Abstract
In 1879, Fritz Müller hypothesized that mimetic resemblance in which defended prey display the same warning signal would share the costs of predator education. Although Müller argued that predators would need to ingest a fixed number of prey with a given visual signal when learning to avoid unpalatable prey, this assumption lacks empirical support. We report an experiment which shows that, as the number of unpalatable prey presented to them increased, avian predators attacked higher numbers of those prey. We calculated that, when predators increase attacks, the fitness costs incurred by unpalatable prey can be substantial. This suggests that the survival benefits of mimicry could be lower than Müller proposed. An important finding is, however, that these costs decline in importance as the total number of available prey increases.
Keywords: Müllerian mimicry, Gallus gallus domesticus, fixed n, fitness
1. Introduction
Müllerian mimicry describes the visual resemblance of two or more sympatric unpalatable prey species that are not closely related (Müller 1879). Though mimicry is taxonomically and ecologically widespread, the theoretical underpinnings of Müllerian mimicry are still the subject of debate (Sherratt 2008). One of the specific assumptions made by Müller remains unresolved: that predators consume a fixed number of unpalatable prey species over a period of time, independent of their density, before learning to avoid that prey's signal. As it stands, no study exists in which the number of unpalatable prey presented to predators is systematically increased, while the number of alternative edible prey is kept constant. This is an important omission, as it leaves us unable to define the survival benefits of mimicry.
An experiment by Lindström et al. (2001) found that predators (great tits, Parus major) attacked a greater number of unpalatable prey as more were presented over a range of values. However, the total number of prey was kept constant, so that as the abundance of defended prey increased, the number of alternative visually distinct edible prey decreased. It is therefore not possible to determine whether increased attack frequency on unpalatable prey is because the absolute number of edible prey decreased or because the absolute number of unpalatable prey increased (experiments by Greenwood et al. (1981, 1989) and Beatty et al. (2004) showed evidence that predation is higher on more abundant prey, but also used fixed populations of aposematic prey; though see Rowe et al. (2004) who investigated the effect of prey density on predator learning rates).
Furthermore, Müller's quantitative model of mimicry does not take into account the abundance of alternative prey (see Kokko et al. (2003) and Lindström et al. (2004) which do take into account the effects of alternative prey abundance). We can form two predictions of the effect of alternative edible prey abundance on predatory behaviour. First, that the number of unpalatable prey attacked during learning would decrease with the addition of alternative palatable prey, since a predator might eliminate unpalatable prey from its diet, with the availability of abundant alternatives (see Kakade & Dayan (2002) for review on massed versus spaced learning). Alternatively, an increased density of alternative palatable prey could slow aversion learning, with predators likely to exhibit longer intervals between aversive contacts with unpalatable prey, resulting in an increase in the number of unpalatable prey attacked during learning (see Matthews 1977).
We therefore tested Müller's assumption of a fixed number of prey being attacked during learning by examining how the number of unpalatable prey attacked was affected by the absolute number of those prey available for attack, and the abundance of alternative edible prey. We use the data to investigate the fitness consequences for prey when predators attack higher numbers of prey.
2. Material and methods
(a). Subjects and housing
Three batches of male chicks (Gallus gallus domesticus), totalling 110 individuals, were obtained from a commercial hatchery on the day of hatching and were randomly assigned to either the experimental group (65 animals) or the buddy group (individuals not used for the learning experiment). Experimental and buddy chicks were housed in separate cages measuring 120 × 50 × 50 cm. All subjects were marked on their heads with non-toxic coloured marker pens for identification. Water was provided ad libitum, as were brown chick starter crumbs, except during training and participation in the experimental task when food deprivation was necessary. All deprivation periods were in accordance with Home Office regulations and guidelines (never longer than 1.5 h). One chick died for reasons unrelated to the experiment.
