Abstract
Aims
Comorbidity, such as myocardial infarction, diabetes, and renal failure, plays a pivotal role in the prognosis of a patient with arrhythmias. However, data on the prognostic impact of comorbiditiy in heart failure patients with cardiac resynchronization therapy and defibrillation (CRT-D) are scarce. The purpose of this study was to determine the impact of comorbidity on survival in CRT-D patients.
Methods and results
The study population consisted of 463 heart failure patients who received a CRT-D between 1999 and 2008 in Rotterdam and Basel. The Charlson comorbidity index (CCI) is often used as an adjusting variable in prognostic models. The Cox proportional hazards analysis was performed to determine the independent effect of comorbidity on survival. During a median follow-up of 30.5 months, 85 patients died. Mortality rates at 1 and 7 years were 6.3 and 32.3%. Cumulative incidence of implantable cardioverter defibrillator (ICD) therapy at 7 years was 50%, and death without ICD therapy was observed in 9% of patients. At least three comorbid conditions were observed in 81% of patients. Patients who died had a higher CCI score compared with those who survived (3.9 ± 1.5 vs. 2.9 ± 1.5; P < 0.001). An age-adjusted CCI score ≥5 was a predictor of mortality (hazard ratio 3.69, 95% CI 2.06–6.60; P < 0.001) independent from indication for ICD therapy, and from ICD interventions during the clinical course.
Conclusion
Comorbidity is often present in heart failure patients, and a high comorbidity burden was a significant predictor of mortality in CRT-D recipients. Comorbidity cannot predict appropriate ICD therapy. Death without prior ICD therapy occurs in a minor proportion of patients.
Keywords: Implantable cardioverter-defibrillator, Cardiac resynchronization therapy, Mortality, Comorbidity, Ventricular arrhythmia
Introduction
Data from randomized clinical trials have demonstrated that implantable cardioverter defibrillators (ICDs) provide a significant reduction in arrhythmic mortality in patients with ischaemic and non-ischaemic cardiomyopathy, both in primary and secondary prevention.1–4 In addition, cardiac resynchronization therapy (CRT) has been shown to improve symptoms, reduce hospitalizations, and to reduce mortality in patients with refractory heart failure (HF).5–7 Despite the advances in medical therapy, the prognosis of HF patients is poor. Commonly used risk factors that predict prognosis in ICD recipients with HF include NYHA functional class, QRS width, and left ventricular ejection fraction (LVEF). However, the majority of patients has more than one condition affecting their health state. Recent studies suggest that comorbidity as renal failure and diabetes further influence the prognosis of ICD recipients.8–10 The role of comorbidity in the short- and long-term prognosis of HF patients implanted with an ICD capable of cardiac resynchronization therapy (CRT-D) has not been adequately studied. The Charlson comorbidity index (CCI) is widely used as an adjustment variable in prognostic models.11 The index is based on comorbid conditions and cardiovascular risk factors of known prognostic value with varying assigned weights. The aim of the present study was to determine whether the pre-implant CCI predicts long-term survival and whether the CCI has the potential to identify those patients who would benefit less from CRT-D implantation.
Methods
Study population
Two prospective ICD registries of the cardiology departments of the Erasmus MC and the University Hospital of Basel were the basis for this study. Out of these registries, we identified all patients who received a CRT-D. The following selection criteria for CRT were applied: symptomatic HF despite optimal drug therapy, impaired LVEF (LVEF ≤35%), inter- or intra-ventricular conduction delay (QRS duration ≥120 ms), and LV end-diastolic diameter >55 mm. Indications for ICD therapy were dichotomized into primary or secondary prevention. Secondary prevention was defined as cardiac arrest without transient or reversible cause or spontaneous symptomatic sustained ventricular arrhythmias. Primary prevention as indication for ICD therapy was defined as the presence of LVEF ≤35% with ischaemic or non-ischaemic cardiomyopathy in the absence of a history of cardiac arrest or sustained ventricular arrhythmia.12 As a certain period of follow-up was warranted, only patients who were followed for at least 12 months were eligible for the analysis.
