Abstract
BACKGROUND
Polycystic ovary syndrome (PCOS) is a common metabolic dysfunction and heterogeneous endocrine disorder in women of reproductive age. Although patients with PCOS are typically characterized by increased numbers of oocytes retrieved during IVF, they are often of poor quality, leading to lower fertilization, cleavage and implantation rates, and a higher miscarriage rate.
METHODS
For this review, we searched the database MEDLINE (1950 to January 2010) and Google for all full texts and/or abstract articles published in English with content related to oocyte maturation and embryo developmental competence.
RESULTS
The search showed that alteration of many factors may directly or indirectly impair the competence of maturating oocytes through endocrine and local paracrine/autocrine actions, resulting in a lower pregnancy rate in patients with PCOS. The extra-ovarian factors identified included gonadotrophins, hyperandrogenemia and hyperinsulinemia, although intra-ovarian factors included members of the epidermal, fibroblast, insulin-like and neurotrophin families of growth factors, as well as the cytokines.
CONCLUSIONS
Any abnormality in the extra- and/or intra-ovarian factors may negatively affect the granulosa cell–oocyte interaction, oocyte maturation and potential embryonic developmental competence, contributing to unsuccessful outcomes for patients with PCOS who are undergoing assisted reproduction.
Keywords: polycystic ovary syndrome, oocyte, fertilization, embryo, IVF
Introduction
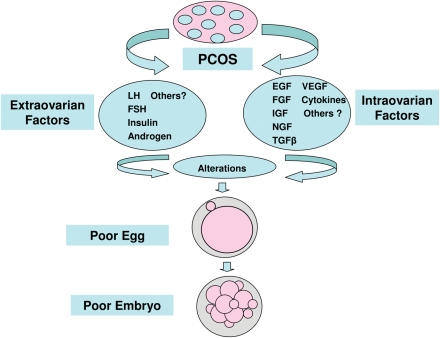
Polycystic ovary syndrome (PCOS) is a common metabolic dysfunction and heterogeneous endocrine disorder in women of reproductive age (Franks, 1995, 2008; Knochenhauer et al., 1998; Diamanti-Kandarakis et al., 2008; Asunción et al., 2000; Azziz, 2004; Wood et al., 2007; Toulis et al., 2009). It is characterized by a clustering of hyperandrogenism, hyperinsulinemia, hypersecretion of LH, menstrual dysfunction, hirsutism, infertility and pregnancy and neonatal complications (Franks, 1995; Moran and Teede, 2009; Stubbs et al., 2007; Toulis et al., 2009). Women with PCOS undergoing IVF treatment have been well-described (Ludwig et al., 1999; Legro, 2001; Mulders et al., 2003; Heijnen et al., 2006; Sahu et al., 2008). Although PCOS patients are typically characterized by producing an increased number of oocytes, they are often of poor quality, leading to lower fertilization, cleavage and implantation rates, and a higher miscarriage rate (Sengoku et al., 1997; Ludwig et al., 1999; Mulders et al., 2003; Heijnen et al., 2006; Weghofer et al., 2007; Sahu et al., 2008; Boomsma et al., 2008). This evidence raises the issue that poor oocyte and embryo quality may contribute to increased aneuploidy rates (Munné et al., 1995; Gianaroli et al., 2003, 2007). However, recent data suggest that women with PCOS yield higher numbers of oocytes and produce more euploid embryos in IVF, but still result in lower pregnancy and increased miscarriage rates, which are not genetically associated with an increased risk for embryonic aneuploidy (Weghofer et al., 2007). Hence, other factors, aside from chromosomal factors, are most likely associated with the significantly increased risk for pregnancy loss in patients with PCOS (Sagle et al., 1988; Carmina and Lobo, 1999; Wood et al., 2007; Weghofer et al., 2007). Impaired oocyte maturation and embryonic developmental competence in PCOS women is possibly linked with abnormal endocrine/paracrine factors, metabolic dysfunction and alterations in the intrafollicular microenvironment during folliculogenesis and follicle maturation (Franks et al., 2002; Dumestic et al., 2007b; Dumesic and Abbott, 2008; Wood et al., 2007). Therefore, a better understanding of how PCOS is related to abnormalities in extra- and intra-ovarian factor (Fig. 1, Table I) and their impact on granulosa cell (GC)–oocyte interactions, oocyte maturation and potential embryonic developmental competence, will be crucial to improving fertility and optimizing clinical stimulation, thus enhancing pregnancy outcomes in women with PCOS undergoing IVF treatment.
Figure 1.
Intra-and extra-ovarian factors associated with the PCOS pathology that negatively affect oocyte and subsequent embryo quality.
Table I.
Factors in serum and follicular fluid of patients with PCOS: impact on quality of oocyte and embryo, fertilization and outcome of pregnancy.
All data are as compared with controls (patients without PCOS).↑, increases or positive impact; ↓, decreases or negative impact; ≈, similar; blank, no data.
Methods
For this review, we searched the database MEDLINE (1950 to January 2010) and GOOGLE for all full texts and/or abstract articles published in English; our own unpublished data were taken into account as well. Search terms included ‘oocyte’, ‘embryo’, ‘oocyte and embryo’, ‘oocyte and embryo quality’, ‘oocyte quality’, ‘embryo quality’, ‘fertilization’, ‘oocyte aneuploidy’, ‘embryo aneuploidy’, ‘oocyte abnormality’, ‘embryo abnormality’, ‘clinical issue’, ‘laboratory issue’, ‘IVF outcome’, ‘follicle fluid’, ‘follicle fluid hormone’, ‘follicle fluid and oocyte’, ‘follicle fluid and embryo’, ‘folliculogenesis’, ‘extra- and intra-ovarian factors’, ‘follicular fluid factors’ and ‘growth factors’ in PCOS. This search resulted in 1596 papers. Upon screening the results for applicable titles and/or abstracts, only articles correlating to PCOS and its relatives were selected for this review. In addition, we hand-searched references of relevant reviews, and conference abstracts, and included ongoing studies to locate other potentially eligible materials.
Extra-ovarian factors
Human folliculogenesis and follicle maturation are complicated developmental processes through which a mature follicle is differentiated from primordial follicles, yielding one mature follicle that is eventually selected to ovulate, releasing a mature oocyte. This developmental process can be disrupted by abnormal extra-ovarian endocrine factors, resulting in ovarian dysfunction. Complex endocrine disorders, such as FSH deficiency, hypersecretion of LH, hyperandrogenemia and hyperinsulinemia with insulin resistance, are responsible for the pathogenesis of PCOS, consequently increasing the risks of impaired oocyte developmental competence, implantation failure and miscarriage (Van der Spuy and Dyer, 2004; Dumesic et al., 2007a; Dumesic and Abbott, 2008; Boomsma et al., 2008).
FSH deficiency
FSH stimulates follicular growth and recruitment of immature follicles from the ovary. FSH is the major survival factor during folliculogenesis, when there is a delicate balance between recruitment and atresia of follicles. Human antral follicles between 2 and 5 mm become responsive to FSH, whereas slightly larger follicles between 6 and 8 mm acquire aromatase activity and potentially increase the estradiol (E2) levels (Dumesic and Abbott, 2008). With the concomitant rise in E2 and inhibin B, FSH levels then decline in the late follicular phase, and eventually only the most advanced and mature follicle is selected to proceed to ovulation. At the end of the luteal phase, there is a slight rise in the FSH level, which is very important in initiating the next ovulatory cycle (Erickson and Shimasaki, 2001; Padhy et al., 2009). In contrast, PCOS patients show lower serum FSH levels as compared with normal cycles (Hillier, 1994). Consequently, FSH deficiency results in an increased accumulation of antral follicles between 2 and 8 mm (Franks et al., 2000, 2008). Clearly, the high number of smaller follicles indicates many have undergone premature arrest and failed to become the dominant follicle (Franks et al., 2008; Padhy et al., 2009). However, the developmental competence of oocytes collected from women with PCOS is normal, potentially leading to similar fertilization and normal cumulative pregnancy rates (Hardy et al., 1995; Ludwig et al., 1999; Jabara and Coutifaris, 2003; Heijnen et al., 2006; Franks et al., 2008).
PCOS patients undergoing IVF commonly demonstrate elevated E2 levels, combined with a significantly higher number of oocytes retrieved, lower number of high-quality oocytes, poor fertilization rates, increased embryonic fragmentation, decreased percentage of blastocyst formation and lower impanation rates (Cano et al., 1997a, b; Urman et al., 2004). High E2 levels in PCOS patients may be detrimental to oocyte maturation and embryonic development (Hardy et al., 1995). In addition, recovery of immature oocytes followed by in vitro maturation (IVM) is a potentially useful treatment option for women with PCOS-related infertility. As an alternative approach, minimal or mild ovarian stimulation with FSH before oocyte collection has been applied in PCOS patients (Chian, 2004). Immature oocytes are then cultured in complex IVM culture medium plus 75 mIU/ml FSH + LH 75 mIU/ml for 24–48 h. ICSI is performed for mature oocytes. Despite the elevated number of immature oocytes obtained from PCOS patients with declining serum FSH levels (Dumesic et al., 2007b; Franks et al., 2008), oocyte maturation in vitro induced by extrinsic FSH and cumulus cell (CC)–oocyte interactions are crucial for the acquisition of oocyte developmental potential (Wynn et al., 1998; Dumesic et al., 2007b). Consequently, oocytes become fertilized embryos and potentially develop into the blastocyst stage (De La Fuente, 2006; Dumesic et al., 2007b). Results suggest that the cumulative pregnancy rate by IVM treatment in women with PCOS is comparable with that of other PCOS women undergoing conventional IVF (Child et al., 2001; Cha et al., 2005; Söderström-Anttila et al., 2005). However, recent studies have suggested that IVM has deleterious effects on the spindle organization and chromosomal configuration of oocytes from PCOS patients (Li et al., 2006; Navarro et al., 2007, 2009; Nichols et al., 2010), possibly explaining the reduced developmental competence of oocytes matured in vitro, compared with those matured in vivo. This may possibly contribute to the decline in the overall clinical outcome observed after IVM treatment (Li et al., 2006; Navarro et al., 2007, 2009).
Hypersecretion of LH
Women with PCOS typically have tonic hypersecreton of LH during the follicular phase of their cycles (Balen et al., 1993; Cano et al., 1997a, b; van der Spuy and Dyer, 2004). High LH levels have been associated with significant decreases in oocyte maturation and fertilization rates, and impaired embryo quality, consequently resulting in impaired pregnancy rates, and higher miscarriage rates (Adams et al., 1985; Stanger and Yovich, 1985; Hombur and Jacobs, 1989; Regan et al., 1990; Sengoku et al., 1997; Ludwig et al., 1999; Jabara and Coutifaris, 2003; Urman et al., 2004; van der Spuy and Dyer, 2004; Santos et al., 2010). Hyperseceretion of LH during folliculogenesis may suppress FSH function, resulting in abnormal GC function by promoting premature GC luteinization and follicular aresia in small antral follicles from women with PCOS, causing premature oocyte maturation via inhibition of oocyte maturation inhibitors (Tesarik, 2003; van der Spuy and Dyer, 2004; Dumesic et al., 2007b; Franks et al., 2008), which all impair the quality of both oocyte and embryo (Tarlatzis and Grimbizis, 1997; Dumesic et al., 2002). LH may also activate premature meiotic processes by damaging the oocyte nucleus, leading to apoptosis via a receptor-coupled signal transduction system (Yoshimura and Wallach, 1987; Kurzawa et al., 2008). Disruption of the endocrine control of meiosis, resulting in impaired extrusion of the first polar body, may compromise the chromosomal normality of oocytes (Sengoku et al., 1997), possibly contributing to embryonic aneuploidy in women with PCOS (Weghofer et al., 2007). Errors in embryogenesis stemming from abnormal and premature oocyte exposure to increased LH stimulation may explain the elevated miscarriage rate in PCOS patients (Balen et al., 1993; Urman et al., 2004).
Hyperandrogenemia
Hyperandrogenemia is a common disorder in PCOS; it is mutifactorial in origin, typically attributed to the ovary with substantial contributions from an adrenal source, and to a lesser extent adipose tissues (van der Spuy and Dyer, 2004; Nisenblat and Norman, 2009). Elevated free circulating levels of bioactive androgen results from either direct increases of ovarian production or an inhibition of hepatic synthesis of sex hormone-binding globin in PCOS patients with insulin resistance (Balen et al., 1995; van der Spuy and Dyer, 2004; Nisenblat and Norman, 2009). Increased androgen concentrations in the follicular fluid (FF) are associated with elevated serum LH levels, which may block dominant follicle development and cause follicular arrest and degeneration (Billig et al., 1993; Kurzawa et al., 2008). It has been suggested that high levels of androgen may have a negative impact on oocyte developmental competence (Brzynski et al., 1995; Teissier et al., 2000; Jabara and Coutifaris, 2003). Incubation of the oocyte with androgen in vitro is associated with decreased oocyte maturation rates (Tesarik and Mendoza, 1995). Data from an in vitro model suggest that testosterone exerts a strong inhibition of meiotic maturation and embryonic development in CC-free mouse oocytes, compared with CC-enclosed oocytes; this demonstrates that CCs can protect oocytes via local aromatase activity in human (Laufer et al., 1984; Dumesic et al., 2007b) and mice (Anderiesz and Trounson, 1995). Such a CC function plays an important role in PCOS folliculogenesis, since small PCOS follicles are hyperandrogenic (Eden et al., 1990; Dumesic et al., 2007b) owing to intrinsically raised androgen biosynthesis by theca cells (Nelson et al., 2001). Further studies have suggested that elevated testosterone, either directly or indirectly, decreases the rates of IVM, fertilization and embryonic development (Dumesic et al., 2007b; Patel and Carr, 2008). The mechanism of testosterone activity within the oocyte may be related to decreased calcium oscillations, consequently inhibiting oocyte cytoplasmic maturation, with effects on meiotic maturation (Tesarik and Mendoza, 1995, 1997; Jabara and Coutifaris, 2003). In addition, elevated testosterone concentrations are associated with higher miscarriage rates in women with PCOS (van der Spuy and Dyer, 2004), suggesting that androgens may have a detrimental effect on folliculogenesis and endometrial function (Okon et al., 1998; Tuckerman et al., 2000).
Hyperinsulinemia
PCOS is an endocrine–metabolic disorder, closely tied to insulin resistance and a compensatory hyperinsulinemia. Metformin is the drug which has been studied most, and is administered to reduce fasting insulin, LH and free testosterone level, in an effort to restore menstrual cyclicity and fertility (Tang et al., 2010). It has been reported that insulin resistance is related to an increased miscarriage rate (Craig et al., 2002); several studies have suggested that metformin can effectively reduce pregnancy loss in women with PCOS (Glueck et al., 2001; Jakubowicz et al., 2002; Kjotrod et al., 2004; Galal and Mitwally, 2009). Hyperinsulinemia may have preferentially impaired oocyte developmental competence, resulting in reduced rates of fertilization, embryonic development and implantation in PCOS patients with obesity (Hamilton-Fairley et al., 1992; Cano et al., 1997a, b; Wang et al., 2001; Wijeyaratne et al., 2002; Jabara and Coutifaris, 2003; Dumesic et al., 2002, 2007b; Dumesic and Abbott, 2008; Palep-Singh et al., 2007; Tian et al., 2007; Boomsma et al., 2008). Data from in vitro cell culture models suggest that co-incubation of insulin and FSH with mouse (Eppig et al., 1998) and bovine (Galal and Mitwally, 2009) oocytes promotes FSH-induced up-regulation of GC LH receptor mRNA expression (Dumesic et al., 2002; Tao and Yan, 2005; Diamanti-Kandarakis, 2008), inhibiting FSH stimulation of aromatase activity (Galal and Mitwally, 2009), thus reducing the percentage of fertilized oocytes that develop into blastocysts (Eppig et al., 1998; Dumesic et al., 2002, 2007b). Insulin may induce local androgen production, which results in oocytes of lower quality, post-maturity (Cano et al., 1997a, b). At the molecular level, insulin binds to its receptor, localized on GC and theca cells, and oocytes, to stimulate follicle recruitment (Dumesic et al., 2002, 2007b; Kezele et al., 2002), consequently altering expression of multiple genes involved in meiotic/mitotic spindle dynamics and centrosome function in PCOS ooctyes (Wood et al., 2007). This indicates that insulin may be an important mediator of oocyte developmental competence via a ligand-receptor regulating system (Dumesic et al., 2007b).
Intra-ovarian factors
Ovarian folliculogenesis is regulated by a fine balance between extra and intra-ovarian factors (Artini et al., 2007). Oogenesis is profoundly dependent upon intra-ovarian factors, in particular follicle fluid factors (FFFs) (Andreani et al., 1996; Hsieh et al., 2009; Padhy et al., 2009), which are positively related to levels of these factors in serum (Table I). Any imbalance or dysfunction between extra- and intra-ovarian factors may result in abnormal folliculogenesis and oogenesis disorder (Frank et al., 2002, 2008; Artini et al., 2007). Recent studies suggest that the main FFFs implicated in polycystic ovary folliculogenesis are members of the growth factor families, cytokines, inhibins and others (Franks et al., 2002; Artini et al., 2007; Diamanti-Kandarakis, 2008). Furthermore, a series of different serum factors, coupled with the intrafollicular fluid microenvironment, may directly impair oocyte developmental competence, should their balance be altered (Yen et al., 1993; Andreani et al., 1996; van der Spuy and Dyer, 2004; Artini et al., 2007; Padhy et al., 2009); this would consequently have a negative impact on the fertilization, embryonic development and outcome of pregnancy in PCOS patients (Table I and Fig. 1).
