Abstract
BACKGROUND
Non-invasive selection of developmentally competent human oocytes may increase the overall efficiency of human assisted reproduction and is regarded as crucial in countries where legal, social or religious factors restrict the production of supernumerary embryos. The purpose of this study was to summarize the predictive value for IVF success of morphological features of the oocyte that can be obtained by light or polarized microscopic investigations.
METHODS
Studies about oocyte morphology and IVF/ICSI outcomes were identified by using a systematic literature search.
RESULTS
Fifty relevant articles were identified: 33 analysed a single feature, 9 observed multiple features and investigated the effect of these features individually, 8 summarized the effect of individual features. Investigated structures were the following: meiotic spindle (15 papers), zona pellucida (15 papers), vacuoles or refractile bodies (14 papers), polar body shape (12 papers), oocyte shape (10 papers), dark cytoplasm or diffuse granulation (12 papers), perivitelline space (11 papers), central cytoplasmic granulation (8 papers), cumulus–oocyte complex (6 papers) and cytoplasm viscosity and membrane resistance characteristics (2 papers). None of these features were unanimously evaluated to have prognostic value for further developmental competence of oocytes.
CONCLUSIONS
No clear tendency in recent publications to a general increase in predictive value of morphological features was found. These contradicting data underline the importance of more intensive and coordinated research to reach a consensus and fully exploit the predictive potential of morphological examination of human oocytes.
Keywords: oocyte quality, IVF/ICSI outcome, embryo development, meiotic spindle, zona pellucida
Introduction
Morphological assessment of preimplantation stage embryos is a key element of the laboratory work in human assisted reproduction. Routine inverted microscopic investigations are performed at predetermined checkpoints, at least every second day of in vitro culture, and internationally acknowledged criteria are applied for quantitative characterization, although there are some concerns regarding the predictive value of these parameters (Cummins et al., 1986; Emiliani et al., 2006). Continuous time-lapse observation of embryo development may provide information with more accurate predictive value (Arav et al., 2008; Lemmen et al., 2008), however, the majority of presently available technologies are extremely expensive and inappropriate for routine use in human IVF.
Curiously, in the everyday work of an average IVF laboratory morphological assessment of the retrieved oocytes is rather superficial. The average investigation of in vitro inseminated oocytes is restricted to assessment of the presence and rough morphology of cumulus using a stereomicroscope.
In the case of ICSI, a rapid evaluation using an inverted microscopic is also performed after denudation, including evaluation of the cytoplasm, the perivitelline space and the zona pellucida. This evaluation provides very superficial and approximate information about the stage of development [germinal vesicle, metaphase I (MI) or MII phase] and the quality [degenerative signs in the cytoplasm, polar body (PB) or zona pellucida]. Subsequently all MII phase oocytes are subjected to ICSI, and from that point the developmental potential of the obtained embryo is estimated exclusively on the basis of the morphology of the embryo proper, regardless of the quality of the oocyte it was derived from.
It has to be acknowledged that the overall light microscopic morphology of oocytes is rather dull compared with that of embryos and spermatozoa. However, oocyte quality is a key limiting factor in female fertility, reflecting the intrinsic developmental potential of an oocyte, and has a crucial role not only in fertilization, but also in subsequent development (Gilchrist et al., 2008). According to some data the phenotype of the adult stage offspring is considerably defined by the quality of the oocytes from which they are derived (Mtango et al., 2008).
Application of ovary stimulation in human reproduction further complicates the situation. In contrast to the in vivo process, where oocyte maturation occurs as the result of a long and meticulous natural selection procedure (Van Soom et al., 2007), common ovary stimulation procedures suppress this selection and allow seemingly successful maturation of oocytes with inherently compromised quality, destined to fertilization failure, compromised embryo development or long-term consequences in vivo (Swain and Pool, 2008). The quality of the oocytes is determined not only by the nuclear and mitochondrial genome, but the microenvironment provided by the ovary and the pre-ovulatory follicle that can modify transcription and translation. Owing to the complex picture it is highly unlikely that a single factor, characteristic or mechanism can adequately indicate the proper developmental competence of oocytes. Accordingly, to obtain full information about oocyte quality, a detailed and non-invasive analysis of key markers would be required.
In spite of the intensive research and some promising results (Patrizio et al., 2007; Marteil et al., 2009; Nagy et al., 2009; Revelli et al., 2009) the application of microarray and mass spectrometry techniques for proteomic and metabolomic characterization of single oocytes is still in the early stages. The general approach in human embryology is at present the postponement of the problem: all available oocytes that meet the basic criteria of MII phase are subjected to fertilization. With the selection based only on the morphological characteristics after fertilization, seemingly appropriate but intrinsically handicapped embryos may be cultured and transferred resulting in compromised in vitro development, or low pregnancy rates, abortions and further negative consequences.
The purpose of this systematic review was to evaluate the results of available publications dealing with the predictive value of morphological features of MII phase human oocytes on their developmental competence. Evaluation of in vitro matured and/or cryopreserved oocytes was beyond the scope of this review owing to the variety of techniques used for these processes and sparse available data about morphological assessment.
Methods
Literature search
The search on the prognostic value of non-invasive morphological features of MII phase human oocytes was performed by using Medline, ISI Web of Knowledge Science Citation Index, Cochrane Controlled Trials Register and Ovid. The search was performed in December, 2009 for all available papers written in English and published in or after January, 1996. The free text search terms ‘human’, ‘oocyte’ or ‘ooplasm’ or ‘zona pellucida’ or ‘meiotic spindle’ and ‘morphology’ and ‘quality’ or ‘prognosis’ or ‘outcome’ were used.
Study selection
Two reviewers (L.R. and G.V.) assessed independently all studies for inclusion or exclusion. Disagreements were solved in discussion with the last author (F.U.).
At the first screening, titles were investigated and studies with lack of any relevance, for example those dealing exclusively with animal oocytes, sperm morphology, in vitro matured, cumulus- or zona-free oocytes as well as with cryopreserved oocytes were excluded. To ensure high sensitivity of the search, uncertain items were not excluded, but subjected to a second screen.
The second screen was performed by reading the abstract of items that were not excluded at the first screen. The criteria mentioned above were used for the abstracts, too. Additionally, all case reports, non-comparative studies, or analyses not reporting the consequences were excluded, as well as studies not reporting original data including reviews, comments, etc. Not excluded or uncertain items were subjected to a third screen.
For the third screen, full papers of all remaining items were collected and read. All above criteria were considered again, together with extraction of the following characteristics: research objective, design of the study, feature investigated (single, multiple, combined), method of investigation, control group, method of evaluation of outcome, conclusion and date of publication. Only studies focusing on consequences of morphological features of oocytes obtained with non-invasive methods were considered for further evaluation. Publications focusing on a specific clinical situation (i.e. polycystic ovary syndrome, ovary hyperstimulation or specific blood parameters) and the related morphological features of oocytes were out of the scope of this study, therefore excluded from the subsequent analyses. Studies focusing on invasive or non-morphological evaluation methods (i.e. apoptosis of cumulus cells, gene expression or chromosomal constitution of polar bodies) or dealing with specific problems (i.e. suitability for somatic cell nuclear transfer) were also excluded.
The remaining studies were grouped according to their scope (single or multiple morphological features investigated either individually or in combination, i.e. by establishing a score), then all specific features and their effect on the further events and developmental phases (fertilization, cleavage, early embryo development, Day 3 embryo quality, aneuploidy, compaction, blastocyst development, blastocyst quality, blastocyst hatching, cryosurvival, implantation, clinical pregnancy, ongoing pregnancy and/or take home baby rates) were investigated and compared.
Results
Literature search
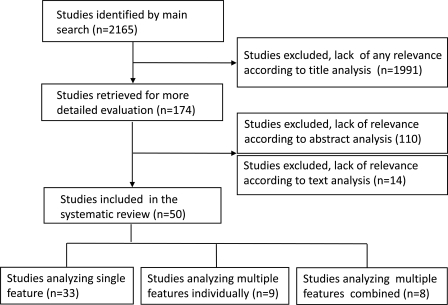
The search of databases resulted in a total of 2165 titles.
