeta genes in infiltrating cells in aortic tissue of patients with Takayasu's arteritis Circulation 1996931788-1790 [DOI] [PubMed] [Google Scholar]
- 69.Beldjord K, Beldjord C, Macintyre E, Even P, Sigaux F.Peripheral selection of V delta 1+ cells with restricted T cell receptor delta gene junctional repertoire in the peripheral blood of healthy donors J Exp Med 1993178121-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boullier S, Cochet M, Poccia F, Gougeon ML.CDR3-independent gamma delta V delta 1+ T cell expansion in the peripheral blood of HIV-infected persons J Immunol 19951541418-1431 [PubMed] [Google Scholar]
- 71.Holtmeier W, Kabelitz D.gammadelta T cells link innate and adaptive immune responses Chem Immunol Allergy 200586151-183 [DOI] [PubMed] [Google Scholar]
- 72.Boehm T, Rabbitts TH.The human T cell receptor genes are targets for chromosomal abnormalities in T cell tumors Faseb J 198932344-2359 [DOI] [PubMed] [Google Scholar]
- 73.Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. Diversity of human alpha beta T cell receptors. Science. 2000;288 doi: 10.1126/science.288.5469.1135a. [DOI] [PubMed] [Google Scholar]
- 74.Ocana E, Bohorquez JC, Perez-Requena J, Brieva JA, Rodriguez C.Characterisation of T and B lymphocytes infiltrating abdominal aortic aneurysms Atherosclerosis 200317039-48 [DOI] [PubMed] [Google Scholar]
- 75.Walton LJ, Powell JT, Parums DV.Unrestricted usage of immunoglobulin heavy chain genes in B cells infiltrating the wall of atherosclerotic abdominal aortic aneurysms Atherosclerosis 199713565-71 [DOI] [PubMed] [Google Scholar]
- 76.Duftner C, Seiler R, Klein-Weigel P, Gobel H, Goldberger C, Ihling C, Fraedrich G, Schirmer M.High prevalence of circulating CD4+CD28- T-cells in patients with small abdominal aortic aneurysms Arterioscler Thromb Vasc Biol 2005251347-1352 [DOI] [PubMed] [Google Scholar]
- 77.Galle C, Schandene L, Stordeur P, Peignois Y, Ferreira J, Wautrecht JC, Dereume JP, Goldman M.Predominance of type 1 CD4+ T cells in human abdominal aortic aneurysm Clin Exp Immunol 2005142519-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT.Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model J Immunol 20041722607-2612 [DOI] [PubMed] [Google Scholar]
- 79.Vainas T, Stassen FR, de Graaf R, Twiss EL, Herngreen SB, Welten RJ, van den Akker LH, van Dieijen-Visser MP, Bruggeman CA, Kitslaar PJ.C-reactive protein in peripheral arterial disease: relation to severity of the disease and to future cardiovascular events J Vasc Surg 200542243-251 [DOI] [PubMed] [Google Scholar]
- 80.Schonbeck U, Sukhova GK, Gerdes N, Libby P.T(H)2 predominant immune responses prevail in human abdominal aortic aneurysm Am J Pathol 2002161499-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN.Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas J Clin Invest 2004114300-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dobrin PB, Baumgartner N, Anidjar S, Chejfec G, Mrkvicka R.Inflammatory aspects of experimental aneurysms. Effect of methylprednisolone and cyclosporine Ann N Y Acad Sci 199680074-88 [DOI] [PubMed] [Google Scholar]
- 83.Lawrence DM, Singh RS, Franklin DP, Carey DJ, Elmore JR.Rapamycin suppresses experimental aortic aneurysm growth J Vasc Surg 200440334-338 [DOI] [PubMed] [Google Scholar]
- 84.Lenk G, Tromp G, Weinsheimer S, Gatalica Z, Berguer R, Kuivaniemi H. Whole genome expression profiling reveals a significant role for immune function in human abdominal aortic aneurysms. BMCGenomics. 2007;8:237. doi: 10.1186/1471-2164-8-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He R, Guo DC, Estrera AL, Safi HJ, Huynh TT, Yin Z, Cao SN, Lin J, Kurian T, Buja LM, Geng YJ, Milewicz DM.Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections J Thorac Cardiovasc Surg 2006131671-678 [DOI] [PubMed] [Google Scholar]
- 86.Tang PC, Yakimov AO, Teesdale MA, Coady MA, Dardik A, Elefteriades JA, Tellides G.Transmural inflammation by interferon-gamma-producing T cells correlates with outward vascular remodeling and intimal expansion of ascending thoracic aortic aneurysms Faseb J 2005191528-1530 [DOI] [PubMed] [Google Scholar]
- 87.Feezor RJ, Baker HV, Xiao W, Lee WA, Huber TS, Mindrinos M, Kim RA, Ruiz-Taylor L, Moldawer LL, Davis RW, Seeger JM.Genomic and proteomic determinants of outcome in patients undergoing thoracoabdominal aortic aneurysm repair J Immunol 20041727103-7109 [DOI] [PubMed] [Google Scholar]
- 88.Verma S, Lindsay TF.Regression of aortic aneurysms through pharmacologic therapy? N Engl J Med 20063542067-2068 [DOI] [PubMed] [Google Scholar]
- 89.Kuivaniemi H, Tromp G.Search for the aneurysm susceptibility gene(s). In: Keen RR, Dobrin PB, eds. Development of Aneurysms Georgetown: Landes Bioscience; 2000 [Google Scholar]
- 90.Kuivaniemi H, Kyo Y, Lenk G, Tromp G.Genome-wide approach to finding abdominal aortic aneurysm susceptibility genes in humans Ann N Y Acad Sci 20061085270-281 [DOI] [PubMed] [Google Scholar]
- 91.Hirose H, Ozsvath KJ, Xia S, Gaetz HP, Tilson MD.Immunoreactivity of adventitial matrix fibrils of normal and aneurysmal abdominal aorta with antibodies against vitronectin and fibrinogen Pathobiology 1998661-4 [DOI] [PubMed] [Google Scholar]