Epidermal growth factor family
Epidermal growth factor (EGF) is a soluble growth factor that plays an important role in the regulation of cell growth, proliferation and differentiation when bound to its receptor, EGFR (ErbB1, ErbB2-4; Hsieh et al., 2009). In the human ovary, EGF is found in the FF, regulating follicular development and oocyte meiotic maturation competence via EGFR signaling transduction system in the CCs (Westergaard and Andersen, 1989; Almahbobi et al., 1998; Jamnongjit et al., 2005; Hsieh et al., 2009). IVM studies show that exposure of the cumulus–oocyte complex (COC) to EGF stimulates CC expansion and improves the nuclear and cytoplasmic maturation of oocytes from the metaphase I (MI) to metaphase II (MII) stage in both humans and other mammals (Goud et al., 1998; Smitz et al., 1998; De La Fuente et al., 1999), significantly facilitating fertilization and embryo development (Singh et al., 1997; Goff et al., 2001). Other studies suggest that FF EGF levels have an inverse correlation with oocyte maturation (Hofmann et al., 1990; Das et al., 1992; Ozornek et al., 1999; Hsieh et al., 2009). In women with PCOS, FF EGF levels are higher than those of normally ovulating women (NOW), which may suggest the involvement of EGF in the maintenance of PCOS (Volpe et al., 1991; Artini et al., 2007). EGF inhibits estrogen synthesis in GCs, which may explain why EGF blocks antral follicle growth and results in follicular arrest in PCOS patients (Artini et al., 2007). Therefore, it is hypothesized that a disruption in the regulatory mechanisms of EGF synthesis and/or physiological function mediated by EGFR may cause anovulatory infertility in women with PCOS (Almahbobi and Trounson, 1996; Almahbobi et al., 1998). Whether an elevated level of EGF in FF is correlated to oocyte quality and embryonic developmental competence is still unclear.
In addition, EGF-like factors, such as amphiregulin, epiregulin and betacellulin, are reportedly involved in oocyte maturation through autocrine and paracrine mechanisms (Ashkenazi et al., 2005; Shimada et al., 2006; Tse and Ge, 2009); however, the physiological function of EGF-like factors in PCOS remains unknown.
Fibroblast growth factor family
Fibroblast growth factors (FGFs) are a group of polypeptides that play a fundamental role in development, cell growth, tissue repair and transformation (Hammadeh et al., 2003). They are expressed in GC and theca cells of growing follicles, and are considered to be physiological regulators of FSH action (Artini et al., 2006, 2007); this may suggest a role for FGF in oocyte maturation by affecting surrounding follicular GC and theca cells (Skinner, 2005; Artini et al., 2007). A previous study shows that FGF levels in the serum and FF are lower in PCOS patients in comparison to patients with endometriosis and tubal factors (Hammadeh et al., 2003). In contrast, another research group reported that FGF concentrations are increased in the FF and serum of PCOS patients when compared with controls, leading to an inverse correlation with oocyte maturity (Artini et al., 2006, 2007): this supports speculation that FGF contributes to alterations in the intra-follicle environment, resulting in arrest of follicle development in patients with PCOS (Artini et al., 2007). Therefore, FGF alterations in the FF and serum remain controversial; the impact of EGF on oocyte maturation and embryonic development requires further elucidation in PCOS patients.
Insulin-like growth factor family
Insulin-like growth factors (IGFs) are multifunctional polypeptides with insulin-like activity. IGFs are part of a complex system used by cells to communicate with their physiological environment. This complex system consists of two surface-receptors (IGF1R and IGF2R), two receptor ligands (IGF-I and IGF-II), six high-affinity IGF binding proteins (IGFBP 1-6) and their specific proteases (Adashi, 1993; Frattali and Pessin, 1993; Yen et al., 1993; Erickson and Shimasaki, 2001; Artini et al., 2007).
Insulin-like growth factor-I/II and IGF binding proteins
IGFs and their binding proteins, IGFBPs, have important regulatory functions in ovarian follicular development (Yen et al., 1993; Artini et al., 2007). Circulating IGFs are produced in the liver, local IGF-I is secreted by theca cells whereas IGF-II is synthesized by GCs, and IGFBPs are present in the FF and expressed by GCs and theca cells (Yen et al., 1993; Erickson and Shimasaki, 2001; Artini et al., 2007). Although how IGFs are involved in the pathogenesis of PCOS remains unknown, the excess insulin concentrations and alterations in IGFs expression may be implicated (Yen et al., 1993). One recent report suggests that the FF IGF-I levels in PCOS women are elevated, although IGF-II and IGFBP-1 levels are lower than NOW (Artini et al., 2007). However, FF IGFBP-2 and -4 levels are significantly greater (Yen et al., 1993; Kwintkiewicz and Giudice, 2009); in contrast, IGFBP-1 is lower in PCOS patients, leading to follicular arrest (Artini et al., 2007). This evidence suggests that an altered IGF system is directly correlated to the oligo-ovulatory disorder of PCOS women (Kwintkiewicz and Giudice, 2009).
Women with PCOS have a higher FF IGFBP-3, but unaltered FF IGF-I levels (Amato et al., 1999). Research shows the levels of IGF-I, IGF-II and IGFBP-3 in mature follicles to be comparable between PCOS patients and controls; however, IGF-I levels in immature follicles in PCOS patients is decreased during ovarian stimulation, and this is associated with the generation of immature oocytes (Eden et al., 1988; Rabinovici et al., 1990; Franchimont et al., 1994; Pellegrini et al., 1995; Barreca et al., 1996; Dragisic et al., 2006; Schoyer et al., 2007), At the same time, IGFBP-3 levels are increased during stimulation, resulting in a greater likelihood of achieving pregnancy in PCOS patients (Schoyer et al., 2007). In infertile IVF patients, the ratio of IGF-1/IGFBP-1 in the serum and FF is significantly increased in women who become pregnant, highlighting the importance of oocyte quality and maturity during ovarian stimulation for IVF (Jimena et al., 1992; Artini et al., 1994; Kawano et al., 1997; Oosterhuis et al., 1998; Fried et al., 2003). Furthermore, results from in vitro culture models demonstrate that IGF-I can significantly increase embryonic development and blastocyst formation (Lighten et al., 1998; Liu et al., 1999; Fried et al., 2003). Hence, study of FF proteins may help to elucidate the roles of IGFs in GC function, meiotic maturity, oocyte chromosomal normality and embryonic developmental competence in PCOS patients.
Neurotrophin growth factor family
Brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), NT-3 and NT-4/5 are major members of the neurotrophin (NT) family of growth factors that are involved in development of the central and peripheral nervous systems (Levi-Montalcini, 1987; Snider, 1994; Buyuk and Seifer, 2008). NTs are not only involved in the nervous system, but also act on the ovaries of humans and other mammals (Seifer et al., 2002a, b, 2003; Buyuk and Seifer, 2008). NTs play a fundamental role in folliculogenesis and cytoplasmic competence of the oocyte (Buyuk and Seifer, 2008). Data from research using in vitro animal models suggest that co-incubation with BDNF promotes nuclear and cytoplasmic maturation of the oocyte, which are essential processes for successful oocyte and preimplantation embryo development (Da Silva et al., 2005; Kawamura et al., 2005). Evidence from some studies shows that increased FF BDNF and NGF levels are closely related to the pathology of women with PCOS (Bai et al., 2004; Johnstone et al., 2008; Dissen et al., 2009). Another report found that FF BDNF and NT-3 levels are increased, but FF NGF is decreased, in women with PCOS (Buyuk and Seifer, 2008) which may be indicative of the differential status of follicles in PCOS patients. Therefore, NT mechanisms in POCS pathogenesis, especially their impact on oocyte and embryo developmental competence, need further clarification at different stages of follicle development.
Transforming growth factor-β family
Among the many intra-ovarian factors, particular members of the transforming growth factor (TGF)-β family play an important biological role in follicle growth and oocyte development. These family members include anti-Müllerian hormone (AMH)/Müllerian inhibiting substance (MIS), activin, follistatin, inhibins, bone morphogenetic protein (BMP)-9 and growth differentiation factor (GDF)-9 (PieK et al., 1999; Artini et al., 2007; Dumesic et al., 2007b; Dumesic and Abbott, 2008). Under different physiological conditions, TGF-β family members may either promote or block ovarian follicle growth and/or differentiation of the GC–oocyte complex, which is also related to the pathogenesis of PCOS (van der Spuy and Dyer, 2004; Diamanti-Kandarakis, 2008; Dumesic and Abbott, 2008).
Anti-Müllerian hormone/Müllerian inhibiting substance
AMH, also known as Müllerian inhibiting factor (MIF), Müllerian inhibiting hormone (MIH) or MIS, is a homodimeric glycoprotein with a molecular weight of 140 kDa (Di Clemente et al., 2003; Artini et al., 2007). It inhibits the development of the Müllerian ducts in the male embryo (Behringer, 1994). AMH is expressed by GCs within ovaries of women of reproductive age, controlling the formation of primary follicles by inhibiting excessive follicular recruitment by FSH and therefore plays an important role in folliculogenesis (Weenen et al., 2004; Sadeu and Smitz, 2008). Some studies have demonstrated that AMH levels reflect some aspects of ovarian function, making AMH levels a potential marker for assessing conditions such as PCOS and premature ovarian failure (Visser et al., 2006; Sir-Petermann and King, 2007; Diamanti-Kandarakis, 2008; Dumesic and Abbott, 2008; Marca et al., 2009). Women with PCOS have elevated serum and FF AMH levels versus those of normal controls (Pigny et al., 2003; Laven et al., 2004; Artini et al., 2007), which is closely associated with increased development of antral follicles and follicular arrest in PCOS patients (Artini et al., 2007; Das et al., 2008; Diamanti-Kandarakis, 2008; Franks et al., 2008). Elevated AMH serum levels are directly correlated with increased testosterone and/or LH levels in women with PCOS, and profoundly impairing oocyte developmental competence and embryo quality (Tarlatzis and Grimbizis, 1997; Dumesic et al., 2002, 2007b; Patel and Carr, 2008; Franks et al., 2008). Also, elevated FF AMH concentrations in women with PCOS are linked to an increased percentage of immature oocyte and lower fertilization rates when compared with women with endometriosis or pelvic adhesions (Fallat et a., 1997); this is supported by evidence from the rat model as well (Takahashi et al., 1986). Recent complementary investigation suggests that increased FF AMH in women with PCOS may have harmful consequences on oocyte quality and maturation, via an unclear molecular mechanism, but does not have an effect on pregnancy rates (Desforges-Bullet et al., 2010).
In a contrasting study conducted among women with PCOS, results suggest that fertilization, implantation and clinical pregnancy rates are significantly better in the group with the highest FF AMH concentration than in any group with a lower concentration (Pabuccu et al., 2009). Additional reports reveal that women with PCOS who have lower FF AMH levels have similar rates of oocyte maturation, fertilization and embryonic development compared with NOW (Wang et al., 2007a, b; Mashiach et al., 2010). However, recent evidence suggests that FF AMH concentrations are only strongly and positively associated with oocyte quality and implantation rates, but not rates of oocyte fertilization, embryo cleavage and embryo morphology in NOW (Ebner et al., 2006; Fanchin et al., 2007; Marca et al., 2009). Still, others demonstrate that lower AMH levels are associated with poor oocyte quality, as supported by decreased fertilization and embryonic developmental rates, and increased miscarriage rates in IVF patients (Lekamge et al., 2007) Therefore, AMH may directly affect cytoplasmic maturation of the oocytes. Based upon all of the above studies, variation in levels of AMH may indicate different physiological conditions during follicle development and oocyte maturation. Hence, AMH may not be a valuable predictor for success in NOW and women with PCOS undergoing assisted reproduction.
Activin, follistatin and inhibin
The activins, follistatin (FS) and inhibins are polypeptides which were originally isolated and characterized from ovarian FF. FS is an activin/inhibin binding protein produced by ovarian GCs, believed to act in an autocrine/paracrine manner to regulate growth and differentiation (Shimonaka et al., 1991; Findlay, 1993; Erickson et al., 1995); over-expression of FS has been associated with increased arrest of follicular development and decreased oocyte developmental competence (Erickson et al., 1995; Norman et al., 2001). Activins are preferentially secreted by these smaller follicles, promoting follicular development by increasing the GC response to FSH stimulation, decreasing androgen synthesis and enhancing oocyte maturation. Inhibins on the other hand, are produced by the dominant follicle and stimulate theca cell androgen production for E2 synthesis (Schwall et al., 1990; Klein et al., 2000; Knight and Glister, 2001; Dumesic et al., 2007b). In NOW, studies suggest that FF levels of inhibin A, inhibin B and activin A reflect changes in follicle size, but are not independent markers of the oocyte's ability to achieve fertilization and pregnancy (Fried et al., 2003; Wen et al., 2006).
An early study failed to demonstrate any correlation between inhibin A and B concentration in the FF and oocyte quality and fertilization rates (Lau et al., 1999). IVF patients with high FF inhibin A and B levels, measured on the day of oocyte retrieval, have better oocyte maturity and fertilization rates, and higher pregnancy rates (Dzik et al., 2000; Ocal et al., 2004). Another study reported that inhibin B levels in the FF are significantly correlated to embryo quality, but not oocyte quality (Change et al., 2002). Interestingly, no differences were found in the levels of FS and activin A in the FF from normal, atretic or polycystic ovaries (Erickson et al., 1995).
Increased FS/activin ratios (high FS and low activin A) are well-known contributors to the pathophysiology of PCOS (Eldar-Geva et al., 2001; Norman et al., 2001); both proteins have an effect on oocyte maturity and developmental competence, with activin enhancing post-fertilization development, and FS blocking this function (Norman et al., 2001). Elevated inhibin B levels are closely related to an elevated risk of developing PCOS (Magoffin and Jakimiuk, 1997; Anderson et al., 1998; Lockwood et al., 1998). In addition, studies have shown that inhibin A and B levels are significantly reduced in the FF of women with PCOS, when compared with FF levels of size-matched follicles from NOW (Lambert-Meserlian et al., 1997; Welt et al., 2005). Therefore, activin, FS and inhibins bring about intra-ovarian actions through paracrine/autocrine systems, playing an important role in maintaining folliculogenesis; their imbalance may be directly correlated to the pathogenesis of PCOS, consequently impairing oocyte maturity, embryo quality and pregnancy outcome.
Growth differentiation factor-9 and bone morphogenetic protein-15
GDF-9 and BMP-15 (also called as GDF-9b) are two closely related members of the TGF-β family of proteins and are highly expressed in growing and full grown oocytes (Teixeira Filho et al., 2002; Gilchrist et al., 2008; Chen et al., 2009). BMP-15 and GDF-9 play fundamental roles in regulating CC functions through the processes of mitosis, proliferation, apoptosis, luteinization, metabolism and expansion through mitogenic signaling transduction mechanisms (Erickson and Shimasaki, 2001; Teixeira Filho et al., 2002; van der Spuy and Dyer, 2004; Gilchrist et al., 2008; Chen et al., 2009). Data from in vitro models demonstrate that co-incubation of COC with either BMP-15 or GDF-9 substantially promotes oocyte maturation and enhances blastocyt production, as well as increases the total number of cells in the trophectoderm (Hussein et al., 2006) and inner cell mass of mouse embryos (Yeo et al., 2008). Following embryo transfer in mice, the rate of fetal survival almost doubles after exposure to BMP-15 or GDF-9, but no differences could be detected in implantation rates (Yeo et al., 2008). Importantly, both GDF-9 and BMP-15 are required for folliculogenesis in humans and their abnormal expression may be related to female infertility (Juengel et al., 2002; Teixeira Filho et al., 2002; Shimasaki et al., 2004; Artini et al., 2007; Wu et al., 2007a, b; Gilchrist et al., 2008), including increased correlations with PCOS pathologies (Franks et al., 2002; Teixeira Filho et al., 2002; van der Spuy and Dyer, 2004; Ciepiela et al., 2007; Dumesic et al., 2007b, Dumesic and Abbott, 2008; Gilchrist et al., 2008; Zhao et al., 2010). In infertile women, elevated FF BMP-15 levels are positively correlated with improved oocyte quality and higher rates of fertilization and embryonic development, suggesting that BMP-15 may be a good indicator of oocyte maturity and fertilization ability (Wu et al., 2007a, b). A recent study demonstrates that the expression of GDF-9 and BMP-15 tended to be higher in PCOS patients when compared with a control group, and thus may be involved in PCOS follicular dysplasia (Zhao et al., 2010). GDF-9 expression in CCs is lower in PCOS patients, which may lead to premature luteinization and decreased oocyte developmental competence and luteal generation (Takebayashi et al., 2000; Artini et al., 2007): this may also be correlated to elevated miscarriage rates in women with PCOS (Zhao et al., 2010). Therefore, the expression of BMP-15 and GDF-9 in both oocytes and CCs may provide valuable support for the ability to regulate the follicular microenvironment during the oocyte maturation process. Further study on the role of BMP-15 or GDF-9 during follicle growth and oocyte meiotic maturation will have important implications in understanding those factors that regulate the mechanisms behind the pathogenesis of PCOS, and help to improve IVM methods for oocytes from women with PCOS.