All implanted devices were capable of storing intracardiac electrograms. A two-zone configuration was programmed in 83% of patients. The mean ventricular tachycardia detection rate was 353 ± 24 ms, the mean ventricular fibrillation detection rate was 284 ± 15 ms. For all devices, antitachycardia pacing in combination with cardioversion/defibrillation therapy features was activated. For resynchronization therapy, the atrio-ventricular delay was optimized by 2D echocardiography to provide the longest diastolic filling time and the highest left ventricular outflow tract velocity timed integral.
Construction of the comorbidity index
Comorbidity as present before ICD implantation was identified via patient history, laboratory values, and review of patients' clinical data. For all patients, the renal function was assessed by estimating the baseline glomerular filtration rate (eGFR) using the abbreviated Modification of Diet in Renal Disease (MDRD) Study equation: eGFR (mL/min/1.73 m2 of body surface area) = 186 × (serum creatinine in mg/dL)−1.154 × (age)−0.203 × 0.742 in female subjects.13 Impaired renal function was defined as an eGFR <60 mL/min/1.73 m2 according to practice guidelines.14 Comorbidity was quantified using the CCI, which is based on 17 different categories of comorbidity with varying assigned weights. The comorbid conditions included in our abbreviated CCI model were myocardial infarction, cerebrovascular disease, chronic obstructive pulmonary disease, diabetes, peripheral vascular disease, renal failure, and any malignancy excluding metastatic tumours. The comorbidity index was calculated by assigning a weight of 2 to renal failure and any malignancy, and a weight of 1 to the other comorbid conditions. The comorbidity score for each patient is the arithmetic sum of the value assigned to each identified comorbid condition. Age is also a risk factor for mortality independent from each comorbid condition. To account for the effects of increasing age, the comorbidity score was adjusted by adding one point to the score for each decade of life over the age of 50 at time of implantation.
Follow-up
Follow-up started at the time of ICD implantation. In Basel, patients were followed at 1, 3, and 6 months after implantation, and then at 6-month intervals. In Rotterdam, patients were seen at 10 days, 3, 6, 9, and 12 months after implantation, and then also at 6-month intervals. All patients were advised to contact the outpatient clinic as soon as possible after symptomatic events. At each visit, arrhythmic events were retrieved from the device's memory. The stored electrograms were analysed to classify the arrhythmias responsible for triggering ICD therapy. Appropriate ICD therapy was defined as an antitachycardia pacing therapy or shock for an arrhythmia determined to be either ventricular tachycardia or ventricular fibrillation.
Deaths were classified according to a modified Hinkle–Thaler system.15,16 The primary endpoint was all-cause mortality. The secondary endpoint was time from ICD implantation until the first appropriate ICD therapy or death (e.g. death without prior appropriate ICD therapy).
Statistical analysis
All statistics were performed using SPSS (version 16.0) for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± SD, if normally distributed, or otherwise by median and interquartile range (IQR). Data were compared with Student's t-test or the Mann–Whitney U test, when appropriate. Categorical data were expressed as percentages and compared with Fisher's exact test. Simultaneous comparison of >2 mean values was performed by one-way analysis of variance. Cumulative actuarial survival rates were calculated according to the Kaplan–Meier method. Differences between pairs of actuarial curves were tested by the log-rank test. Univariate analysis was used to identify variables associated with mortality after ICD implantation. Baseline clinical variables and previously identified variables associated with mortality (P < 0.10) were entered in the multivariate Cox proportional hazards analysis. The proportional hazards assumption was checked graphically by log survival vs. log (−log survival distribution function). In addition, the proportional hazards assumption for all variables was tested using Schoenfeld residuals. Hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) are reported. A two-tailed P-value of 0.05 was considered as significant.
Results
Patient characteristics
The baseline characteristics of the study population are presented in Table 1. The majority of patients was aged <65 years (55%) with a mean age of 62 ± 11 years. Most CRT-D recipients were men (75%). The mean LVEF was 24 ± 7%, and the mean QRS duration was 165 ± 30 ms. Three hundred thirty-four patients (72%) were in NYHA class III. Ischaemic aetiology of HF was present in 50% of the patients. Atrial fibrillation was present in 18% of patients. In the entire study population, the number of comorbid conditions ranged from one to seven per patient. Comorbidity was common among HF patients, with 81% of the patients having at least three comorbid conditions. The most common non-cardiac comorbid conditions were renal disease, diabetes mellitus, cerebrovascular disease, and chronic obstructive pulmonary disease (Table 2). The prevalence of comorbidity increased with older age (all P-value trends <0.001). The comorbidity index scores ranged from 1 to 9, with a mean of 3.1 ± 1.5.