After the first screen based on title search, only evidently irrelevant publications (a total of 1991 items) were excluded, and in many cases the publication failed to meet two or more selection criteria.
The second screen based on the abstract was performed on the remaining 174 publications. One hundred and ten of them did not meet selection criteria and were excluded from the further investigation. Sixty-four were subjected to a third screen.
The third screen was performed by reading the full paper and extracting the specific features. At this thorough investigation, 14 papers did not meet the strict selection criteria and were excluded. The process of selection is illustrated Fig. 1.
Figure 1.
Flow chart for the systematic review of the literature to investigate the predictive value of oocyte morphology in human IVF.
Data extraction was performed in 50 papers. A summary of the extraction results are shown in Table I.
Table I.
Summary of the results from 50 papers identified in a systematic review of the literature in order to investigate the predictive value of oocyte morphology in human IVF.
| Evaluation | Cumul | Zona | Perivit | PBshape | Oshape | Darkdifg | VacRB | Cgran | Spind. | Multiple | Remark | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balaban et al. (1998) | F, EQ, CP, IM | − | No | No | − | No | No | No | − | − | No | |
| Balaban et al. (2008) | CSE, BL, HA | − | No | No | − | No | No | Yes* | Yes* | − | − | *For cryosurvival |
| Balakier et al. (2002) | ED, PL | − | − | − | − | Yes* | − | − | − | − | − | *Giant oocytes for aneuploidy |
| Bertrand et al. (1995) | F | − | Yes* ** | − | − | − | − | − | − | − | − | *Thickness (the thinner the better); **thickness variation not measured |
| Braga et al. (2008) | F, EQ | − | − | − | − | − | − | − | − | Yes* | − | *Presence |
| Chamayou et al. (2006) | EQ, CP, IM | − | − | Yes* | Yes* | No | + | + | + | No | Yes* ** | *For EQ; **cumulative ooplasm evaluation |
| Ciotti et al. (2004) | F, CL, CP, IM | − | − | − | No | − | − | − | − | − | − | |
| Cohen et al. (2004) | F, CL | − | − | − | − | − | − | − | − | Yes* | − | *Presence for F |
| Cooke et al. (2003) | ED, EQ | − | − | − | − | − | − | − | − | Yes* | *Position | |
| De Santis et al. (2005) | F, EQ | − | − | − | No | − | − | − | No* | *Retardation | ||
| De Sutter et al. (1996) | F, EQ | − | No | No | − | No | No | No | − | − | − | |
| Ebner et al. (2000) | F, EQ | − | − | − | Yes | − | − | − | − | − | − | |
| Ebner et al. (2003) | F, EQ, BL | − | − | − | − | (v) Yes | − | − | − | − | − | (v) Cytoplasmic viscosity |
| Ebner et al. (2008a) | F, BL | Yes* | − | − | − | − | − | − | Yes | − | − | *Blood clots in cum. |
| Ebner et al. (2008b) | F, CL, CM, BL | − | − | − | − | Yes* | − | − | − | − | − | *Ovoid: delays |
| Esfandiari et al. (2006) | F, ED, CP | − | No* | − | − | − | No** | − | − | − | *Thick zona; **‘brown eggs’ | |
| Fancsovits et al. (2006) | F, EQ | − | − | − | Yes* | − | − | − | − | − | − | *Fragmented better large impaired |
| Fang et al. (2007) | F | − | − | − | − | − | − | − | Yes* | − | *Presence and position | |
| Farhi et al. (2002) | F, CP, IM | − | − | Yes* | − | − | − | − | − | − | − | *Coarse granules for CP and IM |
| Hassan-Ali et al. (1998) | F, CL, CP, IM | − | − | No* | − | − | − | − | − | − | − | *Coarse dark granules |
| Høst et al. (2002) | EQ | − | Yes* | − | − | − | − | − | − | − | − | *Thickness variation |
| Kahraman et al. (2000) | F, EQ, CP, OP | − | − | − | − | − | − | − | Yes* | − | − | *Only OP affected |
| Kilani et al. (2009) | CP | − | − | − | − | − | − | − | Yes* | − | *Length, retardation: yes; position: no | |
| Konc et al. (2004) | F, CP | − | − | − | − | − | − | − | − | Yes* | − | *Presence for CP only |
| La Sala et al. (2009) | F, PR, THB, MB | + | + | + | + | − | + | + | − | − | No | |
| Lin et al. (2003) | F, CL, EQ, BL | Yes | − | − | − | − | − | − | − | − | − | |
| Loutradis et al. (1999) | F, CL, EQ, CP | − | No | − | − | − | Yes* | Yes* | − | − | − | *For EQ and CP |
| Madaschi et al. (2008) | F, CL, IM, CP | − | − | − | − | − | − | − | − | Yes* | *Presence | |
| Madaschi et al. (2009) | F, IM, CP, OP | − | Yes* | − | − | − | − | − | − | Yes** | − | *Retardation for clinical outcome; **presence |
| Moon et al. (2003) | F, ED, EQ | − | − | − | − | − | − | − | − | Yes* | − | *Presence + angle for EQ |
| Montag et al. (2008) | ED, IM, CP, THB | − | Yes* | − | − | − | − | − | − | − | − | *Inner layer retardation |
| Navarro et al. (2009) | F, CL, EQ | − | − | − | Yes* | − | − | − | − | − | − | *Large polar body |
| Ng et al. (1999) | F, CL, CP | Yes* | − | − | − | − | − | − | − | − | − | *For F and CP |
| Otsuki et al. (2004) | F, CP | − | − | − | − | − | − | Yes* | − | − | − | *Smooth ER clusters for CP |
| Otsuki et al. (2007) | F, BL | − | − | − | − | − | − | Yes* | − | − | *Lipofuchsin | |
| Rama Raju et al. (2007) | F, BL | − | Yes* | − | − | − | − | − | − | Yes** | − | *Thickness., retardation; **presence, retardation |
| Rattanachaiyanont et al. (1999) | F, CL, CP | No | − | − | − | − | − | − | − | − | − | |
| Rienzi et al. (2003) | F, EQ | − | − | − | − | − | − | − | Yes* | − | *Presence and position for the first cleavage | |
| Rienzi et al. (2008) | PNM, F, EQ, CP | − | No | Yes | Yes | No | Yes | Yes | Yes | Yes* | *Scoring for CP THB | |
| Rosenbusch et al. (2002) | PL | − | − | − | − | Yes* | − | − | − | − | − | *Giant oocytes |
| Salumets et al. (2002) | CL, EQ | Yes | − | − | − | − | − | − | − | − | − | |
| Serhal et al. (1997) | CL, EQ, IM, CP | − | − | − | − | − | + | + | + | − | Yes* | *For IM and CP |
| Shen et al. (2005) | F, ED, CP | − | Yes* ** | − | − | − | − | − | − | − | − | *Inner layer retardation and thickness; **for ED and CP |
| Shen et al. (2006) | PNM, F, CP | − | − | − | − | − | − | − | Yes* | − | *Retardation | |
| Ten et al. (2007) | F, EQ | − | No | Yes* | No | No | Yes** | No | − | − | − | *Large space = better EQ; **for EQ |
| Verlinsky et al. (2003) | F, EQ, BL, CP, PL | − | − | − | No | − | − | − | − | − | − | |
| Wang et al. (2001) | F | − | − | − | − | − | − | − | − | Yes* | − | *Presence |
| Wilding et al. (2007) | F, ED, CP | − | − | − | − | (m) Yes* | Yes* ** | Yes* | No | − | Yes*** | (m) Membrane properties *for F; **granulation improves outcome; ***score includes membrane properties |
| Xia (1997) | F, EQ | − | − | + | + | − | − | Yes | − | − | Yes | |
| Yakin et al. (2007) | F, CL, BL, PL | − | + | + | + | No | + | + | + | − | Yes* | *Only BL affected if multiple abnormalities |
Cumul, cumulus–oocyte complex; Zona, zona pellucida; Perivit, perivitelline space; PBshape, shape of the first polar body; Oshape, shape of the oocyte; Darkdifg, dark ooplasm or diffuse granulation; VacRB, vacuoles, inclusions, refractile bodies; Cgran, central granulation; Spind, meiotic spindle; Multiple, multiple features evaluated together (by using a scoring system). PNM, pronuclear morphology; F, fertilization; CL, cleavage; ED, embryo development; EQ, embryo quality; CSE, cryosurvival of embryos; CM, compaction; BL, development to blastrocyst stage; HA, hatching; IM, implantation; CP, clinical pregnancy; OP, ongoing pregnancy; THB, take home baby; MB, multiple birth; PL, aneuploidy. −, not investigated; +, investigated, evaluated together with other features; No, investigated, no correlation found; Yes, investigated, correlation found. Underlined, effect on outcome contradicts the majority of investigations.