Vascular endothelial growth factor family
Vascular endothelial growth factor (VEGF) is a homodimeric glycoprotein belonging to the VEGF/platelet-derived growth factor family (Artini et al., 2007). In the ovary, VEGF is expressed in GCs and theca cells, but rarely in stroma cells (Artini et al., 2007) and is also present in the FF (Artini et al., 1998; Van Blerkom, 2000; Stouffer et al., 2001; Ocal et al., 2004). VEGF exerts its actions by binding to one of three receptors, VEGFR-1/Flt-1, VEGFR-/KDR/Flk-1 or VEGFR-3/Flt-4, functioning via the signal transduction system (De Vries et al., 1992; Terman et al., 1992; Artini et al., 2007, 2009). VEGF plays an important role in angiogenesis, follicular vascularization and intrafollicular oxygenation, consequently impacting follicular maturation, oocyte quality, fertilization and embryo developmental competence (Itskovitz et al., 1991; Van Blerkom et al., 1997; Agrawal et al., 1998, 2002; Loret de Mola et al., 1999; van der Spuy and Dyer, 2004; Bokal et al., 2005).
In vitro culture studies show that VEGF stimulates the maturation of bovine oocytes during IVM, resulting in increased rates of fertilization and embryonic development (Luo et al., 2002; Bokal et al., 2005). In NOW, decreased FF and serum VEGF levels are related to improved ovarian response, consequently increasing the number of oocytes retrieved, and improving the rates of fertilization and pregnancy; the reverse has also been shown, as elevated FF VEGF levels are associated with poor oocyte quality and decreased fertilization and pregnancy rates, especially in older patients (Battaglia et al., 2000a, b; Ocal et al., 2004; Artini et al., 2006, 2007). In women with PCOS, elevated FF VEGF is closely associated with the development of ovarian hyperstimulation syndrome (Agrawal et al., 1998, 2002; Artini et al., 1998; Franks et al., 2002). Furthermore, it is well known that increased FF VEGF levels in PCOS patients is indicative of immature oocytes and poor fertilization rates (Artini et al., 2006, 2009).
An opposing study concluded that follicles containing higher FF VEGF concentrations provide better MII oocytes, compared with those with lower FF VEGF concentrations (Bokal et al., 2004). Among PCOS groups, reports suggest that prolonged hCG action results in elevated FF VEGF, consequently increasing the number of high-quality oocytes and embryos, as well as improving fertilization rates (Bokal et al., 2005): the same researchers also demonstrated that decreases in FF VEGF and E2 levels in PCOS women following GnRH antagonist administration have detrimental effects on follicular development, as compared with those women who were given agonists, consequently reducing oocyte and embryo quality (Bokal et al., 2009). Therefore, FF VEGF may serve as a dynamic indicator for the evaluation of follicular maturity, subsequently predicting oocyte maturity, fertilization success and embryo development in PCOS patients (Bokal et al., 2005, 2009); however, further research is required to uncover the true relationship between VEGF levels and subsequent success in PCOS women.
Cytokine family
Cytokines encompass a large family of soluble polypeptide regulators that are produced widely throughout the body by cells of diverse embryological origin; the family comprises the interleukins (IL1 ∼ 35), leukemia inhibitory factor, tumor necrosis factor (TNF)α, soluble Fas (sFas) and sFas ligand (sFasL) (TNFsubfamily). Within the ovary, the action of cytokines may be autocrine or paracrine, but not endocrine; they exist in the FF, suggesting their production by GCs (Buyalos et al., 1992; Zolti et al., 1992; Jasper and Norman, 1995; Amato et al., 2003; Gallinelli et al., 2003), and have regulatory functions in follicular maturation and subsequent embryonic development (Coskun et al., 1998; Hsieh et al., 2005). In PCOS patients cytokines are believed to play a role in ovarian hyperstimulation (Pellicer et al., 1999) and hyperandrogenism (Escobar-Morreale et al., 2001); however, these reports have been disputed (Gonzalez et al., 1999; Deshpande et al., 2000; Amato et al., 2003).
Interleukins
ILs are a group of cytokines (secreted proteins/signaling molecules) that are expressed by leukocytes (Wu et al., 2007a, b). Studies have elucidated that ILs, namely IL-1, IL-2, IL-6, IL-8, IL-11, IL-12 and other cytokines, play multiple roles in folliculogenesis, ovulation and corpus luteum function (Barak et al., 1992; Naz and Butler, 1996; Branisteanu et al., 1997; Gallinelli et al., 2003). FF IL-12 levels vary within immature and pre-ovulatory follicles (Coskun et al., 1998); the presence of FF IL-12 has been associated with fertilization failure (Gazvani et al., 2000). An important study has demonstrated that decreased FF IL-12 level and increased FF IL-13 level in PCOS patients is correlated with a reduced rate of oocyte maturation, fertilization and pregnancy, but this reduction did not reach statistical significance (Gallinelli et al., 2003).
Tumor necrosis factorα
TNFα is a multifunctional hormone-like polypeptide, which is involved in a wide range of physiological roles in regulating ovarian function, exerting an influence on proliferation, differentiation, follicular maturation, steroidogenesis and apoptosis (Lédée-Bataille et al., 2001; van der Spuy and Dyer, 2004; Artini et al., 2007). In the ovary, TNFα is expressed by the oocyte, theca cells, GCs and corpora lutea (Artini et al., 2007). One IVM model, coupling porcine oocyte co-incubation with high levels of TNFα, reported decreased oocyte maturation and increased proportions of oocytes with abnormal chromosomal alignment and cytoskeleton structure (Ma et al., 2010). Alterations in FF TNFα levels are correlated with poor-quality oocytes in women undergoing IVF (Cianci et al., 1996; Carlberg et al., 2000; Lee et al., 2000), resulting in reduced rates of fertilization, embryonic development and pregnancy outcome (Ma et al., 2010). Furthermore, increased levels of FF TNFα in women with PCOS are significantly and inversely correlated to FF E2 levels, which is again indicative of poor-quality oocytes and embryos (Gallinelli et al., 2003; Amato et al., 2003; Wu et al., 2007a, b; Kim et al., 2009).
Soluble Fas and sFas ligand
sFas and sFasL are transmembrane proteins belonging to the TNF subfamily; sFas and sFasL proteins exert anti- and pro-apoptotic functions, respectively. The binding of sFasL with its receptor induces apoptosis, whereas sFas, acting as a functional antagonist, binds with sFasL to inhibit sFasL-mediated apoptosis by preventing death signal transduction (Ueno et al., 1999; Onalan et al., 2005). sFas can be detected in human sera, oviduct fluid and FF (Srivastava et al., 1998; Onalan et al., 2005, 2006) and sFas levels in the FF are positively correlated to oocyte maturity and survival in IVF patients (Sarandakou et al., 2003). Some studies have demonstrated that the sFas–sFasL system involves apoptosis of theca cells and GCs in PCOS patients (Cataldo et al., 2000; Webber et al., 2003; Onalan et al., 2005). Furthermore, these reports suggest that reduced serum levels of sFas and DNA fragmentation in luteinized GC are found in women with PCOS undergoing IVF treatment. Patients with PCOS who are treated with metformin display anti-apoptotic effects owing to elevated serum sFas levels and reduced FF sFasL levels; GC DNA fragmentation was also reduced, thus increasing implantation and clinical pregnancy rates (Onalan et al., 2005). According to these data, one may speculate that abnormalities in the sFas–sFasL system are indicative of PCOS pathogenesis, further associating decreased oocyte quality, lower fertilization rates and higher miscarriage rates with PCOS.
Other microenvironment factors
Homocysteine
Homocysteine (Hcy) is a homologue of the amino acid cysteine, differing by an additional methylene group, and can be recycled into methionine or converted into cysteine in the presence of B-vitamins. Many studies have established that elevated Hcy levels in serum and FF are inversely associated with oocyte and embryo quality (Steegers-Theunissen et al., 1992; Ebisch et al., 2006; Berker et al., 2009; Nafiye et al., 2010), resulting in decreased fertilization and pregnancy rates, and increased miscarriage rates in PCOS patients undergoing IVF treatment (Ludwig et al., 1999; Plachot et al., 2003; Yarali et al., 2001; Loverro et al., 2002; Schacter et al., 2003; Heijnen et al., 2006; Kaya et al., 2009; Berker et al., 2009; Nafiye et al., 2010). Previous studies demonstrated that IVF patients who have higher E2 levels in FF have improved rates of oocyte fertilization, cleavage and implantation (Botero-Ruiz et al., 1984; Foong et al., 2005; Berker et al., 2009). Furthermore, elevated levels of Hcy in FF and serum may suppress E2 synthesis, and consequently interfere with ovarian follicular developmental competence, oocyte maturation and fertilization in women with PCOS (Boxmeer et al., 2008; Berker et al., 2009). Therefore, all of these results suggest that high levels of FF Hcy have a detrimental effect on oocyte and embryo quality, and may serve as a useful indicator for potential success in PCOS patients undergoing assisted reproduction.
Leptin
Leptin is a 16 kDa protein hormone that plays a key role in regulating energy intake, energy expenditure and a balance between the two. It has also served as a biomarker for body fat. In the field of assisted reproduction, leptin has been used to predict oocyte maturity and embryo quality (Barroso et al., 1999; Georgios et al., 2005). High leptin levels in the FF and serum are closely associated with decreased oocyte maturity, poor fertilization and embryo quality, and lower pregnancy rates in PCOS patients (Mantzoros et al., 2000; Georgios et al., 2005; Li et al., 2007). Some studies show that elevated leptin levels in women with PCOS play an elementary role in the pathogenesis of PCOS (Scarpace et al., 2000; Pasquali et al., 2006; Cervero et al., 2006; Li et al., 2007). Others suggest that elevated leptin levels in the ovary may block E2 production, disturbing follicular development and oocyte maturation (Mantzoros et al., 2000). Hyperleptinemia, or increased FF leptin, in PCOS patients may impair embryo quality and pregnancy rates (Anifandis et al., 2005; De Placido et al., 2006, Li et al., 2007). In contrast, other investigations have shown that FF leptin is decreased in women with PCOS and is not a useful marker for oocyte quality, fertilization or embryo development (Welt et al., 2003; Plati et al., 2009). Hence, the involvement of leptin and its significance in the establishment of PCOS pathophysiology, especially its impact on oocyte maturation competence, needs further clarification.
FF meiosis-activating sterol
FF meiosis-activating sterol (FF-MAS) is an endogenous signaling molecule and an intermediate in the cholesterol biosynthetic pathway, which is present in FF (Byskov et al., 1999, 2002; Bokal et al., 2006; Grondahl, 2008). Many IVM studies demonstrate that exposure to FF-MAS can promote nuclear and cytoplasmic maturation of the oocyte (Tsafriri and Motola, 2007) and improved fertilization and early embryonic development in humans and other mammals (Cukurcam et al., 2003; Bivens et al., 2004; Faerge et al., 2006; Grondahl, 2008). Interestingly, reports show that FF-MAS enhances successful IVM of oocytes retrieved from women with PCOS (Chian et al., 2000; Grondahl, 2008). Furthermore, a leading report suggests that the concentrations of FF-MAS significantly increase during the perio-ovulatory period, between 10–14 and 34–38 h after hCG administration; this may be related to increased numbers of MII stage oocytes retrieved from PCOS patients (Bokal et al., 2006). This knowledge may prove to be useful in the implementation of IVM protocols for PCOS patients.
Immunoreactive corticotrophin-releasing hormone, tissue inhibitor of metalloproteinase-1 & 2 and visfatin
Immunoreactive corticotrophin-releasing hormone (IrCRH) is a 41-amino acid neuropetide (Vales et al., 1981), synthesized by theca cells and/or the mature oocyte itself (Mastorakos et al., 1993, 1994). Study has found that decreased FF IrCRH levels are correlated with oocyte dysfunction in women with PCOS (Mastorakos et al., 1994). Other reports suggest that FF tissue inhibitor of metalloproteinase (TIMP)-1 & 2 levels are significantly lower in women with PCOS than in NOW (Lahav-Bratz et al., 2003). In contrast, there was no difference in basal production of TIMP-1 by cells in culture between women with PCOS and NOW; however, matrix metalloproteinases-2 and 9 are significantly increased in the FF of women with PCOS (Shalev et al., 2001), suggesting an association with inappropriate atresia. In a recent study, serum visfatin levels were significantly increased in women with PCOS, whereas FF visfatin levels do not differ when compared with non-PCOS patients (Plati et al., 2009). On the basis of these studies, it is difficult to assign specific effects to these factors, although their association to physiological or pathological functions in PCOS is evident.
Renin
Renin (also known as angiotensinogenase) participates in the body's renin–angiotensin system. It is known that ovarian renin has an impact on the developmental and fertilization competence of human oocytes (Itskovitz et al., 1991; Van Blerkom et al., 1997; Loret de Mola et al., 1999; Bokal et al., 2005). Investigations suggest that decreased FF renin is related to increased rates of oocyte maturation and fertilization, and better subsequent embryo quality (Bokal et al., 2003, 2004, 2005).
Resistin
Resistin is a 12.5 kDa cyteine-rich protein hormone, synthesized by adipose tissues (Seow et al., 2005). Recent studies demonstrate that there are no significant differences in either serum or FF resistin concentrations between PCOS patients and controls; these are also not significantly correlated with fertilization rates, implantation rates, clinical pregnancy rates or early miscarriage rates in PCOS patients (Seow et al., 2005). These data indicate that resistin is unlikely to be a useful biomarker for oocyte developmental competence during IVF treatment in PCOS women.
Oxidative stress
Reactive oxygen species (ROS) are involved in many physiological functions and act as mediators in a variety of signaling pathways. Damage to biological systems caused by an excess of ROS is referred to as OS (Gupta et al., 2009). In women with PCOS, data show that increased FF ROS and decreased total antioxidant capacity and superoxide dismutase are closely associated with lower rates of oocyte maturation and fertilization, poor embryo quality and decreased pregnancy rates (Sabatini et al., 2000; Ruder et al., 2008; Bausenwein et al., 2010; Chattopadhayay et al., 2010). ROS degrade polyunsaturated lipids, forming malondialdehyde (MDA) (Pryor and Stanley, 1975). Elevated FF MDA levels are directly correlated with increased numbers of immature oocytes retrieved, lower rates of fertilization and embryonic development, and consequently, lower pregnancy rates in PCOS patients (Yildirim et al., 2007; Berker et al., 2009). Therefore, ROS may impair oocyte quality via alterations in the balance of FFFs in the follicular microenvironment.
Concluding remarks
Patients with PCOS are typically characterized by production of an increased numbers of oocytes during stimulation in an IVF cycle; however, these women suffer from poor-quality oocytes and embryos, lower fertilization, cleavage and implantation rates, and higher miscarriage rates. A series of extra- and intra-ovarian factors causing abnormalities during folliculogeneis, follicular growth and oocyte meiotic maturation processes have been identified. Whether these abnormalities have a direct influence on GC–oocyte interactions and oocyte meiotic maturation, fertilization, embryonic development and pregnancy, or whether the influences are through circulating endocrine and local paracrine/autocrine mechanisms, requires further clarification. Although many studies have been performed in all aspects of endocrinology, genetics, metabolism and reproduction in the etiology and pathology of PCOS, it remains a challenge for clinical and academic scientists alike to elucidate the molecular mechanisms involved; in particular, the oocyte's developmental competence and genetic disruption are undoubtedly important considerations. Therefore, systematic screening for key intra-ovarian factors which are related to PCOS (such as AMH, Hcy, growth factors and cytokines) coupled with proper treatment for each PCOS phenotype are essential issues in achieving success for PCOS patients undergoing assisted reproduction, in an effort to effectively improve oocyte maturation and developmental competence.
Authors' roles
J.Q. is responsible for data collection and outline design; H.L.F. is responsible for manuscript preparation.
Funding
This work is partially supported by grants from National Outstanding Young Scientist Grant, China (# 30825038) (Q.J); and Academic Research Project (GRTR-156) from Ferring Pharmaceuticals, NJ, USA (H.L.F).
Acknowledgements
We would like to thank Gerald Scholl, M.D. and Mr Dennis Marchesi for their critical review and comments during the preparation of this manuscript.