Table 1.
Baseline clinical characteristics of the study population
| Total population (n = 463) | |
|---|---|
| Demographic data, n (%) | |
| Male gender | 349 (75) |
| Age (years) | 62 ± 11 |
| Clinical data, n (%) | |
| Indication ICD therapy | |
| Primary prevention | 346 (75) |
| Secondary prevention | 117 (25) |
| NYHA class | |
| II | 129 (28) |
| III | 334 (72) |
| Left ventricular ejection fraction (%) | 24 ± 7 |
| QRS duration (ms) | 165 ± 30 |
| Creatinine (mg/dL) | 1.35 ± 0.70 |
| Ischaemic aetiology | 233 (50) |
| History of myocardial infarction | 200 (43) |
| History of bypass surgery | 95 (21) |
| Pharmacological treatment | |
| Amiodarone | 135 (29) |
| Beta-blockers | 350 (76) |
| ACE-inhibitors/ARBs | 423 (91) |
| Diuretics | 388 (84) |
| Digoxin | 117 (25) |
| Statin | 245 (53) |
Table 2.
Prevalence of comorbid conditions
| Comorbid conditions | Total population (n = 463) |
|---|---|
| Cardiac comorbidities, n (%) | |
| Myocardial infarction | 200 (43) |
| Non-cardiac comorbidities, n (%) | |
| Cerebrovascular disease | 59 (13) |
| Chronic obstructive pulmonary disease | 47 (10) |
| Diabetes | 118 (26) |
| Peripheral vascular disease | 17 (4) |
| Renal disease | 236 (51) |
| Any malignancy (excluding metastatic tumours) | 36 (8) |
Comorbidity and mortality
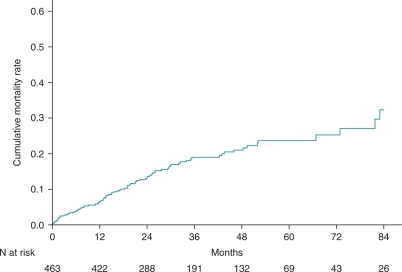
During a median follow-up of 30.5 months (IQR: 18.2–50.7 months), a total of 85 patients (18%) reached the primary endpoint. Of the 85 deaths, 66 (78%) were classified as cardiac and 7 (8%) as non-cardiac. The cause of death was unknown in 12 cases. Figure 1 presents the cumulative mortality for the studied population. The overall mortality rates were 6.3, 12.9, and 32.3%, at 1, 2, and 7 years, respectively. At 7 years, mortality rates were not different between primary and secondary prevention patients (31 vs. 36%).
Figure 1.
Cumulative mortality for heart failure patients treated with cardiac resynchronization therapy and defibrillation.