Of the 50 papers, 33 analysed a single feature. Nine of the remaining 17 papers observing multiple features investigated the effect of these features individually, whereas 8 papers summarized the effect of individual features. For these eight papers, a scoring system was established in seven, and used exclusively for evaluation of all features (two papers), or only for some features although the others were investigated individually (three papers) or for all investigated features that were also analysed individually (2). In one paper that summarized individual results, scoring was not required, as none of the individually investigated features have shown any correlation with the subsequent development. Investigated structures were the following (in parentheses: number of studies dealing with the feature): the meiotic spindle (15), zona pellucida (15), vacuoles or refractile bodies (14), the shape of the PB (12), dark cytoplasm or diffuse granulation (12), perivitelline space (11), the shape of oocytes (10), central granulation (8) cumulus–oocyte complex (COC) (6) and cytoplasm viscosity and membrane resistance characteristics (2). On average, one publication investigated 2.1 morphological features (Figs 2 and 3).
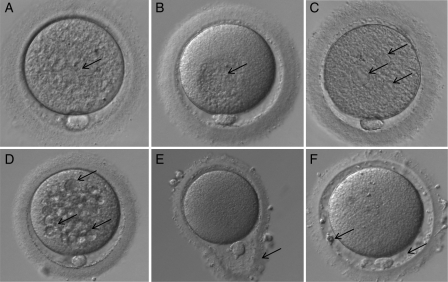
Figure 2.
Different human oocyte morphological abnormalities (arrows) observed by light microscopy (400× magnification): (A) diffuse cytoplasmic granularity, (B) centrally located cytoplasmic granular area, (C) smooth endoplasmic reticulum clusters, (D) vacuoles, (E) abnormal zona pellucida shape, (F) large perivitelline space with fragments.
Figure 3.
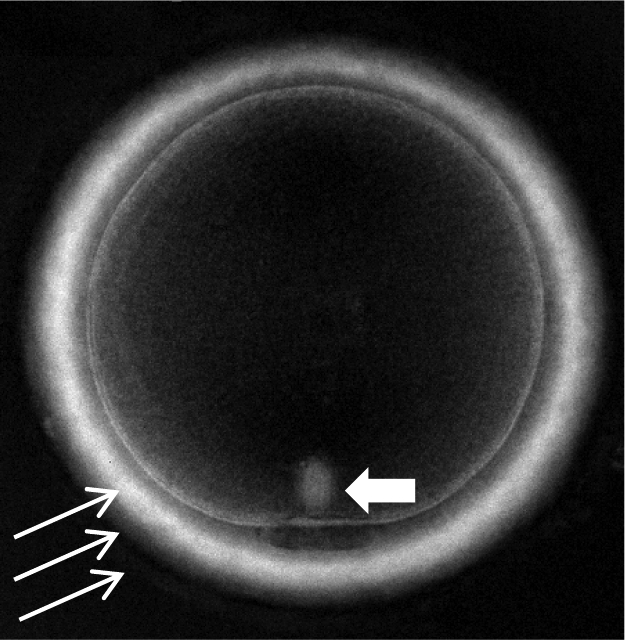
Metaphase II human oocyte observed using polarized light microscopy (400× magnification): meiotic spindle (short arrow) and zona pellucida layers (long arrows).
The outcome was characterized by a total of 15 outcome parameters, with a minimum 1 and maximum 5 in one publication. These parameters included (in parentheses: number of studies investigating the proper parameter): fertilization (40), embryo quality (22), clinical pregnancy rate (22), cleavage rate (13), development to blastocysts (9), implantation rate (9), embryo development (7), aneuploidy (4), ongoing pregnancy (2), take home babies (3), cryosurvival of embryos (1) compaction (1) and hatching (1). On average, one publication investigated 2.7 outcome parameters. Exactly the same parameters were investigated in 8 papers (fertilization and embryo quality), 3 papers (fertilization and blastocyst rate), 4 × 2 papers (fertilization; fertilization and clinical pregnancy rate; fertilization and embryo development; fertilization, cleavage, clinical pregnancy and implantation rates, respectively).
Only two studies used exactly and exclusively the same parameters to investigate the effect of exactly and exclusively the same feature: in these papers meiotic spindle morphology effect was characterized by fertilization and embryo quality.
In the most restricted investigations the consequence of one morphological feature was characterized by one outcome parameter (five papers), although the broadest study was performed in one paper, where the cumulative effect of seven features was compared with five outcome parameters.
Owing to the heterogeneity of approaches, a single direct comparison between the results of the 50 papers was impossible. Therefore, studies were grouped based on the investigated individual morphological features or score systems, and comparisons were performed accordingly.
Cumulus–oocyte complex
Morphological characteristics obtained by non-invasive methods in relation to the further developmental competence were investigated in 5 of the 50 papers. Rattanachaiyanont et al. (1999) have graded the expansion of both cumulus and corona radiata individually. According to their results, there was no correlation between the morphology of COCs and the fertilization, cleavage and clinical pregnancy rates. Ebner et al. (2008a) have performed similar grading and had a similar conclusion; however, they found that presence of blood clots (but not that of amorphous clumps) were associated with dense central granulation of oocytes and had a negative effect on fertilization and blastocyst rates. Both studies have found a correlation between a very dense corona radiata layer and decreased maturity of oocytes.
In contrast, by using a 5-scale scoring system based mostly on the morphology of the cumulus–corona radiata cells, Lin et al. (2003) have found a correlation of the in vitro developmental potential and blastocyst quality. Another scoring system of the quality of the COCs found associations between the observed quality and both fertilization and subsequent pregnancy rates, but not cleavage rates (Ng et al., 1999). In an oocyte donation programme Salumets et al. (2002) have found a strong correlation between the oocyte source and embryo quality whereas cleavage rate was determined by both oocyte and sperm factors.
Zona pellucida
Non-invasive assessment of zona pellucida of oocytes has been performed in 13 investigations. Darkness of the zona did not influence fertilization rates, embryo quality and implantation rates (De Sutter et al., 1996; Balaban et al., 1998; Ten et al., 2007) or cryosurvival of embryos and subsequent blastocyst and hatching rates (Balaban et al., 2008). According to several studies there was no correlation between the thickness (Esfandiari et al., 2006) and thickness associated with darkness (Rienzi et al., 2008) on fertilization, pronuclear morphology, embryo development and clinical pregnancy. Bertrand et al. (1995) have found that oocytes with thinner zonae pellucidae had higher fertilization rates. In contrast, increased inner layer thickness was reported to correlate with increased blastocyst rates (Rama Raju et al., 2007), and higher embryo development and clinical pregnancy rates (Shen et al., 2005). Increased zona pellucida thickness variation was associated with increased embryo quality (Høst et al., 2002).
Elevated birefringence of the zona pellucida's inner layer was found to be correlated with increased in vitro efficiency, including fertilization and embryo development (Shen et al., 2005; Rama Raju et al., 2007; Montag et al., 2008), in contrast to the recent publication of Madaschi et al. (2009), where no association between high or low zona birefringence and fertilization rates or embryo quality was found. On the other hand, the clinical outcome was found improved when oocytes with high birefringence of the zona pellucida were used (Shen et al., 2005; Rama Raju et al., 2007; Montag et al., 2008; Madaschi et al., 2009), and low birefringence was correlated with higher miscarriage rates (Madaschi et al., 2009). In one report analysing the effect of multiple morphological features of oocytes on further development, only drastic morphological alterations (broken or empty zona pellucidae) were regarded as unsuitable for ICSI (Loutradis et al., 1999).