References
- Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, Morris DV, Price J, Jacobs HS. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;ii:1375–1379. doi: 10.1016/s0140-6736(85)92552-8. [DOI] [PubMed] [Google Scholar]
- Adashi EY. Intraovarian regulation: the proposed role of insulin-like growth factors. New York Acad Sci. 1993;687:10–13. doi: 10.1111/j.1749-6632.1993.tb43847.x. doi:10.1111/j.1749-6632.1993.tb43847.x. [DOI] [PubMed] [Google Scholar]
- Agrawal R, Conway G, Sladkevicius P, Tan SL, Engmann L, Payne N, Bekir J, Campbell S, Jacobs H. Serum vascular endothelial growth factor and Doppler blood flow velocities in in vitro fertilization: relevance to ovarian hyperstimulation syndrome and polycystic ovaries. Fertil Steril. 1998;70:651–658. doi: 10.1016/s0015-0282(98)00249-0. doi:10.1016/S0015-0282(98)00249-0. [DOI] [PubMed] [Google Scholar]
- Agrawal R, Jacobs H, Payne N, Conway G. Concentration of vascular endothelial growth factor released by cultured human luteinized granulosa cells is higher in women with polycystic ovaries than in women with normal ovaries. Fertil Steril. 2002;78:1164–1169. doi: 10.1016/s0015-0282(02)04242-5. doi:10.1016/S0015-0282(02)04242-5. [DOI] [PubMed] [Google Scholar]
- Almahbobi G, Trounson AO. The role of intraovarian regulators in the etiology of the polycystic ovarian syndrome. Reprod Med Rev. 1996;5:151–168. doi:10.1017/S0962279900001320. [Google Scholar]
- Almahbobi G, Misajon A, Hutchinson P, Lolatgis N, Trounson AO. Hyperexpression of epidermal growth factor receptors in granulosa cells from women with polycystic ovary syndrome. Fertil Steril. 1998;70:750–758. doi: 10.1016/s0015-0282(98)00252-0. doi:10.1016/S0015-0282(98)00252-0. [DOI] [PubMed] [Google Scholar]
- Amato G, Lzzo A, Tucker AT, Bellastella A. Lack of insulin-like growth factor binding protein-3 variation after follicle-stimulating hormone stimulation in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertil Steril. 1999;72:454–457. doi: 10.1016/s0015-0282(99)00288-5. doi:10.1016/S0015-0282(99)00288-5. [DOI] [PubMed] [Google Scholar]
- Amato G, Conte M, Mazziotti G, Lalli E, Vitolo G, Tucker AT, Bellastella A, Carella C, Izzo A. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol. 2003;101:1177–1182. doi: 10.1016/s0029-7844(03)00233-3. doi:10.1016/S0029-7844(03)00233-3. [DOI] [PubMed] [Google Scholar]
- Anderiesz C, Trounson AO. The effect of testosterone on the maturation and developmental capacity of murine oocytes in vitro. Hum Reprod. 1995;10:2377–2381. doi: 10.1093/oxfordjournals.humrep.a136302. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Groome NP, Baird DT. Inhibin A and inbin B in women with polycystic ovarian syndrome during treatment with FSH to induce mono-ovlation. Clinical Endocrinol. 1998;48:577–684. doi: 10.1046/j.1365-2265.1998.00442.x. doi:10.1046/j.1365-2265.1998.00442.x. [DOI] [PubMed] [Google Scholar]
- Andreani CL, Pierro E, Lazzarin N, Lanzone A, Caruso A, Mancuso S. Effect of follicular fluid on granulosa luteal cells from polycystic ovary. Hum Reprod. 1996;11:2107–2113. doi: 10.1093/oxfordjournals.humrep.a019057. [DOI] [PubMed] [Google Scholar]
- Anifandis G, Koutselini E, Stefanidis I, Liakopoulos V, Leivaditis C, Mantzavinos T, Vamvakopoulos N. Serum and FF leptin levels are correlated with human embryo quality. Reproduction. 2005;130:917–921. doi: 10.1530/rep.1.00705. [DOI] [PubMed] [Google Scholar]
- Artini PG, Battaglia CD, Ambrogio G, Barreca A, Droghini F, Volpe A, Genazzani AR. Relationship between human oocyte maturity, fertilization and follicular fluid growth factors. Hum Reprod. 1994;9:902–906. doi: 10.1093/oxfordjournals.humrep.a138614. [DOI] [PubMed] [Google Scholar]
- Artini PG, Monti M, Fasciani A, Tartaglia ML, D'Ambrogio G, Genazzani AR. Correlation between the amount of follicle stimulating hormone administered and plasma and follicular fluid vascular endothelial growth factor concentrations in women undergoing in vitro fertilization. Gynecol Endocrinol. 1998;12:243–247. doi: 10.3109/09513599809015596. doi:10.3109/09513599809015596. [DOI] [PubMed] [Google Scholar]
- Artini PG, Monteleone P, Toldin MRP, Matteucci C, Ruggiero M, Cela V, Genazzani AR. Growth factors and folliculogenesis in polycystic ovary patients. Expert Rev Endocrinol Metab. 2007;2:215–223. doi: 10.1586/17446651.2.2.215. [DOI] [PubMed] [Google Scholar]
- Artini PG, Monti M, Matteucci C, Valentino V, Cristello F, Genazzani AR. Vascular endothelial growth factor and basic fibroblast growth factor in polycystic ovary syndrome during controlled ovarian hyperstimulation. Gynecol Endocrinol. 2006;22:465–470. doi: 10.1080/09513590600906607. doi:10.1080/09513590600906607. [DOI] [PubMed] [Google Scholar]
- Artini PG, Ruggiero M, Parisen Toldin MR, Monteleone P, Monti M, Cela V, Genazzani AR. Vascular endothelial growth factor and its soluble receptor in patients with polycystic ovary syndrome undergoing IVF. Hum Fertil. 2009;12:40–44. doi: 10.1080/14647270802621358. doi:10.1080/14647270802621358. [DOI] [PubMed] [Google Scholar]
- Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84. doi: 10.1210/en.2004-0588. doi:10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- Asunción M, Calvo RM, San Millán JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- Azziz R. PCOS: a diagnostic challenge. RBM online. 2004;8:644–648. doi: 10.1016/s1472-6483(10)61644-6. [DOI] [PubMed] [Google Scholar]
- Bai YH, Lim SC, Song Ch, Bae CS, Jin CS, Choi BC, Jang CH, Lee SH, Pak SC. Electro-acupuncture reverses nerve growth factor abundance in experimental polycystic ovaries in the rat. Gynecol Obstet Invest. 2004;57:80–85. doi: 10.1159/000075382. doi:10.1159/000075382. [DOI] [PubMed] [Google Scholar]
- Balen AH, Tan SL, Jacobs HS. Hypersecretion of luteinising hormone: a significant cause of infertility and miscarriage. Brit J Obstet Gynaecol. 1993;100:1082–1089. doi: 10.1111/j.1471-0528.1993.tb15170.x. [DOI] [PubMed] [Google Scholar]
- Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, Jacobs HS. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10:2107–2111. doi: 10.1093/oxfordjournals.humrep.a136243. [DOI] [PubMed] [Google Scholar]
- Barak V, Yanai P, Treves AJ, Roisman I, Simon A, Laufer N. Interleukin-1: local production and modulation of human granulosa luteal cells steroidogenesis. Fertil Steril. 1992;58:719–725. doi: 10.1016/s0015-0282(16)55318-7. [DOI] [PubMed] [Google Scholar]
- Barreca A, Del Monte P, Ponzani P, Artini PG, Genazzani AR, Minuto F. Intrafollicular insulin-like growth factor-II levels in normally ovulating women and in patients with polycystic ovary syndrome. Fertil Steril. 1996;65:739–745. doi: 10.1016/s0015-0282(16)58206-5. [DOI] [PubMed] [Google Scholar]
- Barroso G, Barrionuevo M, Rao P, Graham L, Danforth D, Huey S, Abuhamed A, Oehninger S. Vascular endothelial growth factor, nitric oxide, and leptin follicular fluid leptin levels correlate negatively with embryo quality in IVF patients. Fertil Steril. 1999;72:1024–1072. doi: 10.1016/s0015-0282(99)00442-2. doi:10.1016/S0015-0282(99)00442-2. [DOI] [PubMed] [Google Scholar]
- Battaglia C, Genazzani A, Regnani G, Primavera M, Petraglia F, Volpe A. Perifollicular Doppler flow and follicular fluid vascular endothelial growth factor concentrations in poor responders. Fertil Steril. 2000a;74:809–812. doi: 10.1016/s0015-0282(00)01517-x. doi:10.1016/S0015-0282(00)01517-X. [DOI] [PubMed] [Google Scholar]
- Battaglia DE, Woodruff TK, Padmanabhan V, Giudice LC, Bremener WJ, Soules MR. Ovarian follicular concentrations of activin, follistatin, inhibin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-2 (IGFBP-2), IGFBP-3, and vascular endothelial growth factor in spontaneous menstrual cycles of normal women of advanced reproductive age. J Clini Endocrinol Metab. 2000b;85:4520–4525. doi: 10.1210/jcem.85.12.7056. [DOI] [PubMed] [Google Scholar]
- Bausenwein J, Serke H, Eberle K, Hirrlinger J, Jogschies P, Hmeidan FA, Blumenauer V, Spanel-Borowski K. Elevated levels of oxidized low-density lipoprotein and of catalase activity in follicular fluid of obese women. Mol Hum Reprod. 2010;16:117–124. doi: 10.1093/molehr/gap078. doi:10.1093/molehr/gap078. [DOI] [PubMed] [Google Scholar]
- Behringer RR. The in vivo roles of müllerian-inhibiting substance. Curr Top Dev Biol. 1994;29:171–187. doi: 10.1016/s0070-2153(08)60550-5. doi:10.1016/S0070-2153(08)60550-5. [DOI] [PubMed] [Google Scholar]
- Berker B, Kaya CL, Aytac R, Satıroglu H. Homocysteine concentrations in follicular fluid are associated with poor oocyte and embryo qualities in polycystic ovary syndrome patients undergoing assisted reproduction. Hum Reprod. 2009;24:2293–2302. doi: 10.1093/humrep/dep069. doi:10.1093/humrep/dep069. [DOI] [PubMed] [Google Scholar]
- Billig H, Furuta I, Hsueh AJW. Estrogen inhibits and androgen enhances ovarian granulosa cell apoptosis. Endocrinology. 1993;133:2204–2212. doi: 10.1210/endo.133.5.8404672. doi:10.1210/en.133.5.2204. [DOI] [PubMed] [Google Scholar]
- Bivens CIM, Lindenthal BL, Brien MJO, Wigglesworth K, Blume T, Grondahl C, Eppig JJ. A synthetic analogue of meiosis-activating sterol (FF-MAS) is a potent agonist promoting meiotic maturation and preimplantation development of mouse oocytes maturing in vitro. Hum Reprod. 2004;19:2340–2344. doi: 10.1093/humrep/deh436. [DOI] [PubMed] [Google Scholar]
- Bokal EV, Vrtovec HM, Osredkar J, Verdenik I. Follicular fluid renin concentration in patients with polycystic ovaries treated with gonadotrophins in an in vitro fertilization programme. Clin Chem Lab Med. 2003;41:663–667. doi: 10.1515/CCLM.2003.100. [DOI] [PubMed] [Google Scholar]
- Bokal EV, Klun IV, Vrtovee HM. Quality of oocytes and embryos in patients with polycystic ovaries. Int Congr Ser. 2004;1271:112–115. [Google Scholar]
- Bokal EV, Vrtovec HM, Virant Klun I, Verdenik I. Prolonged HCG action affects angiogenic substances and improves follicular maturation, oocyte quality and fertilization competence in patients with polycystic ovarian syndrome. Hum Reprod. 2005;20:1562–1568. doi: 10.1093/humrep/deh789. doi:10.1093/humrep/deh789. [DOI] [PubMed] [Google Scholar]
- Bokal EV, Tacer KF, Vrbnjak M, Leposa S, Klun IV, Verdenik I, Rozmanb D. Follicular sterol composition in gonadotrophin stimulated women with polycystic ovarian syndrome. Mol Cell Endocrinol. 2006;249:92–98. doi: 10.1016/j.mce.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Bokal EV, Klun IV, Verdenik I. Follicular oestradiol and VEGF after GnRH antagonists or GnRH agonists in women with PCOS. Reprod Biomed Online. 2009;18:21–28. doi: 10.1016/s1472-6483(10)60420-8. [DOI] [PubMed] [Google Scholar]
- Boomsma CM, Fauser BCJM, Macklon NS. Pregnancy complications in women with Polyscystic ovary syndrome. Semin Reprod Med. 2008;26:72–84. doi: 10.1055/s-2007-992927. doi:10.1055/s-2007-992927. [DOI] [PubMed] [Google Scholar]
- Botero-Ruiz W, Laufer N, DeCherney A, Polan M, Haseltine F, Behrman H. The relationship between follicular fluid steroid concentration and successful fertilization of human oocytes in vitro. Fertil Steril. 1984;41:820–826. doi: 10.1016/s0015-0282(16)47892-1. [DOI] [PubMed] [Google Scholar]
- Boxmeer JC, Steegers-Theunissen RP, Lindemans J, Wildhagen MF, Martini E, Steegers EA, Macklon NS. Homocysteine metabolism in the pre-ovulatory follicle during ovarian stimulation. Hum Reprod. 2008;23:2570–2576. doi: 10.1093/humrep/den292. doi:10.1093/humrep/den292. [DOI] [PubMed] [Google Scholar]
- Branisteanu I, Pijnenborg R, Spiessens C, Van-der-Auwera I, Keith JC, Van-Assche F. Detection of immunoreactive interleukins-11 in human follicular fluid: correlations with ovarian steroid, insulin-like growth factor I levels and follicular maturity. Fertil Steril. 1997;67:1054–1058. doi: 10.1016/s0015-0282(97)81438-0. doi:10.1016/S0015-0282(97)81438-0. [DOI] [PubMed] [Google Scholar]
- Brzynski RG, Grow DR, Smith JA, Seltman HJ. Increase in androgen: estrogen ratioc specifically during low dose follicle-stimulating hormone therapy for polycystic ovary syndrome. Fertil Steril. 1995;64:693–697. doi: 10.1016/s0015-0282(16)57840-6. [DOI] [PubMed] [Google Scholar]
- Buyalos RP, Watson JM, Martinez-Maza O. Detection of interleukin-6 in human follicular fluid. Fertil Steril. 1992;57:1230–1234. [PubMed] [Google Scholar]
- Buyuk E, Seifer DB. Follicular-fluid neurotrophin levels in women undergoing assisted reproductive technology for different etiologies of infertility. Fertil Steril. 2008;90:1611–1615. doi: 10.1016/j.fertnstert.2007.08.085. doi:10.1016/j.fertnstert.2007.08.085. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Andersen CY, Leonardsen L, Baltsen M. Meiosis activating sterols (MAS) and fertility in mammals and man. J Exp Zool. 1999;285:237–242. doi:10.1002/(SICI)1097-010X(19991015)285:3<237::AID-JEZ6>3.0.CO;2-S. [PubMed] [Google Scholar]
- Byskov AG, Andersen CY, Leonardsen L. Role of meiosis activating sterols, MAS, in induced oocyte maturation. Mol Cell Endocrinol. 2002;87:189–196. doi: 10.1016/s0303-7207(01)00707-9. [DOI] [PubMed] [Google Scholar]
- Cano F, Garcia-Velasco JA, Millet A, Remohi J, Simon C, Pellicer A. Oocyte quality in polycystic ovaries revisited: identification of a particular subgroup of women. J Assist Reprod Genet. 1997a;14:254–260. doi: 10.1007/BF02765826. doi:10.1007/BF02765826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano F, Velasco JAG, Millet A, Remohi J, Simon C, Pellicer A. Oocyte quality in polycystic ovaries revisited: Identification of a particular subgroup of women. J Assist Reprod Genet. 1997b;14:254–261. doi: 10.1007/BF02765826. doi:10.1007/BF02765826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg M, Nejaty J, Froysa B, Guan Y, Soder O, Bergqvist A. Elevated expression of tumour necrosis factor in cultured granulosa cells from women with endometriosis. Hum Reprod. 2000;15:1250–1255. doi: 10.1093/humrep/15.6.1250. doi:10.1093/humrep/15.6.1250. [DOI] [PubMed] [Google Scholar]
- Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. 1999;84:1897–1899. doi: 10.1210/jcem.84.6.5803. doi:10.1210/jc.84.6.1897. [DOI] [PubMed] [Google Scholar]
- Cataldo NA, Giudice LC. Follicular fluid insulin-like growth factor binding protein profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 1992;74:695–697. doi: 10.1210/jcem.74.3.1371292. doi:10.1210/jc.74.3.695. [DOI] [PubMed] [Google Scholar]
- Cataldo NA, Dumesic DA, Goldsmith PC, Jaffe RB. Immunolocalization of Fas and Fas ligand in the ovaries of women with polycystic ovary syndrome: relationship to apoptosis. Hum Reprod. 2000;15:1889–1897. doi: 10.1093/humrep/15.9.1889. [DOI] [PubMed] [Google Scholar]
- Cervero A, Dominguez F, Horcajadas JA, Quinonero A, Pellicer A, Simon C. The role of the leptin in reproduction. Curr Opin Obstet Gynecol. 2006;18:297–303. doi: 10.1097/01.gco.0000193004.35287.89. [DOI] [PubMed] [Google Scholar]
- Cha KY, Chung HM, Lee D, Kwon H, Chng MK, Park LS, Choi DH, Yoon TK. Obstetric outcome of patients with plycystic ovary sysndrome treated by in vitro maturation and in vitro fertilization-embryo transfer. Fertil Steril. 2005;83:1461–1465. doi: 10.1016/j.fertnstert.2004.11.044. doi:10.1016/j.fertnstert.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Chang CL, Wang TH, Horng SG, Wu HM, Wang HS, Soong YK. The concentration of inhibin B in follicular fluid: relation to oocyte maturation and embryo development. Human Reprod. 2002;17:1724–1728. doi: 10.1093/humrep/17.7.1724. doi:10.1093/humrep/17.7.1724. [DOI] [PubMed] [Google Scholar]