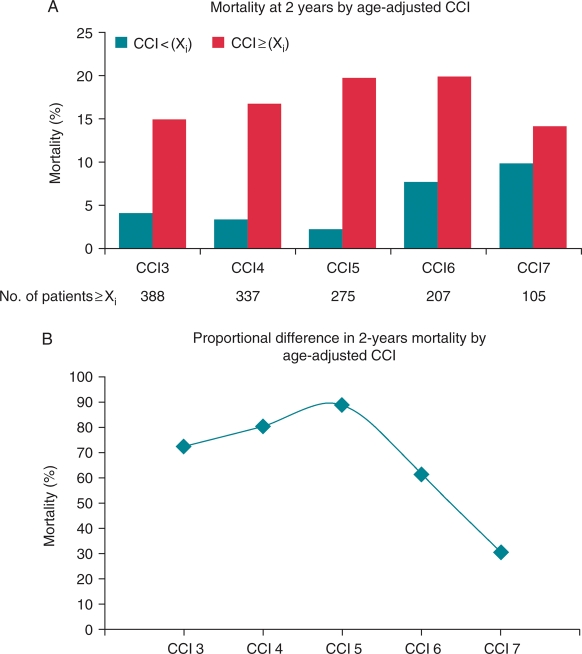
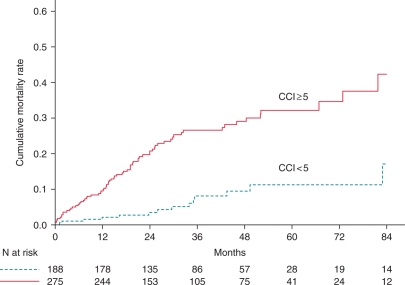
Survivors and non-survivors did not differ significantly with respect to gender, LVEF, QRS duration, and pharmacological treatment (ACE-I, diuretics, beta-blocker, and statin). There was a trend towards a higher prevalence of atrial fibrillation, and use of amiodarone and digoxin was higher in patients who died, but the difference was not statistically significant (P < 0.10). Regarding the comorbidity index score, patients who died had a significantly higher CCI score compared with those who survived (3.9 ± 1.5 vs. 2.9 ± 1.5; P < 0.001). Patients who died during long-term follow-up were significantly older (median 67 vs. 62 years, P < 0.05). Older age at implant is associated with increased mortality risk. In univariate analysis, the HR for all-cause mortality was 1.92 (95% CI 1.25–2.96; P = 0.003) in those aged ≥65 years. Accordingly, the CCI scores were adjusted for age to account for the effects of increasing age by adding one point to the score for each decade of life over the age of 50. The mean age-adjusted CCI score for patients who died was significantly higher compared with those who survived (5.9 ± 1.9 vs. 4.7 ± 2.1; P < 0.001). Figure 2A presents the difference in 2-year mortality rates among patients according to the cut-off value of age-adjusted CCI score. Notably, the difference in mortality rate was most prominent among patients with an age-adjusted CCI ≥5 compared with those with age-adjusted CCI <5, whereas among patients with an age-adjusted CCI ≥7, mortality was slightly higher compared with those with age-adjusted CCI <7. The effect of increasing age-adjusted CCI score on 2-year mortality rates showed an inverted U-shaped curve (Figure 2B). The 7-year mortality rates were significantly higher for patients with age-adjusted CCI score ≥5 compared with those with age-adjusted CCI score <5 (42.3 vs. 17.1%; P < 0.001; Figure 3).
Figure 2.
Inverted U-shaped curve for mortality. (A) Two-year Kaplan–Meier mortality rates for the groups dichotomized according to the cut-off value of age-adjusted Charlson comorbidity index score. (B) The corresponding 2-year mortality increase for patients with age-adjusted Charlson comorbidity index score ≥ cut-off value.
Figure 3.
Cumulative mortality rates for the two groups dichotomized according to an age-adjusted Charlson comorbidity index with cut-off value 5.
Appropriate implantable cardioverter defibrillator therapy, mortality, and comorbidity
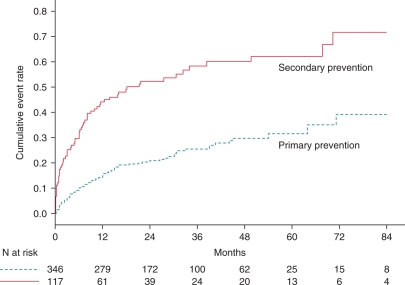
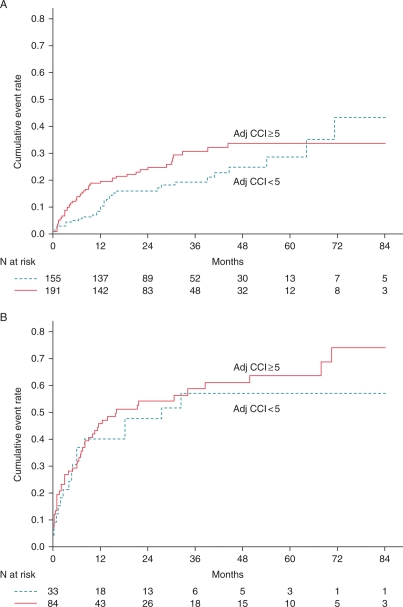
During follow-up, 149 patients (32%) experienced ICD therapy for ventricular tachyarrhythmias. The first appropriate device therapy occurred at median interval of 6.9 months (IQR: 1.7–15.0 months) after implantation. Cumulative event rates for appropriate ICD therapy were 21.7, 28.5, and 50.4%, at 1, 2, and 7 years, respectively. Patients with a secondary prevention indication were more likely to receive appropriate ICD therapy compared with primary prevention (56 vs. 24%, P < 0.001). The 7-year event rate of appropriate ICD therapy was 66.8% for secondary prevention patients, compared with 39.1% for primary prevention patients (Figure 4). The event rates of appropriate ICD therapy were not significantly different in both primary and secondary patients with an age-adjusted CCI ≥5 compared with those with age-adjusted CCI <5 (Figure 5).