Perivitelline space
The prognostic value of this feature was individually analysed in eight publications, and its morphology was part of the scoring system in an additional three papers. Similar to the observations regarding the zona pellucida morphology, no correlation between increased perivitelline space and further developmental characteristics were reported by Balaban et al. (1998, 2008) and De Sutter et al. (1996). Chamayou et al. (2006) have found a correlation between the size of perivitelline space, presence of granulation and subsequent embryo quality, but not with clinical pregnancy and implantation rates. Farhi et al. (2002) found the presence of coarse granules associated with lower pregnancy and implantation rates. In sharp contrast with this observation, no correlation between the presence of perivitelline debris and further in vitro or in vivo development was found by Hassan-Ali et al. (1998) and Ten et al. (2007); however, according to the latter investigation the increased perivitelline space was associated with increased embryo quality. According to Rienzi et al. (2008) large perivitelline space correlated with low fertilization rates and compromised pronuclear morphology, but had no further effect on embryo quality.
Morphology of first polar body
The morphology of first polar body in relation to the further development was investigated in nine papers. No correlation was reported in five of them. According to Verlinsky et al. (2003), irregular shape or fragmentation of the first polar body (PB1) was not related to subsequent embryo quality, blastocyst development, implantation rates or aneuploidies. Similar characteristics, including also the size of the PB1, were investigated by Ciotti et al. (2004), and no effect on the fertilization, cleavage, pregnancy and implantation rate, or embryo quality was reported. De Santis et al. (2005) did not find any correlation between surface characteristics, fragmentation and fertilization rate, embryo quality and blastocyst formation, although there were insufficient data to evaluate the effect of shape of PB1 on the further development. In the report of Chamayou et al. (2006) similar characteristics of PB1 were investigated (surface, fragmentation, size), and a slight influence was found on embryo quality but not on implantation and pregnancy rates. Fertilization rates and embryo quality were not related to the shape (normal, fragmented or irregular) of PB1 in the study of Ten et al. (2007) either.
In contrast to these observations, Ebner et al. (2000) have found a strong correlation between all observed morphological features of PB1 (intact versus rough surface, fragmented or enlarged) and fertilization rates/embryo quality. According to Rienzi et al. (2008), abnormal (large or degenerated) PB1 was related to decreased fertilization rates, but did not show any correlation with pronuclear morphology or embryo quality, whereas fragmentation was not associated with any of these outcomes. Navarro et al. (2009) have found correlation between large PB1 and decreased fertilization, cleavage rates as well as compromised embryo quality. Surprisingly, Fancsovits et al. (2006) found that fragmentation or degeneration of PB1 was related to higher fertilization rates and lower level of fragmentation of embryos, although large PB1s were associated with compromised fertilization and low embryo quality.
Shape of the oocyte
The shape of the oocyte was considered as a potentially predictive individual morphological characteristic in 10 studies. The majority of these studies did not find any correlation with in vitro developmental parameters, including cryosurvival (De Sutter et al., 1996; Balaban et al., 1998, 2008; Ten et al., 2007; Rienzi et al., 2008), aneuploidy (Yakin et al., 2007) and clinical pregnancy/implantation rages (Chamayou et al., 2006). A special feature, ovoid shape of the oocyte, was reported to be associated with delays in in vitro parameters (Ebner et al., 2008b). Embryos developing from giant oocytes were reported to have increased chance for digynic triploidy (Rosenbusch et al., 2002), in spite of the normal in vitro development (Balakier et al., 2002).
Appearance of the whole ooplasm
The appearance of the whole ooplasm was considered as a potential predictive individual factor in eight reviews, although the exact name, the definition and grouping of features differed between publications making comparative analysis very difficult and required some compromises. These different names and groupings included ‘dark cytoplasm’ (De Sutter et al., 1996; Loutradis et al., 1999; Ten et al., 2007), ‘dark cytoplasm–granular cytoplasm’ (Balaban et al., 1998) ‘dark cytoplasm with slight granulation’ (Balaban et al., 2008), ‘dark granular appearance of the cytoplasm’ (Esfandiari et al., 2006) and ‘diffused cytoplasmic granularity’ (Rienzi et al. 2008). In one paper, homogenous granulation was not mentioned, but two groups of heterogenous granulations were distinguished: on one or two sides of the oocyte, or in the centre (Wilding et al., 2007). Oocytes with heterogenous granulations had better fertilization rates than the control ones with clear cytoplasm. In another paper, the dark and the granular cytoplasm (either homogenous or centrally located) was considered as a separate morphological feature (Ten et al., 2007), accordingly in the present analysis only the dark cytoplasm group was involved.
The different terminology may be the cause of the rather controversial outcome of investigation of the predictive value of this feature. The dark cytoplasm, when analysed as an individual feature was found not to be a predictive factor in most investigated in vitro or in vivo parameters (De Sutter et al., 1996; Balaban et al., 1998, 2008; Esfandiari et al., 2006). Compromised quality of embryos which developed from oocytes with dark cytoplasm was reported by Ten et al., (2007) and Loutradis et al. (1999). Diffuse peripheral granulation was found to be associated with compromised pronuclear morphology (Rienzi et al., 2008). According to Wilding et al. (2007), however, any type of cytoplasmic granulation was associated with higher fertilization rates than in oocytes with total absence of granularity.
Presence of vacuoles and/or cytoplasmic inclusions
The presence of vacuoles and/or cytoplasmic inclusions was investigated in 10 publications. Although less heterogeneity in consideration, definition and classification was found than in the previous group, slight variations included observation of vacuoles (saccules, smooth endoplasmic reticulum clusters) only (Otsuki et al., 2004; Ten et al., 2007; Balaban et al., 2008); inclusions (refractile bodies, dark incorporations, fragments, spots, dense granules; lipid droplets; lipofuchsin) only (De Sutter et al., 1996; Xia, 1997; Balaban et al., 1998; Otsuki et al., 2007); or both vacuoles and inclusions (Loutradis et al., 1999; Wilding et al., 2007). In two papers the presence of vacuoles, smooth endoplasmic reticulum clusters and refractile bodies were individually registered and their predictive value were individually analysed (Rienzi et al., 2008). Although the aetiology, structure and potential predictive value of the different structures may differ considerably, owing to the limitations of non-invasive investigation techniques as well as the potential confusion at sorting different structures, here we follow the everyday routine reflected also by these reviews by making tentative groups for analysis of their correlation with the outcome.
Although fertilization rates and embryo quality were not affected (Ten et al., 2007), the presence of vacuoles in oocytes was negatively correlated with cryosurvival and developmental competence of embryos after fertilization (Balaban et al., 2008). Increased biochemical pregnancy rates were followed by decreased clinical pregnancy rates after transfer of embryos derived from oocytes with vacuoles (Otsuki et al., 2004). According to some investigators, cytoplasmic inclusions did not appear to affect fertilization, embryo quality and implantation rates (De Sutter et al., 1996; Balaban et al., 1998). Others, however, reported decreased fertilization and embryo development (Xia, 1997; Otsuki et al., 2007). The presence of both vacuoles and inclusions was related to compromised clinical pregnancy rates in the report by Loutradis et al. (1999). According to Wilding et al. (2007) these oocytes also had lower fertilization, embryo developmental and higher aneuploidy rates. When analysed separately the predictive role of presence of vacuoles, smooth endoplasmic reticulum clusters and refractile bodies, Rienzi et al. (2008) have only found a slight but significant decrease in fertilization rates of vacuolated oocytes. However, none of the three factors affected pronuclear or embryo morphology.
Central granulation or centrally located granular cytoplasm
The correlation between central granulation or centrally located granular cytoplasm of oocytes and developmental competence was discussed in five papers. Pronuclear morphology and embryo quality was compromised after fertilization of these oocytes (Ebner et al., 2008a; Rienzi et al., 2008). Decreased survival and impaired in vitro development after cryopreservation of embryos derived from oocytes with central granulation was reported by Balaban et al. (2008). Centrally located granular cytoplasm was the only feature investigated by Kahraman et al. (2000) who have not found any correlation with fertilization rates, embryo development or pregnancy rates. However, ongoing pregnancy rates were seriously compromised when embryos from centrally granulated oocytes were transferred. In contrast, oocytes with centrally located granulation were not found to have inferior fertilizing and in vitro developmental ability compared with those with completely absent granulation in the cytoplasm (Wilding et al., 2007).