- Chattopadhayay R, Ganesh A, Samanta J, Jana SK, Chakravarty BN, Chaudhury K. Effect of follicular fluid oxidative stress on meiotic spindle formation in infertile women with polycystic ovarian syndrome. Gynecol Obstet Invest. 2010;69:197–202. doi: 10.1159/000270900. doi:10.1159/000270900. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Zhao Sy, Qiao J, Liu P, Lian Y, Zheng XY. Expression of bone morphogenetic protein-15 in human oocyte and cumulus granulosa cells primed with recombinant follicle-stimulating hormone followed by human chorionic gonadotropin. Fertil Steril. 2009;92:2045–2046. doi: 10.1016/j.fertnstert.2009.05.058. doi:10.1016/j.fertnstert.2009.05.058. [DOI] [PubMed] [Google Scholar]
- Chian RC. In-vitro maturation of immature oocytes for infertile women with PCOS. Reprod Biomed Online. 2004;8:547–552. doi: 10.1016/s1472-6483(10)61101-7. [DOI] [PubMed] [Google Scholar]
- Chian RC, Buckett WM, Tulandi T, Tan SL. Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Hum Reprod. 2000;15:165–170. doi: 10.1093/humrep/15.1.165. doi:10.1093/humrep/15.1.165. [DOI] [PubMed] [Google Scholar]
- Child Tj, Jail AKA, Gulekli B, Tan SL. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil Steril. 2001;76:936–942. doi: 10.1016/s0015-0282(01)02853-9. doi:10.1016/S0015-0282(01)02853-9. [DOI] [PubMed] [Google Scholar]
- Cianci A, Calogero AE, Palumbo MA, Burrello N, Ciotta L, Palumbo G, Bernardini R. Relationship between tumour necrosis factor α and sex steroid concentrations in the follicularfluid of women with immunological infertility. Hum Reprod. 1996;11:265–268. doi: 10.1093/humrep/11.2.265. [DOI] [PubMed] [Google Scholar]
- Ciepiela P, Baczkowski T, Brelik P, Antonowicz A, Safranow K, Kurzawa R. Biotechnological and clinical outcome of in vitro fertilization in non-obese patients with polycystic ovarian syndrome. Folia Histoch Cytobiol. 2007;45(Suppl. y):65–71. [PubMed] [Google Scholar]
- Coskun S, Uzumcu M, Jaroudi K, Hollanders JM, Parhar RS, al-Sedairy ST. Presence of leukemia inhibitory factor and interleukin-12 in human follicular fluid during follicular growth. Am J Reprod Immunol. 1998;40:13–18. doi: 10.1111/j.1600-0897.1998.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Craig LB, Ke RW, Kutteh WH. Increased prevalence of insulin resistance in women with a history of recurrent pregnancy loss. Fertil Steril. 2002;78:487–490. doi: 10.1016/s0015-0282(02)03247-8. doi:10.1016/S0015-0282(02)03247-8. [DOI] [PubMed] [Google Scholar]
- Cukurcam S, Hegele-Hartung C, Eichenlaub-Ritter U. Meiosis activating sterol protects oocytes from precocious chromosome segregation. Hum Reprod. 2003;18:1908–1917. doi: 10.1093/humrep/deg378. doi:10.1093/humrep/deg378. [DOI] [PubMed] [Google Scholar]
- Da Silva SJM, Gardner JO, Taylor JE, Springbett A, De Sousa PA, Anderson RA. Brain-derived neurotrophic factor promotes bovine oocyte cytoplasmic competence for embryo development. Reproduction. 2005;129:423–434. doi: 10.1530/rep.1.00471. [DOI] [PubMed] [Google Scholar]
- Das K, Phipps WR, Hensleigh HC, Tagatz GE. Epidermal growth factor in human follicular fluid stimulates mouse oocyte maturation in vitro. Fertil Steril. 1992;57:895–901. doi: 10.1016/s0015-0282(16)54977-2. [DOI] [PubMed] [Google Scholar]
- Das M, Gillott DJ, Saridoan E, Djahanbakhch O. Anti-Mullerian hormone is increased in follicular fluid from unstimulated ovaries in women with polycystic ovary syndrome. Hum Reprod. 2008;23:2122–2126. doi: 10.1093/humrep/den185. doi:10.1093/humrep/den185. [DOI] [PubMed] [Google Scholar]
- De La Fuente R. Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Dev Biol. 2006;292:1–12. doi: 10.1016/j.ydbio.2006.01.008. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, O'Brien MJ, Eppig JJ. Epidermal growth factor enhances preimplantation developmental competence of maturing mouse oocytes. Hum Reprod. 1999;14:3060–3068. doi: 10.1093/humrep/14.12.3060. doi:10.1093/humrep/14.12.3060. [DOI] [PubMed] [Google Scholar]
- De Placido G, Alviggi C, Clarizia R, Mollo A, Alviggi E, Strina I, Fiore E. Intra-follicular leptin concentration as a predictive factor for in vitro oocyte fertilization in assisted reproductive techniques. J Endocrinol Invest. 2006;29:719–726. doi: 10.1007/BF03344182. [DOI] [PubMed] [Google Scholar]
- De Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. doi:10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- Desforges-Bullet V, Gallo C, Lefebvre C, Pigny P, Dewailly D, Jonard CJ. Increased antimüllerian hormone and decreased FSH levels in follicular fluid obtained in women with polycystic ovaries at the time of follicle puncture for in vitro fertilization. Fertil Steril. 2010;94:198–204. doi: 10.1016/j.fertnstert.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Deshpande RR, Chang MY, Chapman JC, Micheal SD. Alteration of cytokine production in follicular cystic ovaries induced in mice by neonatal estradiol injection. Am J Reprod Immunol. 2000;44:80–88. doi: 10.1111/j.8755-8920.2000.440203.x. doi:10.1111/j.8755-8920.2000.440203.x. [DOI] [PubMed] [Google Scholar]
- Di Clemente N, Josso N, Gouedard L, Belville C. Components of the anti-Mullerian hormone signaling pathway in gonads. Mol Cell Endocrinol. 2003;211:9–14. doi: 10.1016/j.mce.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E. Polycystic ovarian sysndrome: pathophysiology, molecular aspects and clinical implications. Expert Rev Mol Med. 2008;10:e31–e21. doi: 10.1017/S1462399408000598. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Katsikis I, Piperi C, Kandaraki E, Piouka A, Papavassiliou AG, Panidis D. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2008;69:634–641. doi: 10.1111/j.1365-2265.2008.03247.x. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Garcia-Rudaz C, Paredes A, Mayer C, Mayerhofer A, Ojeda SR. Excessive ovarian production of Nerve Growth Factor facilitates development of cystic ovarian morphology in mice and is a feature of polycystic ovarian syndrome in humans. Endocrinol. 2009;150:2906–2914. doi: 10.1210/en.2008-1575. doi:10.1210/en.2008-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragisic K, Liu HC, He ZY, Witkin S, Rosenwaks Z, Spandorfer S. Association of IGF-I, IGF-II and IGFBP-3 with individual oocyte maturity in PCOS patients: Evaluation of follicular fluid protein and granulosa cell gene expression. Fertil Steril. 2006;86(Suppl.):S456. [Google Scholar]
- Dumesic DA, Abbott DH. Implications of polycystic ovary syndrome on oocyte development. Seminar Reprod Med. 2008;26:53–61. doi: 10.1055/s-2007-992925. doi:10.1055/s-2007-992925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. JCEM. 2002;87:1111–1119. doi: 10.1210/jcem.87.3.8287. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Discord. 2007a;8:127–141. doi: 10.1007/s11154-007-9046-0. doi:10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Padmanabhan V, Abbott DH. Polycystic ovary syndrome and oocyte developmental competence. Obst Gyn Surv. 2007b;63:39–48. doi: 10.1097/OGX.0b013e31815e85fc. doi:10.1097/OGX.0b013e31815e85fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzik A, Lambert-Messerlian G, Izzo VM, Soares JB, Pinotti JA, Seifer DB. Inhibin B response to EFORT is associated with the outcome of oocyte retrieval in the subsequent in vitro fertilization cycle. Fertil Steril. 2000;74:1114–1117. doi: 10.1016/s0015-0282(00)01627-7. doi:10.1016/S0015-0282(00)01627-7. [DOI] [PubMed] [Google Scholar]
- Ebisch IMW, Peters WH, Thomas CM, Wetzels AM, Peer PG, Steegers-Theunissen RP. Homocysteine, glutathione and related thiols affect fertility parameters in the (sub) fertile couple. Hum Reprod. 2006;21:1725–1733. doi: 10.1093/humrep/del081. doi:10.1093/humrep/del081. [DOI] [PubMed] [Google Scholar]
- Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022–2026. doi: 10.1093/humrep/del127. doi:10.1093/humrep/del127. [DOI] [PubMed] [Google Scholar]
- Eden JA, Jones J, Carter GD, Alaghband-Zadeh J. A comparison of follicular fluid levels of insulin-like growth factor-1 in normal dominant and cohort follicles, polycystic and multicystic ovaries. Clin Endocrinol (Oxf) 1988;29:327–336. doi: 10.1111/j.1365-2265.1988.tb01231.x. doi:10.1111/j.1365-2265.1988.tb01231.x. [DOI] [PubMed] [Google Scholar]
- Eden JA, Jones J, Carter GD, Alaghband-Zadeh J. Follicular fluid concentrations of insulin-like growth factor 1, epidermal growth factor, transforming growth factor-alpha and sex-steroids in volume matched normal and polycystic human follicles. Clin Endocrinol. 1990;32:395–405. doi: 10.1111/j.1365-2265.1990.tb00879.x. doi:10.1111/j.1365-2265.1990.tb00879.x. [DOI] [PubMed] [Google Scholar]
- Eldar-Geva T, Spitz IM, Groome NP, Margalioth EJ, Homburg R. Follistatin and activivin A serum concentrations n obese and non-obese patients with polycystic ovary syndrome. Human Reprod. 2001;16:2552–2556. doi: 10.1093/humrep/16.12.2552. doi:10.1093/humrep/16.12.2552. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle stimulating hormone and insulin. Biol Reprod. 1998;59:1445–1453. doi: 10.1095/biolreprod59.6.1445. doi:10.1095/biolreprod59.6.1445. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Shimasaki S. The physiology of folliculogenesis: the role of novel growth factors. Fertil Steril. 2001;76:943–949. doi: 10.1016/s0015-0282(01)02859-x. doi:10.1016/S0015-0282(01)02859-X. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Chung DG, Sit A, DePaolo LV, Shimasaki S, Ling N. Follistatin concentrations in follicular fluid of normal and polycystic ovaries. Human Reprod. 1995;10:2120–2124. doi: 10.1093/oxfordjournals.humrep.a136246. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Calvo RM, Sancho J, San Millan JL. TNF-α and hyperandrogenism: A clinical, biochemical, and molecular genetic study. J Clin Endocrinol Metab. 2001;86:3761–3767. doi: 10.1210/jcem.86.8.7770. doi:10.1210/jc.86.8.3761. [DOI] [PubMed] [Google Scholar]
- Faerge I, Strejcek F, Laurincik J, Rath D, Niemann H, Schellander K, Rosenkranz C, Hyttel PM, Grøndahl C. The effect of FF-MAS on porcine cumulus–oocyte complex maturation, fertilization and pronuclear formation in vitro. Zygote. 2006;14:189–199. doi: 10.1017/S0967199406003765. doi:10.1017/S0967199406003765. [DOI] [PubMed] [Google Scholar]
- Fallat ME, Cook C, Siow Y, Carrillo A, Marra M. Müllerian-inhibiting substance in follicular fluid and serum: a comparison of patients with tubal factor infertility, polycystic ovary syndrome, and endometriosis. Fertil Steril. 1997;67:962–965. doi: 10.1016/s0015-0282(97)81417-3. doi:10.1016/S0015-0282(97)81417-3. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Mendez Lozano DH, Frydman N, Gougeon A, di Clemente N, Frydman R, Taieb J. Anti-Müllerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1796–1802. doi: 10.1210/jc.2006-1053. doi:10.1210/jc.2006-1053. [DOI] [PubMed] [Google Scholar]
- Findlay JK. An update on the roles of inhibin, activin and follistatin as local regulators of folliculogenesis. Biol. Reprod. 1993;48:15–23. doi: 10.1095/biolreprod48.1.15. doi:10.1095/biolreprod48.1.15. [DOI] [PubMed] [Google Scholar]
- Foong SC, Abbott DH, Lesnick TG, Session DR, Walker DL, Dumesic DA. Diminished intrafollicular estacadiol levels in in vitro fertilization cycles from women with reduced ovarian response to recombinant human follicle-stimulating hormone. Fertil Steril. 2005;83:1377–1383. doi: 10.1016/j.fertnstert.2004.11.041. doi:10.1016/j.fertnstert.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Franchimont P, Hazout A, Menezo Y, Colette J. Insulin-like growth factors I and II in follicular and oocyte maturation. Nucl Med Biol. 1994;21:523–530. doi: 10.1016/0969-8051(94)90074-4. doi:10.1016/0969-8051(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- Franks S. Polycystic ovary syndrome in adolescents. Int J Obes (Lond) 2008;32:1035–1041. doi: 10.1038/ijo.2008.61. [DOI] [PubMed] [Google Scholar]
- Franks S, Mason H Willis D. Follicular dynamics in the polycystic ovary syndrome. Mol Cell Endocrinol. 2000;163:49–52. doi: 10.1016/s0303-7207(99)00239-7. doi:10.1016/S0303-7207(99)00239-7. [DOI] [PubMed] [Google Scholar]
- Franks S, Robberts R, Hardy K. Gonadotrophin regimens and oocyte quality in women with polycystic ovaries. Reprod Biomed Online. 2002;6:181–184. doi: 10.1016/s1472-6483(10)61708-7. [DOI] [PubMed] [Google Scholar]
- Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14:367–378. doi: 10.1093/humupd/dmn015. doi:10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- Frattali AL, Pessin JE. Molecular Defects of Insulin/IGF-1 Receptor Transmembrane Signaling. Ann NY Acad Sci. 1993;687:77–89. doi: 10.1111/j.1749-6632.1993.tb43856.x. doi:10.1111/j.1749-6632.1993.tb43856.x. [DOI] [PubMed] [Google Scholar]
- Fried G, Remaeus K, Harlin J, Krog E, Csemiczky G, Aanesen A, Tall M. Inhibin B predicts oocyte number and the ratio IGF-I/IGFBP-1 may indicate oocyte quality during ovarian hyperstimulation for in vitro fertilization. J Assist Reprod Genet. 2003;20:167–176. doi: 10.1023/A:1023656225053. doi:10.1023/A:1023656225053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galal A, Mitwally MF. Insulin sensitizers for women with polycystic ovarian syndrome. Expert Rev Endocrinol Metab. 2009;4:183–192. doi: 10.1586/17446651.4.2.183. doi:10.1586/17446651.4.2.183. [DOI] [PubMed] [Google Scholar]
- Gallinelli A, Ciaccio I, Giannella L, Salvatori M, Marsella T, Volpe A. Correlations between concentrations of interleukin-12 and interleukin-13 and lymphocyte subsets in the follicular fluid of women with and without polycystic ovary syndrome. Fertil Steril. 2003;79:1365–1372. doi: 10.1016/s0015-0282(03)00344-3. doi:10.1016/S0015-0282(03)00344-3. [DOI] [PubMed] [Google Scholar]
- Gazvani MR, Bates M, Vince G, Christmas S, Lewis-Jones DI, Kingsland C. Follicular fluid concentrations of interleukin-12 and interleukin-8 in IVF cycles. Fertil Steril. 2000;74:953–958. doi: 10.1016/s0015-0282(00)01538-7. [DOI] [PubMed] [Google Scholar]
- Georgios A, Eleni K, Ioannis S, Vassilios L, Constantinos L, Themis M, Nikolaos V. Serum and follicular fluid leptin levels are correlated with human embryo quality. Reproduction. 2005;130:917–921. doi: 10.1530/rep.1.00705. doi:10.1530/rep.1.00705. [DOI] [PubMed] [Google Scholar]
- Gianaroli L, Magli MC, Ferraretti AP, Fortini D, Grieco N. Pronuclear morphology and chromosomal abnormalities as scoring criteria for embryo selection. Fertil Steril. 2003;80:341–349. doi: 10.1016/s0015-0282(03)00596-x. [DOI] [PubMed] [Google Scholar]
- Gianaroli L, Magli MC, Ferraretti AP, Lappi M, Borghi E, Ermini B. Oocyte euploidy, pronuclear zygote morphology and embryo chromosomal complement. Hum Reprod. 2007;22:241–249. doi: 10.1093/humrep/del334. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality function and oocyte quality. Hum Reprod Update. 2008;14:159–177. doi: 10.1093/humupd/dmm040. doi:10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Phillips H, Cameron D, Sieve-Smith L, Wang P. Continuing metformin throughout pregnancy in women with polycystic ovary syndrome appears to safely reduce first-trimester spontaneous abortion: a pilot study. Fertil Steril. 2001;75:46–52. doi: 10.1016/s0015-0282(00)01666-6. doi:10.1016/S0015-0282(00)01666-6. [DOI] [PubMed] [Google Scholar]