Figure 4.
Cumulative rates of appropriate device therapy for patients with a primary or secondary prevention indication.
Figure 5.
Cumulative rates of appropriate device therapy for patients dichotomized according to an age-adjusted Charlson comorbidity index with cut-off value 5 and a primary prevention indication (A) or secondary prevention indication (B).
A total of 189 patients (41%) reached the secondary endpoint of time to first appropriate ICD therapy or death. Death without prior appropriate ICD therapy was observed in 40 patients, whereas 45 patients received appropriate ICD therapy prior to death, and 104 patients received appropriate ICD therapy. The estimated proportion of patients free of appropriate ICD therapy or death was 40.9% at 7 years. The majority of patients (85%) who died without prior appropriate ICD therapy had a primary prevention indication. The 7-year mortality rates between patients with and without prior appropriate ICD therapy were significantly different (44.9 vs. 21.9%, P < 0.001). In univariate analysis, appropriate ICD therapy was associated with an increased risk for all-cause mortality (HR 2.06, 95% CI 1.34–3.17; P < 0.001).
The multivariate Cox proportional hazard regression analysis identified an age-adjusted CCI score ≥5 (HR 3.69, 95% CI 2.06–6.60; P < 0.001) and appropriate device therapy (HR 1.80, 95% CI 1.17–2.77; P = 0.008) as predictors of all-cause mortality independent from the prevention indication for ICD therapy. Atrial fibrillation, use of amiodarone and digoxin was not significantly associated with mortality in the multivariate analysis.
Discussion
The present study evaluated the prognostic impact of comorbidity using an abbreviated modified CCI in HF patients who received a CRT-D for primary or secondary prevention of sudden cardiac death. We found that comorbidity was common in our study population of HF patients, with 81% of the patients having at least three comorbid conditions. Comorbidity was significantly associated with mortality after device implantation. Patients with a high comorbidity burden, defined as an age-adjusted CCI ≥5 had an increased risk for mortality, independent from the prevention indication.
Previous studies have examined factors associated with increased mortality in ICD recipients. Parkash et al.17 developed a risk score using baseline characteristics to predict early mortality after ICD implantation. Independent factors were age >80 years, history of atrial fibrillation, renal dysfunction (creatinine 1.8 mg/dL), and NYHA class III or IV. Patients with two or more of these factors had an increased risk for early mortality after device implantation, with renal dysfunction as comorbidity. A recent analysis of the second Multicenter Automatic Defibrillator Implantation Trial (MADIT II) demonstrated that a point score based on clinical variables (age >65 years, diabetes, NYHA class >II, blood urea nitrogen >28 mg/dL, and non-sinus rhythm) can identify patients at high risk for death during long-term follow-up.18 At 6-years follow-up, patients with high risk (at least three risk factors), medium risk (one to two risk factors), and low risk (no risk factors) had a cumulative mortality of 68, 43, and 19%, respectively. In a study by Bruch et al.19 the prognostic impact of comorbidities in HF patients treated with an ICD was examined. In this study, the presence of renal dysfunction, age, and NYHA functional class predicted mortality. Patients with all of these variables had an event-free survival of 15% at 1400 days follow-up. The previous studies identified renal failure as a predictor of mortality, next to traditional markers as NYHA functional class and advanced age. The effect of cardiac and non-cardiac conditions on survival has been evaluated in a large community-based examination of ICD recipients.20 This study revealed that the presence of non-cardiac comorbidity and prior HF was a significant predictor of death. The adjusted HR for death was 2.98 for patients with at least three comorbid conditions. In our study all patients had HF, but consistent results were observed with increased mortality for those with a high comorbidity burden.