Presence and morphology of the meiotic spindle
Fifteen papers of the 50 selected were dealing with the correlation between the presence and morphology of the meiotic spindle and further development including fertilizing ability and in vitro/in vivo developmental competence. Computer assisted polarization microscopy systems were used to evaluate the presence and other morphological features of the spindle including position, length and birefringence.
Only one study, that of Chamayou et al. (2006), found the presence or absence of the meiotic spindle irrelevant regarding the further developmental competence, including embryo quality and clinical pregnancy rates. The presence of meiotic spindle has been associated with higher birefringence of the zona pellucida (Madaschi et al., 2009) and higher fertilization rates (Moon et al., 2003; Cohen et al., 2004; Konc et al., 2004; Fang et al., 2007; Rama Raju et al., 2007; Braga et al., 2008, Madaschi et al., 2008; Wang et al., 2001). Results regarding the correlation between the presence of the spindle and early embryo development were controversial, with improved results (Moon et al., 2003; Rama Raju et al., 2007; Madaschi et al., 2008), versus no difference as observed by Cohen et al. (2004). Pregnancy rates were reported to be higher when embryos were derived from oocytes with the presence of spindle (Konc et al., 2004; Madaschi et al., 2008, 2009). Position of the meiotic spindle close to the PB was correlated with fertilization and cleavage rates (Fang et al., 2007) and early embryo development and quality (Cooke et al., 2003), whereas Moon et al. (2003) did not find any significant difference. According to Rienzi et al. (2003) high degrees of misalignment between the meiotic spindle and the first PB increased risk of fertilization abnormalities. However, when normal fertilization had occurred, the cleavage potential of embryos developing from such oocytes was not impaired. De Santis et al. (2005) did not find significant correlation between the spindle retardance and embryo quality; on the other hand, Shen et al. (2006) found that better pronuclear scores and higher pregnancy rates correlated with higher retardance. Higher blastocyst rates were also found to be related to higher retardance in the report of Rama Raju et al. (2007). Pregnancy rates were strongly related to the normal morphology (complete, barrel shaped, with strong birefringence) and also to the retardance of the spindle (Kilani et al., 2009).
Viscosity of the cytoplasm and the resistance of the cell membrane at ICSI
Two special features, the viscosity of the cytoplasm and the resistance of the cell membrane at ICSI, were analysed by Ebner et al. (2003) and Wilding et al. (2007). Although these parameters were not strictly morphological ones, resistance (together with two morphological features) was included into the grading system of Wilding et al. (2007) and does not seem to be sharply distinct from the viscosity analysed by Ebner et al. (2003), accordingly both of them were included in this review. Both viscosity and resistance had a significant effect on some investigated outcome parameters (fertilization, embyro quality, blastocyst rates and fertilization for viscosity and resistance, respectively).
Cumulative effect of multiple features
Investigations regarding the cumulative effect of multiple features were performed in eight studies. As the applied systems were different in almost all of these investigations, here we summarize briefly the method and the outcome.
Balaban et al. (1998) have compared the outcome of embryo transfers of cycles where all transferred embryos were derived from morphologically intact oocytes with those where all oocytes had morphological abnormalities, including extracytoplasmic, cytoplasmic, shape and multiple defects. No difference between the pregnancy and implantation rates was found.
A similar system was used and the same conclusion was obtained by La Sala et al. (2009). Investigated features involved the morphology of the COC, zona pellucida, perivitelline space, PB, ooplasm and presence of vacuoles or granulations. Outcomes (fertilization, pregnancy, take home baby and multiple pregnancy rates) were similar when all embryos were derived from intact oocytes, or all from morphologically ‘handicapped’ oocytes.
According to Yakin et al. (2007), the impaired morphology of oocytes characterized by almost all previously listed morphological features—except for the morphology of the COC and the investigation of the meiotic spindle—affected only blastocyst development, but not fertilization, cleavage and aneuploidy rates.
Chamayou et al. (2006) have used a cumulative evaluation for cytoplasmic features of oocytes including texture, inclusions, vacuoles and central granulation. The presence of these features was correlated with impaired embryo quality, but did not influence pregnancy and implantation rates.
In the study of Serhal et al. (1997) similar features (excessive granularity, cytoplasmic inclusions, smooth endoplasmic reticulum clustering and refactile bodies) were tentatively investigated with a completely different conclusion: in vitro developmental parameters were not influenced, but implantation and pregnancy rates were lower, when embryos were derived from oocytes with cytoplasmic abnormalities.
The oocyte grading system of Xia (1997) was based on the evaluation of the perivitelline space, PB morphology and cytoplasmic inclusions, and a strong correlation with fertilization rates and embryo quality was found.
In the study of Wilding et al. (2007), two morphologic and one functional parameters were involved in a grading system: oocyte granularity, vacuoles and inclusions (as one parameter) and injection properties (the resistance and fragility of the plasma membrane at ICSI). Fertilization, embryo development and clinical pregnancy rates were significantly different between the high and low quality group.
A complex grading system (MOMS; MII oocyte morphological score) has been established recently by Rienzi et al. (2008) according to the impact of different oocyte morphological features (vacuoles, abnormal PB1, large perivitelline space, diffused cytoplasmic granularity and/or centrally located granular area) on fertilization rate, and pronuclear and embryo morphology. A significant relationship between the MOMS rate and clinical pregnancy was found.
In general, out of the 92 studies of different parameters (including both single features and cumulative scores) investigating direct association with the further prognosis, 57 resulted in a significant correlation with the outcome, whereas in 35 no predictive value of the microscopic feature was found. The diversity of observations and results did not allow statistical comparison; however, there was no clear tendency of improved accuracy regarding the predictive value in recent publications compared with the earlier ones. Twenty four of 42 observations performed between 1997 and 2005 have found correlations with the outcome, whereas these values between 2006 and 2009 were 33 of 50.
Discussion
In a recent review Swain and Pool (2008) summarize the short list of features of an oocyte that is regarded healthy on the basis of morphological investigations during the routine IVF programme: single PB, ‘normal-looking’ cytoplasm, appropriate zona thickness, proper perivitelline space. According to the authors, the common experience is that these features often fail to predict the future fertilizing ability and developmental competence. This seems to be a widely accepted and shared opinion between human embryologists. On the other hand, most embryologists may also agree that some morphologically detectable features of MII phase oocytes indicate seriously compromised developmental competence. Morphological alterations may also be related to the patient and the cycle characteristics, and may involve most or all oocytes in the cohort.
The purpose of our review was to investigate if any non-invasive morphological feature or group of features of MII phase human oocytes has a strong predictive value for further development according to the literature between 1996 and 2009. Out of the 2159 titles identified by the initial search of relevant electronic databases, only 50 met the selection criteria (full papers dealing with light or polarized microscopic morphological features of in vivo matured, not cryopreserved MII phase human oocytes, and analysing the prognostic value of these features for further development). Unfortunately the majority (27) of the papers restricted the outcome investigation to the in vitro period, with limited value regarding the primary outcome including clinical pregnancy, implantation and ongoing pregnancy rates. Only three publications (Montag et al., 2008; Rienzi et al., 2008; La Sala et al., 2009) extended the investigation to the ultimate parameter, the number of take home babies. Moreover, because of the heterogeneity of the approaches and the unavailability of clinical data, the correlation between oocyte morphology and patient and cycle characteristics was not included in the analysis.
The grouping of morphological features was the result of an unavoidable compromise, as the use of terms and description of alterations were inconsistent between papers. Experimental designs also varied considerably, with wide differences between papers regarding the investigated features and outcome parameters. The only exception was the morphology of the meiotic spindle, where the reasonably homogenous material (relatively well-defined morphological features and similar outcome parameters) would allow meta-analysis. However, for this feature a recent review provided an excellent comparative analysis (Petersen et al., 2009): as the full papers investigated by this review were the same that were recovered by our independent search, the results of Petersen et al. (2009) were acknowledged and considered when conclusions were made.
As a result of these facts, and the relatively small number of publications investigating the predictive value of other morphological features, no strict selection according to the experimental designs (retrospective, prospective, randomized studies etc.) was applied; statistical calculations of authors were not re-evaluated or ranked. Each statistically supported result (correlation or no correlation between the feature and the outcome) was included and considered as a proven individual observation.