- Goff A, Yang Z, Cortvrindt R, Smitz J, Miron P. Protein synthesis during maturation of bovine oocytes, effect of epidermal growth factor. Reprod Domest Anim. 2001;36:19–24. doi: 10.1046/j.1439-0531.2001.00263.x. doi:10.1046/j.1439-0531.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Thusu K, Abdel-Rahman E, Prabhala A, Tomani M, Dandona P. Elevated serum levels of tumor necrosis factor alpha in normal-weight women with polycystic ovary syndrome. Metabolism. 1999;48:437–441. doi: 10.1016/s0026-0495(99)90100-2. doi:10.1016/S0026-0495(99)90100-2. [DOI] [PubMed] [Google Scholar]
- Goud PT, Goud AP, Qian C, Laverge H, Van der Elst J, De Sutter P, Dhont M. In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod. 1998;13:1638–1644. doi: 10.1093/humrep/13.6.1638. doi:10.1093/humrep/13.6.1638. [DOI] [PubMed] [Google Scholar]
- Grondahl C. Oocyte maturation. Dan Med Bull. 2008;55:1–16. [PubMed] [Google Scholar]
- Gupta S, Malhotra N, Sharma D, Chandra A, Ashok A. Oxidative stress and its role in female infertility and assisted reproduction: Clinical implications. Int J Fertil Steril. 2009;2:147–164. [Google Scholar]
- Hamilton-Fairley D, Kiddy D, Watson H, Paterson C, Franks S. Association of moderate obesity with a poor pregnancy outcome in women with polycystic ovary syndrome treated with low dose gonadotrophin. Br J Obstet Gynaecol. 1992;99:128–131. doi: 10.1111/j.1471-0528.1992.tb14470.x. [DOI] [PubMed] [Google Scholar]
- Hammadeh ME, Fischer-Hammadeh C, Hoffimeister H, Huebner U, George T, Rosenbaum P, Schmidt W. Fibroblast Growth Factor (FGF), Intracellular Adhesion Molecule (sICAM-1) level in serum and follicular fluid of infertile women with polycystic ovarian syndrome, endometriosis and tubal damage, and their effect on ICSI outcome. Am J Reprod Immunol. 2003;50:124–130. doi: 10.1034/j.1600-0897.2003.00056.x. doi:10.1034/j.1600-0897.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- Hardy K, Robinson FM, Paraschos T, Wicks R, Franks S, Winston RM. Normal development and metabolic activity of preimplantation embryos in vitro from patients with polycystic ovaries. Hum Reprod. 1995;10:2125–2135. doi: 10.1093/oxfordjournals.humrep.a136247. [DOI] [PubMed] [Google Scholar]
- Heijnen EM, Eijkemans MJ, Hughes EG, Laven JS, Macklon NS, Fauser BC. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:13–21. doi: 10.1093/humupd/dmi036. doi:10.1093/humupd/dmi036. [DOI] [PubMed] [Google Scholar]
- Hillier SG. Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod. 1994;9:188–191. doi: 10.1093/oxfordjournals.humrep.a138480. [DOI] [PubMed] [Google Scholar]
- Hofmann GE, Scott RT, Jr, Brzyski RG, Jones HW., Jr Immunoreactive epidermal growth factor concentrations in follicular fluid obtained from in vitro fertilization. Fertil Steril. 1990;54:303–307. [PubMed] [Google Scholar]
- Homburg R, Jacobs HS. Etiology of miscarriage in polycystic ovary syndrome. Fertil Steril. 1989;51:196–198. doi: 10.1016/s0015-0282(16)60457-0. [DOI] [PubMed] [Google Scholar]
- Hsieh YY, Chang CC, Tsai HD, Lin CS. Leukemia inhibitory factor in follicular fluid is not related to the number and quality of embryos as well as implantation and pregnancy rates. Biochem Genet. 2005;43:501–506. doi: 10.1007/s10528-005-8166-z. doi:10.1007/s10528-005-8166-z. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Zamah AM, Conti M. Epidermal Growth Factor-Like Growth Factors in the Follicular Fluid: Role in Oocyte Development and Maturation. Semin Reprod Med. 2009;27:52–61. doi: 10.1055/s-0028-1108010. doi:10.1055/s-0028-1108010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein TS, Thompson JG, Gilchrist RB. Oocyte-secreted factors enhance oocyte developmental competence. Dev Biol. 2006;296:514–521. doi: 10.1016/j.ydbio.2006.06.026. doi:10.1016/j.ydbio.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Itskovitz J, Rubattu S, Rosenwaks Z, Liu HC, Sealey JE. Relationship of follicular fluid prorenin to oocyte maturation, steroid levels, and outcome of in vitro fertilization. J Clin Endocrinol Metab. 1991;72:165–171. doi: 10.1210/jcem-72-1-165. doi:10.1210/jcem-72-1-165. [DOI] [PubMed] [Google Scholar]
- Jabara S, Coutifaris C. In vitro fertilization in the PCOS patient: clinical considerations. Semin Reprod Med. 2003;21:317–324. doi: 10.1055/s-2003-43310. [DOI] [PubMed] [Google Scholar]
- Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Roberts KA, Nestler JE. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:524–529. doi: 10.1210/jcem.87.2.8207. doi:10.1210/jc.87.2.524. [DOI] [PubMed] [Google Scholar]
- Jamnongjit M, Gill A, Hammes SR. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. PNAS. 2005;102:16257–16262. doi: 10.1073/pnas.0508521102. doi:10.1073/pnas.0508521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper M, Norman RJ. Immunoactive interleukin-1 beta and tumour necrosis factor-alpha in thecal, stromal and cell granulosa cell cultures from normal and polycystic ovaries. Hum Reprod. 1995;10:1352–1354. doi: 10.1093/humrep/10.6.1352. [DOI] [PubMed] [Google Scholar]
- Jimena P, Castilla JA, Peran F, Molina R, Ramirez JP, Acebal M, Vergara F, Herruzo A. Insulin and insulin-like growth factor I in follicular fluid after induction of ovulation in women undergoing in vitro fertilization. J Reprod Fertil. 1992;96:641–647. doi: 10.1530/jrf.0.0960641. [DOI] [PubMed] [Google Scholar]
- Johnstone EB, Shelly WB, Mellon S, Cedars MI. Brain derived neurotrophic factor is elevated in follicular fluid of women with PCOS. Fertil Steril. 2008;90(Suppl.):s256. [Google Scholar]
- Juengel JL, Hudson NL, Heath DA, Smith P, Reader KL, Lawrence SB, O'Connell AR, Laitinen MP, Cranfield M, Groome NP, et al. Growth differentiation factor 9 and bone morphogenetic protein 15 are essential for ovarian follicular development in sheep. Biol Reprod. 2002;67:1777–1789. doi: 10.1095/biolreprod.102.007146. doi:10.1095/biolreprod.102.007146. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Kawamura N, Mulders SM, Sollewijn Gelpke MD, Hsueh AJ. Ovarian brain-derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. Proc Natl Acad Sci USA. 2005;102:9206–9211. doi: 10.1073/pnas.0502442102. doi:10.1073/pnas.0502442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Narahara H, Matsui N, Nasu K, Miyamura K, Miyakawa I. Insulin-like growth factor-binding protein-1 in human follicular fluid: A marker for oocyte maturation. Gynecol Obstet Invest. 1997;44:145–148. doi: 10.1159/000291507. doi:10.1159/000291507. [DOI] [PubMed] [Google Scholar]
- Kaya C, Cengiz SD, Berker B, Demirtas S, Cesur M, Erdogan G. Comparative effects of atorvastatin and simvastatin on the plasma total homocysteine levels in women with polycystic ovary syndrome: a prospective, randomized study. Fertil Steril. 2009;92:635–642. doi: 10.1016/j.fertnstert.2008.06.006. doi:10.1016/j.fertnstert.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Kezele PR, Nilsson EE, Skinner MK. Insulin but not insul inlike growth factor-I promotes the primordial to primary follicle transition. Mol Cell Endocrinol. 2002;192:37–43. doi: 10.1016/s0303-7207(02)00114-4. doi:10.1016/S0303-7207(02)00114-4. [DOI] [PubMed] [Google Scholar]
- Kim CH, Cheon KH, Park EH, Koo YH, Jung KS, Kang BM. Pioglitazone treatment decreases follicular fluid levels of TNFα and IL-6, and improves ovarian response to FSH and IVF outcome in patients with PCOS under IVF. Fertil Steril. 2009;92(Suppl.):s104–s105. [Google Scholar]
- Kjotrod SB, von During V, Carlsen SM. Metormin treatment before IVF/ICSI in women with polycystic ovary syndrome: a prospective, randomized, double blind study. Hum Reprod. 2004;19:1315–1322. doi: 10.1093/humrep/deh248. doi:10.1093/humrep/deh248. [DOI] [PubMed] [Google Scholar]
- Klein NA, Battaglia DE, Woodruff TK, Padmanabhan V, Giudice LC, Bremner WJ, Soules MR. Ovarian follicular concentrations of activin, follistatin, inhibin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-2 (IGFBP-2), IGFBP-3, and vascular endothelial growth factor in spontaneous menstrual cycles of normal women of advanced reproductive age. J Clin Endocrinol Metab. 2000;85:4520–4525. doi: 10.1210/jcem.85.12.7056. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. Potential local regulatory functions of inhibins, activins and follistatin in the ovary. Reproduction. 2001;121:503–512. doi: 10.1530/rep.0.1210503. doi:10.1530/rep.0.1210503. [DOI] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- Kurzawa R, Ciepiela P, Baczkowski T, Safranow K, Brelik P. Comparison of embryological and clinical outcome in GnRH antagonist vs. GnRH agonist protocols for in vitro fertilization in PCOS non-obese patients. A prospective randomized study. J Assist Reprod Genet. 2008;25:365–374. doi: 10.1007/s10815-008-9249-7. doi:10.1007/s10815-008-9249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwintkiewicz J, Giudice LC. The Interplay of Insulin-Like Growth Factors, Gonadotropins, and Endocrine Disruptors in Ovarian Follicular Development and Function. Semin Reprod Med. 2009;27:43–51. doi: 10.1055/s-0028-1108009. doi:10.1055/s-0028-1108009. [DOI] [PubMed] [Google Scholar]
- Lahav-Bratz S, Kraiem Z, Shiloh H, Koifman M, Ishai D, Dirnfeld M. Decreased expression of tissue inhibitor of matrix metalloproteinases in follicular fluid from women with polycystic ovaries compared with normally ovulating patients undergoing in vitro fertilization. Fertil Steril. 2003;79:567–571. doi: 10.1016/s0015-0282(02)04838-0. [DOI] [PubMed] [Google Scholar]
- Lambert-Meserlian G, Taylor A, Leykin L, Isaacson K, Toth T, Chang YC, Schneyer A. Characterization of intrafollicular steroid hormones, inhibin, and follistatin in women with and without polycystic ovarian syndrome following gonadotropin hyperstimulation. Biol Reprod. 1997;57:1211–1216. doi: 10.1095/biolreprod57.5.1211. doi:10.1095/biolreprod57.5.1211. [DOI] [PubMed] [Google Scholar]
- Lau Cp, Ledger WL, Groome NP, Barlow DH, Mutttukrishna S. Dimeric inhibins and activin A in human follicular fluid and oocyte–cumulus culture medium. Human Reprod. 1999;14:2525–2530. doi: 10.1093/humrep/14.10.2525. doi:10.1093/humrep/14.10.2525. [DOI] [PubMed] [Google Scholar]
- Laufer N, DeCherney AH, Haseltine FP, Behrman HR. Steroid secretion by the human egg–corona–cumulus complex in culture. J Clin Endocrinol Metab. 1984;58:1153–1157. doi: 10.1210/jcem-58-6-1153. doi:10.1210/jcem-58-6-1153. [DOI] [PubMed] [Google Scholar]
- Laven JS, Mulders AG, Thermmen AP. Anti-Müllerian hormone (AMH) serum concentrations in normo-ovulatory and anovulatory women. J Clin Endocrinol Metab. 2004;89:318–323. doi: 10.1210/jc.2003-030932. doi:10.1210/jc.2003-030932. [DOI] [PubMed] [Google Scholar]
- Lédée-Bataille N, Delage GL, Taupin JL, Dubanchet S, Taieb J, Moreau JF, Chaouat G. Follicular fluid concentration of leukaemia inhibitory factor is decreased among women with polycystic ovarian syndrome during assisted reproduction cycles. Hum Reprod. 2001;16:2073–2078. doi: 10.1093/humrep/16.10.2073. doi:10.1093/humrep/16.10.2073. [DOI] [PubMed] [Google Scholar]
- Lee KS, Joo BS, Na YJ, Yoon MS, Choi OH, Kim WW. Relationships between concentrations of tumor necrosis factor-a and nitric oxide in follicular fluid and oocyte quality. J Assis Reprod Genet. 2000;17:222–228. doi: 10.1023/A:1009495913119. doi:10.1023/A:1009495913119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS. Polycystic ovary syndrome: the new millennium. Mol Cell Endocrinol. 2001;184:87–93. doi: 10.1016/s0303-7207(01)00640-2. doi:10.1016/S0303-7207(01)00640-2. [DOI] [PubMed] [Google Scholar]
- Lekamge DN, Barry M, Kolo M, Lane M, Gilchrist RB, Tremellen KP. Anti-Müllerian hormone as a predictor of IVF outcome. Reprod Biomed Online. 2007;14:602–610. doi: 10.1016/s1472-6483(10)61053-x. doi:10.1016/S1472-6483(10)61053-X. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. doi:10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Li Y, Feng HL, Cao YJ, Zheng GJ, Yang Y, Mullen S, Critser JK, Chen ZJ. Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertil Steril. 2006;85:827–832. doi: 10.1016/j.fertnstert.2005.06.064. doi:10.1016/j.fertnstert.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Li MG, Ding GL, Chen XJ, Lu XP, Dong LJ, Dong MY, Yang XF, Lu XE, Huang HF. Association of serum and follicular fluid leptin concentrations with granulosa cell phosphorylated signal transducer and activator of transcription 3 expression in fertile patients with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2007;92:4771–4776. doi: 10.1210/jc.2007-0978. doi:10.1210/jc.2007-0978. [DOI] [PubMed] [Google Scholar]
- Lighten AD, Moore GE, Winston RM, Hardy K. Routine addition of human insulin-like growth factor-I ligand could benefit clinical in-vitro fertilization culture. Hum Reprod. 1998;13:3144–3150. doi: 10.1093/humrep/13.11.3144. doi:10.1093/humrep/13.11.3144. [DOI] [PubMed] [Google Scholar]
- Liu HC, He ZY, Mele CA, Veeck LL, Davis O, Rosenwaks Z. Human endometrial stromal cells improve embryo quality by enhancing the expression of insulin-like growth factors and their receptors in cocultured human preimplantation embryos. Fertil Steril. 1999;71:361–367. doi: 10.1016/s0015-0282(98)00451-8. doi:10.1016/S0015-0282(98)00451-8. [DOI] [PubMed] [Google Scholar]
- Lockwood GM, Muttukrisshna S, Ledger WL. Inhibins and activins in human ovulation, conception and pregnancy. Hum Reprod Update. 1998;4:284–295. doi: 10.1093/humupd/4.3.284. doi:10.1093/humupd/4.3.284. [DOI] [PubMed] [Google Scholar]
- Loret de Mola JR, Goldfarb JM, Hecht BR, Babbo CJ, Friedlander MA. Gonadotropins induce higher active renin levels in the follicular fluid of normal and hyperstimulated cycles. Gynecol Endocrinol. 1999;13:155–160. doi: 10.3109/09513599909167549. doi:10.3109/09513599909167549. [DOI] [PubMed] [Google Scholar]
- Loverro G, Lorusso F, Mei L, Depalo R, Cormio G, Selvaggi L. The plasma homocysteine levels are increased in polycystic ovary syndrome. Gynecol Obstet Invest. 2002;53:157–162. doi: 10.1159/000058367. doi:10.1159/000058367. [DOI] [PubMed] [Google Scholar]
- Lu XE, Huang HF, Li MG, Zhu YM, Qiang YL, Dong MY. Resistin levels of serum and follicular fluid in non-obese patients with polycystic ovary syndrome during IVF cycles. J Zhejiang Univ Sci B. 2005;6:897–902. doi: 10.1631/jzus.2005.B0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Finas DF, al-Hasani S, Diedrich K, Ortmann O. Oocyte quality and treatment outcome in intracytoplasmic sperm injection cycles of polycystic ovarian syndrome patients. Hum Reprod. 1999;14:354–358. doi: 10.1093/humrep/14.2.354. doi:10.1093/humrep/14.2.354. [DOI] [PubMed] [Google Scholar]
- Luo H, Kimura K, Aoki M, Hirako M. Effect of vascular endothelial growth factor on maturation, fertilization and developmental competence of bovine oocytes. J Vet Med Sci. 2002;64:803–806. doi: 10.1292/jvms.64.803. doi:10.1292/jvms.64.803. [DOI] [PubMed] [Google Scholar]