The results of the present study may have implications for the translation of the expanded indications for ICD therapy from randomized trials into clinical practice. Evidence from randomized clinical trials indicates that the ICD reduces arrhythmic mortality in HF patients with ischaemic and non-ischaemic cardiomyopathy, both in primary and secondary prevention.1–4 In addition, the efficacy and survival benefits of CRT have been proved in observational studies as well as in randomized trials.5,6,21 Taken this together, the addition of defibrillation to CRT is reasonable, as the CRT-D targets both HF and arrhythmic mortality. Despite the effectiveness of terminating ventricular tachyarrhythmias by the implantable defibrillator, competing non-cardiac comorbidity is associated with increased mortality in ICD patients.22 Bai et al.,23 demonstrated that renal failure and diabetes are strong independent predictors of mortality in patients treated with CRT. Both comorbid conditions are incorporated in the Charlson model as applied in the present study.
The rate of appropriate ICD therapy was twice as likely in secondary prevention compared with primary prevention. This finding is consistent with previous studies.24,25 In addition, we found increased mortality in patients who experienced appropriate ICD therapy. This result is consistent with other published studies.26,27 In clinical practice, it is of particular interest to identify patients who might not benefit from ICD implantation. In our study, the majority of patients who died prior to appropriate ICD therapy had a primary prevention indication. Taken all together, the findings of this study may assist the clinician contemplating ICD insertion as primary prevention. However, it is still a personal decision about how high the risk of death from non-cardiac comorbidity should be before ICD therapy is no longer justified. Unfortunately, appropriate ICD interventions cannot be predicted by the comorbidity index.
Study limitations
The present study has some limitations. Even though the design was non-randomized, data were collected prospectively. Another possible limitation is the assessment of comorbidity, which was partly based on administrative data sources. These sources have a likelihood of underreporting. However, an analysis of administrative data suggested a high degree of specificity.28
Conclusion
Comorbidity is common in HF patients with a CRT-D, which has a major impact on survival. A high comorbidity burden was a significant predictor of death in CRT-D recipients. The abbreviated CCI is a useful and easy tool to assess the burden of comorbidity at baseline. Our findings may assist clinicians contemplating ICD insertion as primary prevention in patients with HF in the presence of non-cardiac comorbidity. However, further research is still needed to characterize other specific comorbid conditions as predictors of survival, and to predict who will receive appropriate ICD therapy.
Conflict of interest: none declared.
Funding
B.A.S. was supported by a grant from the Swiss National Fund (IZK0Z3-12146/1) and from St Jude Medical. Funding to pay the Open Access publication charges was provided by the Department of Cardiology, Thoraxcenter, Erasmus MC, Rotterdam, the Netherlands.