The tentative summary of our work is rather disappointing: in the 50 relevant papers recovered from databases from the past 15 years, none of the investigated 9 single features was unanimously correlated with normal or compromised development, when evaluated by 15 outcome parameters.
Among extracytoplasmic features, only dysmorphism of the COC was found to be indicative for further development in the majority of papers (four of five) dealing with the subject. For the zona pellucida, only 6 of 13 papers found correlation between the morphology and developmental competence including four papers dealing with the birefringence of the zona (one of them did not find correlation with the in vitro period; another one did not extend the investigation to the in vivo period). The commonly assessed light microscopic alterations (thickness and thickness variations, other dysmorphism) were found to be related to improved/compromised development almost randomly by the relevant papers.
Other extracytoplasmic features, including the size and granulations of the perivitelline space, and the shape of the oocyte or the PB, were related to developmental competence only in approximately half of the publications focusing on the issue, questioning their commonly supposed indicative value.
Among intracytoplasmic features, the presence, position and retardance of the spindle were found to be related to the developmental competence in 12 of 13 papers. In accordance with the recent meta-analysis of Petersen et al. (2009), based essentially on the same material, the significantly correlated outcome parameters were restricted to the in vitro development, whereas in vivo outcome analyses were relatively rare and the statistical analysis failed to show significant differences. Among features observable with simple transmitted light, the presence of central granulation resulted in impaired further development in four of five publications, and two of these also found a correlation with in vivo development. Regarding other cytoplasmic features, the assessment of their predictive value has shown less consistent results.
Complex analyses of multiple features based on a scoring system seems to be a promising alternative to single feature analysis, and this seems to be supported by the fact that six of the eight papers applying this strategy have found a correlation between the cumulative results and the prognosis. Unfortunately the applied scoring systems have shown extreme variability regarding the type and individual scoring of investigated features including combination with functional tests, and outcome parameters. Accordingly, at present, they can only be regarded as diverse approaches to the same strategy, but their comparative evaluation is impossible. Paradoxically, two recent publications investigating similar parameters and extending the outcome evaluation to take home babies (Rienzi et al., 2008; La Sala et al., 2009) resulted in contradicting conclusions about the predictive value of their system.
Additionally, the slight increase in IVF efficiency in recent publications can mostly be attributed to the application of the Polscope microscope for detecting retardance of the zona, or presence, position and retardance of the spindle, a method that is applied in a limited number of assisted reproduction technology (ART) units worldwide.
Conclusion
The analysis of 50 papers published in the past 15 years about predictive value of non-invasive morphological parameters of MII phase oocytes has produced contradicting results, and did not entirely support the average opinion about the features of ‘good’ and ‘bad’ quality and respective developmental competence. This outcome may be explained both with the common misjudgement of predictive value of some features, and the extremely diverse approaches these papers used for investigations regarding terms, features, outcome parameters and grouping. As oocyte selection before insemination may have important benefits in certain situations, including that in countries with legal restrictions, our finding underlines the importance of more intensive and coordinated research to reach a consensus and exploit fully the predictive potential of morphological examination, particularly as that is expected to remain, for several years to come, the only available selection method for many ART units.
Supplementary data
Supplementary data are available at http://humupd.oxfordjournals.org/.
Funding
Funding to pay the Open Access publication charges for this article was provided by G.EN.E.R.A.
Supplementary Material
References
- Arav A, Aroyo A, Yavin S, Roth Z. Prediction of embryonic developmental competence by time-lapse observation and ‘shortest half’ analysis. Reprod BioMed Online. 2008;17:669–675. doi: 10.1016/s1472-6483(10)60314-8. doi:10.1016/S1472-6483(10)60314-8. [DOI] [PubMed] [Google Scholar]
- Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod. 1998;13:3431–3433. doi: 10.1093/humrep/13.12.3431. doi:10.1093/humrep/13.12.3431. [DOI] [PubMed] [Google Scholar]
- Balaban B, Ata B, Isiklar A, Yakin K, Urman B. Severe cytoplasmic abnormalities of the oocyte decrease cryosurvival and subsequent embryonic development of cryopreserved embryos. Hum Reprod. 2008;23:1778–1785. doi: 10.1093/humrep/den127. doi:10.1093/humrep/den127. [DOI] [PubMed] [Google Scholar]
- Balakier H, Bouman D, Sojecki A, Librach C, Squire JA. Morphological and cytoenetic analysis of human giant oocytes and giant embryos. Hum Reprod. 2002;17:2394–2401. doi: 10.1093/humrep/17.9.2394. doi:10.1093/humrep/17.9.2394. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Van den Bergh M, Englert Y. Does zona pellucida thickness influence the fertilization rate? Hum Reprod. 1995;10:1189–1193. doi: 10.1093/oxfordjournals.humrep.a136116. [DOI] [PubMed] [Google Scholar]
- Braga DP, Figueira RC, Rodrigues D, Madaschi C, Pasqualotto FF, Iaconelli A, Jr, Borges E., Jr Prognostic value of meiotic spindle imaging on fertilization rate and embryo development in in vitro-matured human oocytes. Fertil Steril. 2008;90:429–433. doi: 10.1016/j.fertnstert.2007.06.088. doi:10.1016/j.fertnstert.2007.06.088. [DOI] [PubMed] [Google Scholar]
- Chamayou S, Ragolia C, Alecci C, Storaci G, Maglia E, Russo E, Guglielmino A. Meiotic spindle presence and oocyte morphology do not predict clinical ICSI outcomes: a study of 967 transferred embryos. Reprod Biomed Online. 2006;13:661–667. doi: 10.1016/s1472-6483(10)60656-6. doi:10.1016/S1472-6483(10)60656-6. [DOI] [PubMed] [Google Scholar]
- Ciotti PM, Notarangelo L, Morselli-Labate AM, Felletti V, Porcu E, Venturoli S. First polar body morphology before ICSI is not related to embryo quality or pregnancy rate. Hum Reprod. 2004;19:2334–2339. doi: 10.1093/humrep/deh433. doi:10.1093/humrep/deh433. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Malcov M, Schwartz T, Mey-Raz N, Carmon A, Cohen T, Lessing JB, Amit A, Azem F. Spindle imaging: a new marker for optimal timing of ICSI? Hum Reprod. 2004;19:649–654. doi: 10.1093/humrep/deh113. doi:10.1093/humrep/deh113. [DOI] [PubMed] [Google Scholar]
- Cooke S, Tyler JPP, Driscoll GL. Meiotic spindle location and identification and its effect on embryonic cleavage plane and early development. Hum Reprod. 2003;11:2397–2405. doi: 10.1093/humrep/deg447. [DOI] [PubMed] [Google Scholar]
- Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in-vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf. 1986;3:284–295. doi: 10.1007/BF01133388. doi:10.1007/BF01133388. [DOI] [PubMed] [Google Scholar]
- De Santis L, Cino I, Rabellotti E, Calzi F, Persico P, Borini A, Coticchio G. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod Biomed Online. 2005;11:36–42. doi: 10.1016/s1472-6483(10)61296-5. doi:10.1016/S1472-6483(10)61296-5. [DOI] [PubMed] [Google Scholar]
- De Sutter P, Dozortsev D, Qian C, Dhont M. Oocyte morphology does not correlate with fertilization rate and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1996;11:595–597. doi: 10.1093/humrep/11.3.595. [DOI] [PubMed] [Google Scholar]
- Ebner T, Yaman C, Moser M, Sommergruber M, Feichtinger O, Tews G. Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection. Hum Reprod. 2000;15:427–430. doi: 10.1093/humrep/15.2.427. doi:10.1093/humrep/15.2.427. [DOI] [PubMed] [Google Scholar]
- Ebner T, Moser M, Sommergruber M, Puchner M, Wiesinger R, Tews G. Developmental competence of oocytes showing increased cytoplasmic viscosity. Hum Reprod. 2003;18:1294–1298. doi: 10.1093/humrep/deg232. doi:10.1093/humrep/deg232. [DOI] [PubMed] [Google Scholar]
- Ebner T, Moser M, Shebl O, Sommergruber M, Yaman C, Tews G. Blood clots in the cumulus–oocyte complex predict poor oocyte quality and post-fertilization development. Reprod Biomed Online. 2008a;16:801–807. doi: 10.1016/s1472-6483(10)60145-9. doi:10.1016/S1472-6483(10)60145-9. [DOI] [PubMed] [Google Scholar]
- Ebner T, Shebl O, Moser M, Sommergruber M, Tews G. Developmental fate of ovoid oocytes. Hum Reprod. 2008b;23:62–66. doi: 10.1093/humrep/dem280. doi:10.1093/humrep/dem280. [DOI] [PubMed] [Google Scholar]
- Emiliani S, Fasano G, Vandamme B, et al. Impact of the assessment of early cleavage in a single embryo transfer policy. Reprod BioMed Online. 2006;13:255–260. doi: 10.1016/s1472-6483(10)60623-2. doi:10.1016/S1472-6483(10)60623-2. [DOI] [PubMed] [Google Scholar]
- Esfandiari N, Burjaq H, Gotlieb L, Casper RF. Brown oocytes: implications for assisted reproductive technology. Fertil Steril. 2006;86:1522–1525. doi: 10.1016/j.fertnstert.2006.03.056. doi:10.1016/j.fertnstert.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Fancsovits P, Tothne ZG, Murber A, Takacs FZ, Papp Z, Urbancsek J. Correlation between first polar body morphology and further embryo development. Acta Biol Hung. 2006;57:331–338. doi: 10.1556/ABiol.57.2006.3.7. doi:10.1556/ABiol.57.2006.3.7. [DOI] [PubMed] [Google Scholar]
- Fang C, Tang M, Li T, Peng WL, Zhou CQ, Zhuang GL, Leong M. Visualization of meiotic spindle and subsequent embryonic development in in vitro and in vivo matured human oocytes. J Assist Reprod Genet. 2007;24:547–551. doi: 10.1007/s10815-007-9171-4. doi:10.1007/s10815-007-9171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhi J, Nahum H, Weissman A, Zahalka N, Glezerman M, Levran D. Coarse granulation in the perivitelline space and IVF-ICSI outcome. J Assist Reprod Genet. 2002;19:545–549. doi: 10.1023/A:1021243530358. doi:10.1023/A:1021243530358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–177. doi: 10.1093/humupd/dmm040. doi:10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- Hassan-Ali H, Hisham-Saleh A, El-Gezeiry D, Baghdady I, Ismaeil I, Mandelbaum J. Perivitelline space granularity: a sign of human menopausal gonadotrophin overdose in intracytoplasmic sperm injection. Hum Reprod. 1998;13:3425–3430. doi: 10.1093/humrep/13.12.3425. doi:10.1093/humrep/13.12.3425. [DOI] [PubMed] [Google Scholar]
- Høst E, Gabrielsen A, Lindenberg S, Smidt-Jensen S. Apoptosis in human cumulus cells in relation to zona pellucida thickness variation, maturation stage, and cleavage of the corresponding oocyte after intracytoplasmic sperm injection. Fertil Steril. 2002;77:511–515. doi: 10.1016/s0015-0282(01)03006-0. doi:10.1016/S0015-0282(01)03006-0. [DOI] [PubMed] [Google Scholar]
- Kahraman S, Yakin K, Donmez E, Samli H, Bahce M, Cengiz G, Sertyel S, Samli M, Imirzalioglu N. Relationship between granular cytoplasm of oocytes and pregnancy outcome following intracytoplasmic sperm injection. Hum Reprod. 2000;15:2390–2393. doi: 10.1093/humrep/15.11.2390. doi:10.1093/humrep/15.11.2390. [DOI] [PubMed] [Google Scholar]
- Kilani S, Cooke S, Kan A, Chapman M. Are there non-invasive markers in human oocytes that can predict pregnancy outcome? Reprod Biomed Online. 2009;18:674–680. doi: 10.1016/s1472-6483(10)60013-2. doi:10.1016/S1472-6483(10)60013-2. [DOI] [PubMed] [Google Scholar]
- Konc J, Kanyo K, Cseh S. Visualization and examination of the meiotic spindle in human oocytes with polscope. J Assist Reprod Genet. 2004;21:349–353. doi: 10.1023/B:JARG.0000046202.00570.1d. doi:10.1023/B:JARG.0000046202.00570.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Sala GB, Nicoli A, Villani MT, Di Girolamo R, Capodanno F, Blickstein I. The effect of selecting oocytes for insemination and transferring all resultant embryos without selection on outcomes of assisted reproduction. Fertil Steril. 2009;91:96–100. doi: 10.1016/j.fertnstert.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Lemmen JG, Agerholm I, Ziebe S. Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICSI-fertilized oocytes. Reprod Biomed Online. 2008;17:385–391. doi: 10.1016/s1472-6483(10)60222-2. doi:10.1016/S1472-6483(10)60222-2. [DOI] [PubMed] [Google Scholar]
- Lin YC, Chang SY, Lan KC, Huang HW, Chang CY, Tsai MY, Kung FT, Huang FJ. Human oocyte maturity in vivo determines the outcome of blastocyst development in vitro. J Assist Reprod Genet. 2003;20:506–512. doi: 10.1023/B:JARG.0000013651.37866.0c. doi:10.1023/B:JARG.0000013651.37866.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutradis D, Drakakis P, Kallianidis K, Milingos S, Dendrinos S, Michalas S. Oocyte morphology correlates with embryo quality and pregnancy rate after intracytoplasmic sperm injection. Fertil Steril. 1999;72:240–244. doi: 10.1016/s0015-0282(99)00233-2. doi:10.1016/S0015-0282(99)00233-2. [DOI] [PubMed] [Google Scholar]
- Madaschi C, de Souza Bonetti TC, de Almeida Ferreira Braga DP, Pasqualotto FF, Iaconelli A, Jr, Borges E., Jr Spindle imaging: a marker for embryo development and implantation. Fertil Steril. 2008;90:194–198. doi: 10.1016/j.fertnstert.2007.05.071. doi:10.1016/j.fertnstert.2007.05.071. [DOI] [PubMed] [Google Scholar]
- Madaschi C, Aoki T, de Almeida Ferreira Braga DP, de Cassia Savio FR, Semiao FL, Iaconelli A, Jr, Borges E., Jr Zona pellucida birefringence score and meiotic spindle visualization in relation to embryo development and ICSI outcomes. Reprod Biomed Online. 2009;18:681–686. doi: 10.1016/s1472-6483(10)60014-4. doi:10.1016/S1472-6483(10)60014-4. [DOI] [PubMed] [Google Scholar]
- Marteil G, Richard-Parpaillon L, Kubiak JZ. Role of oocyte quality in meiotic maturation and embryonic development. Reprod Biol. 2009;9:203–224. doi: 10.1016/s1642-431x(12)60027-8. [DOI] [PubMed] [Google Scholar]
- Montag M, Schimming T, Köster M, Zhou C, Dorn C, Rösing B, van der Ven H, Ven der Ven K. Oocyte zona birefringence intensity is associated with embryonic implantation potential in ICSI cycles. Reprod Biomed Online. 2008;16:239–244. doi: 10.1016/s1472-6483(10)60580-9. doi:10.1016/S1472-6483(10)60580-9. [DOI] [PubMed] [Google Scholar]
- Moon JH, Hyun CS, Lee SW, Son WY, Yoon SH, Lim JH. Visualization of the metaphase II meiotic spindle in living human oocytes using the Polscope enables the prediction of embryonic developmental competence after ICSI. Hum Reprod. 2003;18:817–820. doi: 10.1093/humrep/deg165. doi:10.1093/humrep/deg165. [DOI] [PubMed] [Google Scholar]