- Ma CH, Yan LY, Qiao J, Sha W, Li L, Chen Y, Sun QY. Effects of tumor necrosis factor-alpha on porcine oocyte meiosis progression, spindle organization, and chromosome alignment. Fertil Steril. 2010;93:920–926. doi: 10.1016/j.fertnstert.2009.01.131. doi:10.1016/j.fertnstert.2009.01.131. [DOI] [PubMed] [Google Scholar]
- Magoffin DA, Jakimiuk AJ. Inhibin A, inhibin B and activin A in the follicular fluid of regularly cycling women. Human Reprod. 1997;12:1714–1719. doi: 10.1093/humrep/12.8.1714. doi:10.1093/humrep/12.8.1714. [DOI] [PubMed] [Google Scholar]
- Magoffin DA, Jakimiuk AJ. Inhibin A, inhibin B and activin A concentrations in follicular fluid from women with polycystic ovary syndrome. Hum Reprod. 1998;13:2693–2698. doi: 10.1093/humrep/13.10.2693. [DOI] [PubMed] [Google Scholar]
- Mantzoros CS, Cramer DW, Liberman RF, Barbieri RL. Predictive value of serum and follicular fluid leptin concentrations during assisted reproductive cycles in normal women and in women with the polycystic ovarian syndrome. Hum Reprod. 2000;15:539–544. doi: 10.1093/humrep/15.3.539. [DOI] [PubMed] [Google Scholar]
- Marca AL, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, Stabile G, Volpe A. Anti-Müllerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2009;16:113–130. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- Mashiach R, Amit A, Hasson J, Amzalzg S, Almog B, Yosef DB, Lessing JB, Limor R, Azem F. Follicular fluid levels of anti-Mullerian hormone as a predictor of oocyte maturation, fertilization rate, and embryonic development in patients with polycystic ovary syndrome. Fertil Steril. 2010;93:2299–2302. doi: 10.1016/j.fertnstert.2009.01.125. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Webster EL, Friedman TC, Chrousos GP. Immunoreactive corticotrophin-releasing hormone and its binding sites in the rat ovary. J Clin Invest. 1993;92:961–968. doi: 10.1172/JCI116672. doi:10.1172/JCI116672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos G, Scopa CD, Vryonidou A, Friedman TC, Kattis D, Phenekos C, Merino MJ, Chrousos GP. Presence of immunoreactive corticotrophin-releasing hormone in normal and polycystic human ovaries. JCEM. 1994;79:1191–1197. doi: 10.1210/jcem.79.4.7525629. [DOI] [PubMed] [Google Scholar]
- Moran L, Teede H. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod Update. 2009;15:477–488. doi: 10.1093/humupd/dmp008. doi:10.1093/humupd/dmp008. [DOI] [PubMed] [Google Scholar]
- Mulders AG, Laven JS, Imani B, Eijkemans MJ, Fauser BC. IVF outcome in anovulatory infertility (WHO group 2)—including polycystic ovary syndrome following previous unsuccessful ovulation induction. Reprod Biomed Online. 2003;7:50–58. doi: 10.1016/s1472-6483(10)61728-2. doi:10.1016/S1472-6483(10)61728-2. [DOI] [PubMed] [Google Scholar]
- Munné S, Dailey T, Sultan KM, Grifo J, Cohen J. The use of first polar bodies for preimplantation diagnosis of aneuploidy. Hum Reprod. 1995;10:1014–1020. doi: 10.1093/oxfordjournals.humrep.a136027. [DOI] [PubMed] [Google Scholar]
- Nafiye Y, Sevtap K, Muammer D, Emre O, Senol K, Leyla M. The effect of serum and intrafollicular insulin resistance parameters and homocysteine levels of nonobese, nonhyperandrogenemic polycystic ovary syndrome patients on in vitro fertilization outcome. Fertil Steril. 2010;93:1864–1869. doi: 10.1016/j.fertnstert.2008.12.024. doi:10.1016/j.fertnstert.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Navarro PA, Barcelos ID, Viera RC, Ferreira EM, Reis RM, Ferriani RA. Evaluation meiotic spindle and chromosome configuration of in vitro matured human oocytes from PCOS and endometriotic patients submitted to ovarian stimulation: Preliminary results. Fertil Steril. 2007;88(Suppl.):S297. [Google Scholar]
- Navarro PA, Barcelos ID, Ferreira EM, Giorgenon RC, Araujo MC, Viera RC. Comparative analysis of the spindle and chromosome configurations of in vitro-matured oocytes from patients with polycystic ovary syndrome and from controls: a pilot study. Fertil Steril. 2009;92(Suppl.):S68. doi: 10.1016/j.fertnstert.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Naz RK, Butler A. Interleukins-6 and -8 levels in sera and cervical mucus of fertile, idiopathic infertile, and immuno-infertile women: implication in infertility. Am J Reprod Immunol. 1996;35:534–540. doi: 10.1111/j.1600-0897.1996.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Nelson VL, Qin K, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss JF, 3rd, McAllister JM. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:5925–5933. doi: 10.1210/jcem.86.12.8088. doi:10.1210/jc.86.12.5925. [DOI] [PubMed] [Google Scholar]
- Nichols SM, Gierbolini L, Gonzalez-Martinez JA, Bavister BD. Effect of in vitro maturation and age on oocyte quality in the rhesus macaque Macaca mulatta. Fertil Steril. 2010;93:1591–1600. doi: 10.1016/j.fertnstert.2008.12.141. [DOI] [PubMed] [Google Scholar]
- Norman RJ, Milner CR, Groome NP, Robertson DM. Circulating follistatin concentration are higher and activin concentrations are lower in polycystic ovarian syndrome. Hum Reprod. 2001;16:668–672. doi: 10.1093/humrep/16.4.668. doi:10.1093/humrep/16.4.668. [DOI] [PubMed] [Google Scholar]
- Nisenblast V, Norman RJ. Androgens and polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes. 2009;16:224–231. doi: 10.1097/MED.0b013e32832afd4d. [DOI] [PubMed] [Google Scholar]
- Ocal P, Aydin S, Cepni I, Idil S, Idil M, Uzun H, Benian A. Follicular fluid concentrations of vascular endothelial growth factor, inhibin A and inhibin B in IVF cycles: are they markers for ovarian response and pregnancy outcome? Eur J Obstet Gynecol Reprod Biol. 2004;115:194–199. doi: 10.1016/j.ejogrb.2004.01.034. doi:10.1016/j.ejogrb.2004.01.034. [DOI] [PubMed] [Google Scholar]
- Okon MA, Laird SM, Tuckerman EM, Li TC. Serum androgen levels in women who have recurrent miscarriages and their correlation with markers of endometrial function. Fertil Steril. 1998;69:682–690. doi: 10.1016/s0015-0282(98)00007-7. doi:10.1016/S0015-0282(98)00007-7. [DOI] [PubMed] [Google Scholar]
- Onalan G, Selam B, Baran Y, Cincik M, Onalan R, Gündüz U, Ural AU, Pabuccu R. Serum and follicular fluid levels of soluble Fas, soluble Fas ligand and apoptosis of luteinized granulosa cells in PCOS patients undergoing IVF. Hum Reprod. 2005;20:2391–2395. doi: 10.1093/humrep/dei068. doi:10.1093/humrep/dei068. [DOI] [PubMed] [Google Scholar]
- Onalan G, Selam B, Onalan R, Ceyhan T, Cincik M, Pabuccu R. Serum and follicular fluid levels of soluble Fas and soluble Fas ligand in IVF cycles. Eur J Obstet Gynecol Reprod Biol. 2006;125:85–91. doi: 10.1016/j.ejogrb.2005.08.002. doi:10.1016/j.ejogrb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Oosterhuis GJ, Vermes I, Lambalk CB, Michgelsen HW, Schoemaker J. Insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations in fluid from human stimulated follicles. Hum Reprod. 1998;13:285–289. doi: 10.1093/humrep/13.2.285. [DOI] [PubMed] [Google Scholar]
- Ozornek MH, Bielfeld P, Krussel JS, Hirchenhain J, Jeyendran RS, Koldovsky U. Epidermal growth factor and leukemia inhibitory factor levels in follicular fluid. Association with in vitro fertilization outcome. J Reprod Med. 1999;44:367–369. [PubMed] [Google Scholar]
- Pabuccu R, Kaya C, Caglar GS, Oztas E, Satiroglu H. Follicular-fluid anti-Mullerian hormone concentrations are predictive of assisted reproduction outcome in PCOS patients. Reprod Biomed Online. 2009;19:631–637. doi: 10.1016/j.rbmo.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Padhy N, Sathya ML, Varma TR. Antral follicle size in the down regulated cycle and is relation to in vitro fertilization outcome. J Hum Reprod Sci. 2009;2:68–71. doi: 10.4103/0974-1208.57225. doi:10.4103/0974-1208.57225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palep-Singh M, Picton HM, Vrotsou K, Maruthini D, Balen AH. South Asian women with polycystic ovary syndrome exhibit greater sensitivity to gonadotropin stimulation with reduced fertilization and ongoing pregnancy rates than their Caucasian counterparts. Eur J Obstet Gynecol Reprod Biol. 2007;134:202–207. doi: 10.1016/j.ejogrb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Gambineri A, Pagotto U. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG. 2006;113:1148–1159. doi: 10.1111/j.1471-0528.2006.00990.x. [DOI] [PubMed] [Google Scholar]
- Patel SS, Carr BR. Oocyte quality in adult polycystic ovary syndrome. Semin Reprod Med. 2008;26:196–203. doi: 10.1055/s-2008-1042958. doi:10.1055/s-2008-1042958. [DOI] [PubMed] [Google Scholar]
- Pellegrini S, Fuzzi B, Pratesi S, Mannelli M, Criscuoli L, Messeri G, Forti G. In-vivo studies on ovarian insulin-like growth factor I concentrations in human preovulatory follicles and human ovarian circulation. Hum Reprod. 1995;10:1341–1345. doi: 10.1093/humrep/10.6.1341. [DOI] [PubMed] [Google Scholar]
- Pellicer A, Albert C, Mercader A, Bonilla-Musoles F, Remohi' J, Simon C. The pathogenesis of ovarian hyperstimulation syndrome: in vivo studies investigating the role of interleukin-1_, interleukin-6, and vascular endothelial growth factor. Fertil Steril. 1999;71:482–489. doi: 10.1016/s0015-0282(98)00484-1. doi:10.1016/S0015-0282(98)00484-1. [DOI] [PubMed] [Google Scholar]
- Piek E, Heldin CH, Ten DP. Specificity, diversity, and regulation in TGF-beta super family signaling. FASEB J. 1999;13:2105–2124. [PubMed] [Google Scholar]
- Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, Dewailly D. Elevated serum level of Anti-Müllerian hormone (AMH) in polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–5962. doi: 10.1210/jc.2003-030727. doi:10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- Plachot M, Belaisch-Allart J, Chouraqui A, Tesquier A, Serkine AM, Agabeyrached F. Oocyte and embryo quality in polycystic ovary syndrome. Gynecol Obstet Fertil. 2003;31:350–354. doi: 10.1016/s1297-9589(03)00059-6. doi:10.1016/S1297-9589(03)00059-6. [DOI] [PubMed] [Google Scholar]
- Plati E, Kouskouni E, Malamitsi-Puchner A, Boutsikou M, Kaparos G, Baka S. Visfatin and leptin levels in women with polycystic ovaries undergoing ovarian stimulation. Fertil Steril. 2009 doi: 10.1016/j.fertnstert.2009.04.055. Jun 10 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Pryor WA, Stanley JP. Letter: a suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J Org Chem. 1975;40:3615–3617. doi: 10.1021/jo00912a038. doi:10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- Rabinovici J, Dandekar P, Angle MJ, Rosenthal S, Martin MC. Insulin like growth factor I (IGF-I) levels in follicular fluid from human revelatory follicles: correlation with serum IGF-I levels. Fertil Steril. 1990;54:428–433. doi: 10.1016/s0015-0282(16)53756-x. [DOI] [PubMed] [Google Scholar]
- Regan I, Owen EJ, Jacobs H. Hypersecretion of luteinizing hormone, infertility and miscarriage. Lancet. 1990;ii:1141–1144. doi: 10.1016/0140-6736(90)92765-a. [DOI] [PubMed] [Google Scholar]
- Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update. 2008;14:345–357. doi: 10.1093/humupd/dmn011. doi:10.1093/humupd/dmn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini L, Shawaf TA, Wilson C, Lower A, Grudzinskas JG. Follicular Fluid Superoxide Dismutase (SOD) Activity in Women With Polycystic Ovarian Syndrome (PCOS) Fertil Steril. 2000;74(Suppl.):s253–s254. [Google Scholar]
- Sadeu JC, Smitz J. Growth differentiation factor-9 and anti-Müllerian hormone expression in cultured human follicles from frozen-thawed ovarian tissue. Reprod Biomed Online. 2008;17:537–548. doi: 10.1016/s1472-6483(10)60242-8. doi:10.1016/S1472-6483(10)60242-8. [DOI] [PubMed] [Google Scholar]
- Sagle M, Bishop K, Ridley N, Alexander FM, Michel M, Bonney RC, Beard RW, Franks S. Recurrent early miscarriage and polycystic ovaries. Br Med J. 1988;297:1027–1028. doi: 10.1136/bmj.297.6655.1027. doi:10.1136/bmj.297.6655.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu B, Ozturk O, Ranierri M, Serhal P. Comparison of oocyte quality and intracytoplasmic sperm injection outcome in women with isolated polycystic ovaries or polycystic ovarian syndrome. Arch Gynecol Obstet. 2008;277:239–244. doi: 10.1007/s00404-007-0462-x. doi:10.1007/s00404-007-0462-x. [DOI] [PubMed] [Google Scholar]
- Samanta J, Sanyal A, Chattopadhayay R, Goswanmi SK. Could oxidative stress be responsible for poor oocyte competence in women with polycystic ovary syndrome? Fertil Steril. 2008;90(Suppl.):s133–s134. [Google Scholar]
- Santos MA, Kuijk EW, Macklon NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. 2010;139:23–34. doi: 10.1530/REP-09-0187. doi:10.1530/REP-09-0187. [DOI] [PubMed] [Google Scholar]
- Sarandakou A, Malamitsi-Puchner A, Baka S, Rizos D, Hassiakos D, Creatsas G. Apoptosis and proliferation factors in serum and follicular fluid from women undergoing in vitro fertilization. Fertil Steril. 2003;79:634–636. doi: 10.1016/s0015-0282(02)04802-1. doi:10.1016/S0015-0282(02)04802-1. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Shek EW. Impaired leptin signal transduction with age-related obesity. Neuropharmacology. 2000;39:1872–1879. doi: 10.1016/s0028-3908(00)00014-9. doi:10.1016/S0028-3908(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Schacter M, Raziel A, Friedler S, Starssburger D, Bern O, Ron-El R. Insulin resistance in patients with polycystic ovary syndrome is associated with elevated plasma homocysteine. Hum Reprod. 2003;8:721–727. doi: 10.1093/humrep/deg190. [DOI] [PubMed] [Google Scholar]
- Schoyer KD, Liu HC, Witkin S, Rosenwaks Z, Spandorfe SD. Serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3) in IVF patients with polycystic ovary syndrome: correlations with outcome. Fertil Steril. 2007;88:139–144. doi: 10.1016/j.fertnstert.2006.11.108. doi:10.1016/j.fertnstert.2006.11.108. [DOI] [PubMed] [Google Scholar]
- Schwall R, Mason A, Wilcox J, Bassett S, Zeleznik A. Localization of inhibin/activin subunit mRNAs within the primate ovary. Mol Endocrinol. 1990;4:75–79. doi: 10.1210/mend-4-1-75. doi:10.1210/mend-4-1-75. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Feng B, Shelden R, Chen S, Dreyfus SF. Brain-derived neurotrophic factor: a novel human ovarian follicular protein. J Clin Endocrinol Metab. 2002a;87:655–659. doi: 10.1210/jcem.87.2.8213. doi:10.1210/jc.87.2.655. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Feng B, Shelden R, Chen S, Dreyfus SF. Neurotrophin-4/5 and neurotrophin-3 are present within the human ovarian follicle but appear to have different paracrine/autocrine functions. J Clin Endocrinol Metab. 2002b;87:4569–4571. doi: 10.1210/jc.2002-020499. doi:10.1210/jc.2002-020499. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Lambert-Messerlian G, Schneyer AL. Ovarian Brain-Derived Neurotrophic Factor (BDNF) is present in follicular fluid from normally cycling women. Fertil Steril. 2003;79:451–452. doi: 10.1016/s0015-0282(02)04669-1. doi:10.1016/S0015-0282(02)04669-1. [DOI] [PubMed] [Google Scholar]
- Sengoku K, Tamate K, Takuma N, Yoshida T, Goishi K, Ishikawa M. The chromosomal normality of unfertilized oocytes from patients with polycystic ovarian syndrome. Hum Reprod. 1997;12:474–477. doi: 10.1093/humrep/12.3.474. [DOI] [PubMed] [Google Scholar]
- Seow KM, Juan CC, Hsu YP, Ho LT, Wang YY, Hwang JL. Serum and follicular resistin levels in women with polycystic ovarian syndrome during IVF-stimulated cycles. Hum Reprod. 2005;20:117–121. doi: 10.1093/humrep/deh589. doi:10.1093/humrep/deh589. [DOI] [PubMed] [Google Scholar]