References
- 1.The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–83. doi: 10.1056/NEJM199711273372202. doi:10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. doi:10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 3.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–8. doi: 10.1056/NEJMoa033088. doi:10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 4.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. doi:10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 5.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. doi:10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 6.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. doi:10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 7.Rivero-Ayerza M, Theuns DA, Garcia-Garcia HM, Boersma E, Simoons M, Jordaens LJ. Effects of cardiac resynchronization therapy on overall mortality and mode of death: a meta-analysis of randomized controlled trials. Eur Heart J. 2006;27:2682–8. doi: 10.1093/eurheartj/ehl203. doi:10.1093/eurheartj/ehl203. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg I, Moss AJ, McNitt S, Zareba W, Andrews ML, Hall WJ, et al. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006;98:485–90. doi: 10.1016/j.amjcard.2006.03.025. doi:10.1016/j.amjcard.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Soliman OI, van Dalen BM, Theuns DA, ten Cate FJ, Nemes A, Jordaens LJ, et al. The ischemic etiology of heart failure in diabetics limits reverse left ventricular remodeling after cardiac resynchronization therapy. J Diabetes Complications. 2009;23:365–70. doi: 10.1016/j.jdiacomp.2008.04.003. doi:10.1016/j.jdiacomp.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Schefer T, Wolber T, Binggeli C, Holzmeister J, Brunckhorst C, Duru F. Long-term predictors of mortality in ICD patients with non-ischaemic cardiac disease: impact of renal function. Europace. 2008;10:1052–9. doi: 10.1093/europace/eun186. doi:10.1093/europace/eun186. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. doi:10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, III, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–e62. doi: 10.1016/j.jacc.2008.02.032. doi:10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 14.Foundation NK. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 15.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–64. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg H, Case RB, Moss AJ, Brown MW, Carroll ER, Andrews ML. Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II) J Am Coll Cardiol. 2004;43:1459–65. doi: 10.1016/j.jacc.2003.11.038. doi:10.1016/j.jacc.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 17.Parkash R, Stevenson WG, Epstein LM, Maisel WH. Predicting early mortality after implantable defibrillator implantation: a clinical risk score for optimal patient selection. Am Heart J. 2006;151:397–403. doi: 10.1016/j.ahj.2005.04.009. doi:10.1016/j.ahj.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Cygankiewicz I, Gillespie J, Zareba W, Brown MW, Goldenberg I, Klein H, et al. Predictors of long-term mortality in Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) patients with implantable cardioverter-defibrillators. Heart Rhythm. 2009;6:468–73. doi: 10.1016/j.hrthm.2008.12.023. doi:10.1016/j.hrthm.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Bruch C, Sindermann J, Breithardt G, Gradaus R. Prevalence and prognostic impact of comorbidities in heart failure patients with implantable cardioverter defibrillator. Europace. 2007;9:681–6. doi: 10.1093/europace/eum097. doi:10.1093/europace/eum097. [DOI] [PubMed] [Google Scholar]
- 20.Lee DS, Tu JV, Austin PC, Dorian P, Yee R, Chong A, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol. 2007;49:2408–15. doi: 10.1016/j.jacc.2007.02.058. doi:10.1016/j.jacc.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 21.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. doi:10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 22.Koller MT, Schaer B, Wolbers M, Sticherling C, Bucher HC, Osswald S. Death without prior appropriate implantable cardioverter-defibrillator therapy: a competing risk study. Circulation. 2008;117:1918–26. doi: 10.1161/CIRCULATIONAHA.107.742155. doi:10.1161/CIRCULATIONAHA.107.742155. [DOI] [PubMed] [Google Scholar]
- 23.Bai R, Di Biase L, Elayi C, Ching CK, Barrett C, Philipps K, et al. Mortality of heart failure patients after cardiac resynchronization therapy: identification of predictors. J Cardiovasc Electrophysiol. 2008;19:1259–65. doi: 10.1111/j.1540-8167.2008.01234.x. doi:10.1111/j.1540-8167.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- 24.Wilkoff BL, Hess M, Young J, Abraham WT. Differences in tachyarrhythmia detection and implantable cardioverter defibrillator therapy by primary or secondary prevention indication in cardiac resynchronization therapy patients. J Cardiovasc Electrophysiol. 2004;15:1002–9. doi: 10.1046/j.1540-8167.2004.03625.x. doi:10.1046/j.1540-8167.2004.03625.x. [DOI] [PubMed] [Google Scholar]
- 25.Theuns DA, Thornton AS, Klootwijk AP, Scholten MF, Vantrimpont PJ, Balk AH, et al. Outcome in patients with an ICD incorporating cardiac resynchronisation therapy: differences between primary and secondary prophylaxis. Eur J Heart Fail. 2005;7:1027–32. doi: 10.1016/j.ejheart.2005.05.006. doi:10.1016/j.ejheart.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–5. doi: 10.1161/01.CIR.0000150390.04704.B7. doi:10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 27.Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–17. doi: 10.1056/NEJMoa071098. doi:10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee DS, Donovan L, Austin PC, Gong Y, Liu PP, Rouleau JL, et al. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43:182–8. doi: 10.1097/00005650-200502000-00012. doi:10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]