- Mtango NR, Potireddy S, Latham K. Oocyte quality and maternal control of development. Int Rev Cell Mol Biol. 2008;268:223–290. doi: 10.1016/S1937-6448(08)00807-1. doi:10.1016/S1937-6448(08)00807-1. [DOI] [PubMed] [Google Scholar]
- Nagy ZP, Jones-Colon S, Roos P, Botros L, Greco E, Dasig J, Behr B. Metabolomic assessment of oocyte viability. Reprod Biomed Online. 2009;18:219–225. doi: 10.1016/s1472-6483(10)60259-3. doi:10.1016/S1472-6483(10)60259-3. [DOI] [PubMed] [Google Scholar]
- Navarro PA, de Araujo MM, de Araujo CM, Rocha M, dos RR, Martins W. Relationship between first polar body morphology before intracytoplasmic sperm injection and fertilization rate, cleavage rate, and embryo quality. Int J Gynaecol Obstet. 2009;104:226–229. doi: 10.1016/j.ijgo.2008.11.008. doi:10.1016/j.ijgo.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Ng ST, Chang TH, Jackson Wu TC. Prediction of the rates of fertilization, cleavage, and pregnancy success by cumulus-coronal morphology in an in vitro fertilization program. Fertil Steril. 1999;72:412–417. doi: 10.1016/s0015-0282(99)00290-3. doi:10.1016/S0015-0282(99)00290-3. [DOI] [PubMed] [Google Scholar]
- Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19:1591–1597. doi: 10.1093/humrep/deh258. doi:10.1093/humrep/deh258. [DOI] [PubMed] [Google Scholar]
- Otsuki J, Nagai Y, Chiba K. Lipofuscin bodies in human oocytes as an indicator of oocyte quality. J Assist Reprod Genet. 2007;24:263–270. doi: 10.1007/s10815-007-9130-0. doi:10.1007/s10815-007-9130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizio P, Fragouli E, Bianchi V, Borini A, Wells D. Molecular methods for selection of the ideal oocyte. Reprod Biomed Online. 2007;15:346–353. doi: 10.1016/s1472-6483(10)60349-5. doi:10.1016/S1472-6483(10)60349-5. [DOI] [PubMed] [Google Scholar]
- Petersen CG, Oliveira JB, Mauri AL, Massaro FC, Baruffi RL, Pontes A, Franco JG., Jr Relationship between visualization of meiotic spindle in human oocytes and ICSI outcomes: a meta-analysis. Reprod Biomed Online. 2009;18:235–243. doi: 10.1016/s1472-6483(10)60261-1. doi:10.1016/S1472-6483(10)60349-5. [DOI] [PubMed] [Google Scholar]
- Rama Raju GA, Prakash GJ, Krishna KM, Madan K. Meiotic spindle and zona pellucida characteristics as predictors of embryonic development: a preliminary study using PolScope imaging. Reprod Biomed Online. 2007;14:166–174. doi: 10.1016/s1472-6483(10)60784-5. [DOI] [PubMed] [Google Scholar]
- Rattanachaiyanont M, Leader A, Leveille MC. Lack of correlation between oocyte–corona–cumulus complex morphology and nuclear maturity of oocytes collected in stimulated cycles for intracytoplasmic sperm injection. Fertil Steril. 1999;71:937–940. doi: 10.1016/s0015-0282(99)00100-4. doi:10.1016/S0015-0282(99)00100-4. [DOI] [PubMed] [Google Scholar]
- Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;4:7–40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienzi L, Ubaldi F, Martinez F, Iacobelli M, Minasi MG, Ferrero S, Tesarik J, Greco E. Relationship between meiotic spindle location with regard to the polar body position and oocyte developmental potential after ICSI. Hum Reprod. 2003;18:1289–1293. doi: 10.1093/humrep/deg274. doi:10.1093/humrep/deg274. [DOI] [PubMed] [Google Scholar]
- Rienzi L, Ubaldi FM, Iacobelli M, Minasi MG, Romano S, Ferrero S, Sapienza F, Baroni E, Litwicka K, Greco E. Significance of metaphase II human oocyte morphology on ICSI outcome. Fertil Steril. 2008;90:1692–1700. doi: 10.1016/j.fertnstert.2007.09.024. doi:10.1016/j.fertnstert.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Rosenbusch B, Schneider M, Gläser B, Brucker C. Cytogenetic analysis of giant oocytes and zygotes to assess their relevance for the development of digynic triploidy. Hum Reprod. 2002;17:2388–2393. doi: 10.1093/humrep/17.9.2388. doi:10.1093/humrep/17.9.2388. [DOI] [PubMed] [Google Scholar]
- Salumets A, Suikkari AM, Mols T, Soderstrom-Anttila V, Tuuri T. Influence of oocytes and spermatozoa on early embryonic development. Fertil Steril. 2002;78:1082–1087. doi: 10.1016/s0015-0282(02)04215-2. doi:10.1016/S0015-0282(02)04215-2. [DOI] [PubMed] [Google Scholar]
- Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12:1267–1270. doi: 10.1093/humrep/12.6.1267. [DOI] [PubMed] [Google Scholar]
- Shen Y, Stalf T, Mehnert C, Eichenlaub-Ritter U, Tinneberg HR. High magnitude of light retardation by the zona pellucida is associated with conception cycles. Hum Reprod. 2005;20:1596–1606. doi: 10.1093/humrep/deh811. doi:10.1093/humrep/deh811. [DOI] [PubMed] [Google Scholar]
- Shen Y, Stalf T, Mehnert C, De SL, Cino I, Tinneberg HR, Eichenlaub-Ritter U. Light retardance by human oocyte spindle is positively related to pronuclear score after ICSI. Reprod Biomed Online. 2006;12:737–751. doi: 10.1016/s1472-6483(10)61086-3. doi:10.1016/S1472-6483(10)61086-3. [DOI] [PubMed] [Google Scholar]
- Swain JE, Pool TB. ART failure: ooctye contributions to unsuccessful fertilization. Hum Reprod Update. 2008;14:431–446. doi: 10.1093/humupd/dmn025. doi:10.1093/humupd/dmn025. [DOI] [PubMed] [Google Scholar]
- Ten J, Mendiola J, Vioque J, de JJ, Bernabeu R. Donor oocyte dysmorphisms and their influence on fertilization and embryo quality. Reprod Biomed Online. 2007;14:40–48. doi: 10.1016/s1472-6483(10)60762-6. doi:10.1016/S1472-6483(10)60762-6. [DOI] [PubMed] [Google Scholar]
- Van Soom A, Vandaele L, Goossens K, de Kruif A, Peelman L. Gamete origin in relation to early embryo development. Theriogenology. 2007;68(Suppl. 1):131–137. doi: 10.1016/j.theriogenology.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Testart J, Lassalle B, Frydman R, Belaisch JC. A study of factors affectingthe success of human fertilization in vitro. II. Influence of semen quality and oocyte maturity on fertilization and cleavage. Biol Reprod. 1983;28:425–431. doi: 10.1095/biolreprod28.2.425. doi:10.1095/biolreprod28.2.425. [DOI] [PubMed] [Google Scholar]
- Verlinsky Y, Lerner S, Illkevitch N, Kuznetsov V, Kuznetsov I, Cieslak J, Kuliev A. Is there any predictive value of first polar body morphology for embryo genotype or developmental potential? Reprod Biomed Online. 2003;7:336–341. doi: 10.1016/s1472-6483(10)61874-3. doi:10.1016/S1472-6483(10)61874-3. [DOI] [PubMed] [Google Scholar]
- Wang WH, Meng L, Hackett RJ, Odenbourg R, Keefe DL. The spindle observation and its relationship with fertilization after intracytoplasmic sperm injection in living human oocytes. Fertil Steril. 2001;75:348–353. doi: 10.1016/s0015-0282(00)01692-7. doi:10.1016/S0015-0282(00)01692-7. [DOI] [PubMed] [Google Scholar]
- Wilding M, Di ML, D'Andretti S, Montanaro N, Capobianco C, Dale B. An oocyte score for use in assisted reproduction. J Assist Reprod Genet. 2007;24:350–358. doi: 10.1007/s10815-007-9143-8. doi:10.1007/s10815-007-9143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P. Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod. 1997;12:1750–1755. doi: 10.1093/humrep/12.8.1750. doi:10.1093/humrep/12.8.1750. [DOI] [PubMed] [Google Scholar]
- Yakin K, Balaban B, Isiklar A, Urman B. Oocyte dysmorphism is not associated with aneuploidy in the developing embryo. Fertil Steril. 2007;88:811–816. doi: 10.1016/j.fertnstert.2006.12.031. doi:10.1016/j.fertnstert.2006.12.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.