- Shalev E, Goldman S, Ben-Shlomo B. The balance between MMp-9 and MMp-2 and their tissue inhibitor (TIMP)-1 in luteinized granulose cells: comparison between women with PCOS and normal ovulatory women. Hum Reprod. 2001;7:325–331. doi: 10.1093/molehr/7.4.325. [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–1365. doi: 10.1210/me.2005-0504. doi:10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. doi:10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- Shimonaka M, Inouye S, Shimasaki S, Ling N. Follistatin binds to both activin and inhibin through the common beta-subunit. Endocrinology. 1991;128:3313–3315. doi: 10.1210/endo-128-6-3313. [DOI] [PubMed] [Google Scholar]
- Singh B, Meng L, Rutledge JM, Armstrong DT. Effects of epidermal growth factor and follicle-stimulating hormone during in vitro maturation on cytoplasmic maturation of porcine oocytes. Mol Reprod Dev. 1997;46:401–407. doi: 10.1002/(SICI)1098-2795(199703)46:3<401::AID-MRD20>3.0.CO;2-#. doi:10.1002/(SICI)1098-2795(199703)46:3<401::AID-MRD20>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, King NC. Polycystic ovary syndrome: a focus on Anti-Müllerian hormone levels. Expert Rev Endocrinol Metab. 2007;2:751–758. doi: 10.1586/17446651.2.6.751. doi:10.1586/17446651.2.6.751. [DOI] [PubMed] [Google Scholar]
- Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–471. doi: 10.1093/humupd/dmi020. doi:10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- Smitz J, Cortvrindt R, Hu Y. Epidermal growth factor combined with recombinant human chorionic gonadotrophin improves meiotic progression in mouse follicle-enclosed oocyte culture. Hum Reprod. 1998;13:664–669. doi: 10.1093/humrep/13.3.664. doi:10.1093/humrep/13.3.664. [DOI] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. doi:10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Söderström-Anttila V, Mäkinen S, Tuuri T, Suikkari AM. Favourable pregnancy results with insemination of in vitro matured oocytes from unstimulated patients. Hum Reprod. 2005;20:1534–1540. doi: 10.1093/humrep/deh768. doi:10.1093/humrep/deh768. [DOI] [PubMed] [Google Scholar]
- Srivastava MLJ, Fichorova R, De los Santos, Anderson DJ. Soluble Fas (sFas) and soluble Fas ligand (sFasL) in human reproductive tract fluids. In: Proceedings of the 45th Annual Meeting of the Society for Gynecologic Investigation, Chicago, IL, 1998; Abstract T203 [Google Scholar]
- Stanger JD, Yovich JL. Reduced in-vitro fertilization of human oocyte from patients with based basal luteinizing hormone levels during the follicular phase. Br J Obstet Gynaecol. 1985;92:385–393. doi: 10.1111/j.1471-0528.1985.tb01113.x. [DOI] [PubMed] [Google Scholar]
- Steegers-Theunissen RP, Boers GH, Blom HJ, Trijbels FJ, Eskes TK. Hyperhomocysteinaemia and recurrent spontaneous abortion or abruption placentace. Lancet. 1992;339:1122–1123. doi: 10.1016/0140-6736(92)90725-i. doi:10.1016/0140-6736(92)90725-I. [DOI] [PubMed] [Google Scholar]
- Stouffer RL, Martinez-Chequer JC, Molskness TA, Xu F, Hazzard TM. Regulation and action of angiogenic factors in the primate ovary. Arch Med Res. 2001;32:567–575. doi: 10.1016/s0188-4409(01)00323-x. doi:10.1016/S0188-4409(01)00323-X. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Koide SS, Donahoe PK. Miillerian inhibiting substance as oocyte meiosis inhibitor. Mol Cell Endocrinol. 1986;47:225–234. doi: 10.1016/0303-7207(86)90116-4. doi:10.1016/0303-7207(86)90116-4. [DOI] [PubMed] [Google Scholar]
- Takebayashi K, Takakura K, Wang H, Kimura F, Kasahara K, Noda Y. Mutation analysis of the growth differentiation factor-9 and -9B genes in patients with premature ovarian failure and polycystic ovary syndrome. Fertil Steril. 2000;74:976–979. doi: 10.1016/s0015-0282(00)01539-9. doi:10.1016/S0015-0282(00)01539-9. [DOI] [PubMed] [Google Scholar]
- Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitizing drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2010;(1):CD003053. doi: 10.1002/14651858.CD003053.pub4. [DOI] [PubMed] [Google Scholar]
- Tao Z, Yan L. Luteinizing hormone and insulin inducing earlier and excess expression of luteinizing hormone receptor messenger ribonucleic acids in granulosa cells of polycystic ovary syndrome. Fertil Steril. 2005;84(Suppl.):S426–S427. [Google Scholar]
- Tarlatzis BC, Grimbizis G. The significance of high follicular-phase luteinizing hormone levels in the treatment of women with polycystic ovarian syndrome by in vitro fertilization. J Assist Reprod Genet. 1997;14:1–4. doi: 10.1007/BF02765740. doi:10.1007/BF02765740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissier MP, Chable H, Paulhac S, Aubard Y. Comparison of follicle steroidogenesis from normal and polycystic ovaries in women undergoing IVF: relationship between steroid concentrations, follicle size, oocyte quality and fecundability. Hum Reprod. 2000;15:2471–2477. doi: 10.1093/humrep/15.12.2471. doi:10.1093/humrep/15.12.2471. [DOI] [PubMed] [Google Scholar]
- Teixeira Filho FL, Baracat EC, Lee TH, Suh CS, Matsui M, Chang RJ, Shimasaki S, Erickson GF. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1337–1344. doi: 10.1210/jcem.87.3.8316. doi:10.1210/jc.87.3.1337. [DOI] [PubMed] [Google Scholar]
- Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Bohlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial growth factor. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. doi:10.1016/0006-291X(92)90483-2. [DOI] [PubMed] [Google Scholar]
- Tesarik J. Effects of LH on oocyte yield and developmental competence. Hum Reprod. 2003;18:1358–1360. doi: 10.1093/humrep/deg266. doi:10.1093/humrep/deg267. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Mendoza C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80:1438–1443. doi: 10.1210/jcem.80.4.7714121. doi:10.1210/jc.80.4.1438. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Mendoza C. Direct non-genomic effects of follicular steroids on maturing human oocytes: oestrogen versus androgen antagonism. Hum Reprod Update. 1997;3:95–100. doi: 10.1093/humupd/3.2.95. doi:10.1093/humupd/3.2.95. [DOI] [PubMed] [Google Scholar]
- Tian L, Shen H, Lu Q, Norman RJ, Wang J. Insulin resistance increases the risk of spontaneous abortion after assisted reproduction technology treatment. J Clin Endocrinol Metab. 2007;92:1430–1433. doi: 10.1210/jc.2006-1123. doi:10.1210/jc.2006-1123. [DOI] [PubMed] [Google Scholar]
- Toulis KA, Gouli DG, Farmakiotis D, Georgopoulos NA, Katsikis I, Tarlatzis BC, Papadima I, Panidis D. Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Hum Reprod Update. 2009;15:297–307. doi: 10.1093/humupd/dmp006. doi:10.1093/humupd/dmp006. [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Motola S. Are steroids dispensable for meiotic resumption in mammals? Trends Endocrinol Metab. 2007;18:321–327. doi: 10.1016/j.tem.2007.08.005. doi:10.1016/j.tem.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Tse AC, Ge W. Differential regulation of betacellulin and heparin-binding EGF-like growth factor in cultured zebrafish ovarian follicle cells by EGF family ligands. Comp Biochem Physiol A Mol Integr Physiol. 2009;153:13–17. doi: 10.1016/j.cbpa.2008.10.011. doi:10.1016/j.cbpa.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Tuckerman EM, Okon MA, Li T, Laird SM. Do androgens have a direct effect on endometrial function? An in vitro study. Fertil Steril. 2000;74:771–779. doi: 10.1016/s0015-0282(00)00711-1. doi:10.1016/S0015-0282(00)00711-1. [DOI] [PubMed] [Google Scholar]
- Ueno T, Toi M, Tominaga T. Circulating soluble Fas concentration in breast cancer patients. Clin Cancer Res. 1999;5:3529–3533. [PubMed] [Google Scholar]
- Urman B, Tiras B, Yakin K. Assisted reproduction in the treatment of polycystic ovarian syndrome. Reprod Biomed Online. 2004;8:419–430. doi: 10.1016/s1472-6483(10)60926-1. [DOI] [PubMed] [Google Scholar]
- Vales W, Spiess J, Rivier C, River J. Characterization of 41-residue ovine hypothalamic peptide that stimulates secretion of corticotrophin and β-endophin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod. 1997;12:1047–1055. doi: 10.1093/humrep/12.5.1047. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. Intrafollicular influences on human oocyte developmental competence: perifollicular vascularity, oocyte metabolism and mitochondrial function. Hum Reprod. 2000;15(Suppl. 2):173–188. doi: 10.1093/humrep/15.suppl_2.173. [DOI] [PubMed] [Google Scholar]
- Van Der Spuy ZM, Dyer SJ. The pathogenesis of infertility and early pregnancy loss in polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18:755–771. doi: 10.1016/j.bpobgyn.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Visser J, de Jong F, Laven J, Themmen A. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. doi:10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- Volpe A, Coukos G, D'Ambrogio G, Artini PG, Genazzani AR. Follicular fluid steroid and epidermal growth factor content, and in vitro estrogen release by granulosa-luteal cells from patients with polycystic ovaries in an IVF/ET program. Eur J Obstet Gynecol Reprod Biol. 1991;42:195–199. doi: 10.1016/0028-2243(91)90219-b. doi:10.1016/0028-2243(91)90219-B. [DOI] [PubMed] [Google Scholar]
- Wang JX, Davies MJ, Norman RJ. Polycystic ovarian syndrome and the risk of spontaneous abortion following assisted reproductive technology treatment. Hum Reprod. 2001;16:2606–2609. doi: 10.1093/humrep/16.12.2606. doi:10.1093/humrep/16.12.2606. [DOI] [PubMed] [Google Scholar]
- Wang JG, Nakhuda GS, Guarnaccia MM, Sauer MV, Lobo RA. Müllerian inhibiting substance and disrupted folliculogenesis in polycystic ovary syndrome. Am J Obstet Gynecol. 2007a;196:77.e1–77.e5. doi: 10.1016/j.ajog.2006.07.046. doi:10.1016/j.ajog.2006.07.046. [DOI] [PubMed] [Google Scholar]
- Wang YT, Tang L, Cai J, Lu XE, Xu J, Zhu XM, Luo Q, Huang HF. High bone morphogenetic protein-15 level in follicular fluid is associated with high quality oocyte and subsequent embryonic development. Hum Reprod. 2007b;22:1526–1531. doi: 10.1093/humrep/dem029. [DOI] [PubMed] [Google Scholar]
- Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 362:1017–1021. doi: 10.1016/s0140-6736(03)14410-8. 2003. [DOI] [PubMed] [Google Scholar]
- Weenen C, Laven J, Von Bergh A, Cranfield M, Groome N, Visser J, Kramer P, Fauser B, Themmen A. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. doi:10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- Weghofer A, Munne S, Chen S, Barad D, Gleicher N. Lack of association between polycystic ovary syndrome and embryonic aneuploidy. Fertil Steril. 2007;88:900–905. doi: 10.1016/j.fertnstert.2006.12.018. doi:10.1016/j.fertnstert.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Welt CK, Schneyer AL, Heist K, Mantzoros CS. Leptin and soluble leptin receptor in follicular fluid. J Assist Reprod Genet. 2003;20:495–501. doi: 10.1023/B:JARG.0000013649.38415.2a. doi:10.1023/B:JARG.0000013649.38415.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welt CK, Taylor AE, Fox J, Messerlian GM, Adams JM, Schneyer AL. Follicular Arrest in Polycystic Ovary Syndrome is Associated with Deficient Inhibin A and B Biosynthesis. J Clin Endocrinol Metab. 2005;90:5582–5587. doi: 10.1210/jc.2005-0695. doi:10.1210/jc.2005-0695. [DOI] [PubMed] [Google Scholar]
- Wen XS, Tozer AJ, Butler SA, Bell CM, Docherty SM, Iles RK. Follicular fluid levels of inhibin A, inhibin B, and activin A levels reflect changes in follicle size but are not independent markers of the oocyte's ability to fertilize. Fertil Steril. 2006;85:1723–1729. doi: 10.1016/j.fertnstert.2005.11.058. doi:10.1016/j.fertnstert.2005.11.058. [DOI] [PubMed] [Google Scholar]
- Westergaard LG, Andersen CY. Epidermal growth factor (EGF) in human preovulatory follicles. Hum Reprod. 1989;4:257–260. doi: 10.1093/oxfordjournals.humrep.a136883. [DOI] [PubMed] [Google Scholar]
- Wijeyaratne CN, Balen AH, Barth JH, Belchetz PE. Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome (PCOS) among South Asians and Caucasians: is there a difference? Clin Endocrinol (Oxf) 2002;57:343–350. doi: 10.1046/j.1365-2265.2002.01603.x. doi:10.1046/j.1365-2265.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- Wood JR, Dumesic DA, Abbott DH, Strauss JF., III Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–713. doi: 10.1210/jc.2006-2123. doi:10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- Wu R, Fujii S, Ryan NK, Van der Hoek KH, Jasper MJ, Sini I, Robertson SA, Robker RL, Norman RJ. Ovarian leukocyte distribution and cytokine/chemokine mRNA expression in follicular fluid cells in women with polycystic ovary syndrome. Hum Reprod. 2007a;22:527–535. doi: 10.1093/humrep/del371. doi:10.1093/humrep/del371. [DOI] [PubMed] [Google Scholar]
- Wu YT, Tang L, Cai J, Lu XE, Xu J, Zhu XM, Luo Q, Huang HF. High bone morphogenetic protein-15 level in follicular fluid is associated with high quality oocyte and subsequent embryonic development. Hum Reprod. 2007b;22:1526–1531. doi: 10.1093/humrep/dem029. doi:10.1093/humrep/dem029. [DOI] [PubMed] [Google Scholar]
- Wynn P, Picton HM, Krapez JA, Rutherford AJ, Balen AH, Gosden RG. Pretreatment with follicle stimulating hormone promotes the numbers of human oocytes reaching metaphase II by in vitro maturation. Hum Reprod. 1998;13:3132–3138. doi: 10.1093/humrep/13.11.3132. doi:10.1093/humrep/13.11.3132. [DOI] [PubMed] [Google Scholar]
- Yarali H, Yildirir A, Aybar F, Kabakci G, Bukumez O, Akgul E, Oto A. Diastolic dysfunction and increased serum homocysteine concentrations may contribute to increased cardiovascular risk in patients with polycystic ovary syndrome. Fertil Steril. 2001;76:511–516. doi: 10.1016/s0015-0282(01)01937-9. doi:10.1016/S0015-0282(01)01937-9. [DOI] [PubMed] [Google Scholar]
- Yen SSc, Laughlin GA, Morales AJ. Interface beween extra-and intraovarian factors in polycystic ovarian syndrome. Ann NY Acad Sci. 1993;687:98–111. doi: 10.1111/j.1749-6632.1993.tb43858.x. doi:10.1111/j.1749-6632.1993.tb43858.x. [DOI] [PubMed] [Google Scholar]
- Yeo CX, Gilchrist RB, Thompson JG, Lane M. Exogenous growth differentiation factor 9 in oocyte maturation media enhances subsequent embryo development and fetal viability in mice. Hum Reprod. 2008;23:67–73. doi: 10.1093/humrep/dem140. doi:10.1093/humrep/dem140. [DOI] [PubMed] [Google Scholar]
- Yildirim B, Demir S, Temur I, Erdemir R, Kaleli B. Lipid Peroxidation in follicular fluid of women with polystic ovary syndrome during assisted reproduction cycles. J Reprod Med. 2007;52:722–726. [PubMed] [Google Scholar]
- Yoshimura Y, Wallach EE. Studies of the mechanism(s) of mammalian ovulation. Fertil Steril. 1987;47:22–34. [PubMed] [Google Scholar]
- Zhao SY, Qiao J, Chen YJ, Liu P, Li J, Yan J. Expression of growth differentiation factor-9 and bone morphogenetic protein-15 in oocytes and cumulus granulosa cells of patients with polycystic ovary syndrome. Fertil Steril. 2010;94:261–267. doi: 10.1016/j.fertnstert.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Zolti M, Bider D, Seidman DS, Mashiach S, Ben-Rafael Z. Cytokine levels in follicular fluid of polycystic ovaries in patients treated with dexamethasone. Fertil Steril. 1992;57:501–504. doi: 10.1016/s0015-0282(16)54891-2. [DOI] [PubMed] [Google Scholar]