(b). Artificial prey
Palatable and unpalatable crumbs were produced by spraying 150 g of chick starter crumbs with either 100 ml of water or 6 g of chloroquine phosphate dissolved in 100 ml of water. Crumbs were coloured either green (edible) or red (unpalatable) by spraying 150 g of the crumbs with 0.5 ml of Sugarflair spruce green diluted to 90 ml with tap water and 2 ml of Supercook red food dye diluted to 90 ml with tap water. These concentrations produced similar colour saturation in the crumbs. All crumbs were allowed to dry for 24 h and then sieved to ensure that they were of similar size.
(c). Experimental arena
The arena was identical to the holding cage but partitioned using wire mesh into two sections: the experimental arena (80 × 50 cm) and the buddy arena (40 × 50 cm). The floor of the experimental arena was covered in white paper divided into 80 equally sized squares. On days 1 and 2 the experimental chicks were trained to eat brown crumbs in the experimental arena, following methods employed by Skelhorn et al. (2008).
(d). Learning trials and analysis
On day 3, chicks were assigned to one of six treatments (see table 1). Treatments differed in the numbers of unpalatable (5, 15 or 30) and edible prey (20 or 30). We used a full factorial design. After approximately 1.5 h of food deprivation a lone chick was placed in the experimental arena where it encountered a mixture of green palatable and red unpalatable crumbs at specified densities. Each chick was required to attack (peck or eat) 16 crumbs to complete a trial, and participated in eight learning trials in total (two trials on days 3 to 6).
Table 1.
Details of numbers and proportions taken plus values of revised per capita attack rates.
| treatment number | unpalat. prey per treatment | number of edible prey | mean total number taken | total presented over 8 trials | observed per capita (pc) attack | revised pc value | increase in pc |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 20 | 21.27 | 40 | 0.532 | ||
| 2 | 15 | 20 | 28.56 | 120 | 0.238 | 0.177 | 0.061 |
| 3 | 30 | 20 | 34.00 | 240 | 0.142 | 0.089 | 0.053 |
| 4 | 5 | 30 | 21.82 | 40 | 0.546 | ||
| 5 | 15 | 30 | 31.60 | 120 | 0.263 | 0.182 | 0.081 |
| 6 | 30 | 30 | 35.12 | 240 | 0.146 | 0.091 | 0.055 |
Data were recorded by a single researcher using a Psion Workabout Pro with Observer 8.0 software. Following Skelhorn et al. (2008), if a chick attacked the same crumb several times in quick succession, we counted one attack. However, if a chick attacked a crumb, moved away from it, attacked a different crumb and then returned to attack the first crumb, this was counted as two separate attacks. Consequently, we have a small overestimate of per capita attack rates when birds are naive and there are only five unpalatable prey presented (in the first trial of treatments 1 and 4, attack numbers in the first trial are c. 6.5 and c. 5, respectively). In all other cases, the birds attacked fewer prey than the total presented.
The number of unpalatable red crumbs attacked in the first and last trial was analysed by paired sample t-tests. The total number of unpalatable prey attacked across all eight trials was analysed by GLM ANOVA in SPSS v. 16.0, with number of unpalatable prey, number of edible prey and chick's batch number as fixed factors. We also calculated relative predation risk by dividing the number of each prey type taken by the predicted number that would have been killed assuming random predation. For example, because the predators were allowed to attack 16 prey items during the trial, the expected predation for five unpalatable prey when presented with 20 edible prey, would be 3.2 unpalatable and 12.8 edible prey. We analysed predation risk by GLM ANOVA with the Bonferroni-corrected estimated marginal means (EMM). The higher order interaction with batch was nonsignificant, and was removed from all models.
3. Results
Chicks in all treatment groups readily learned to discriminate between quinine-coated red prey and water-coated green prey. The birds increased their attacks on green prey in all treatments from trial 1 to trial 8 (see the electronic supplementary material, figure S1). Birds in all treatments learned to reduce the number of quinine-coated red prey attacked from trial 1 to trial 8 (paired sample t-test, all p < 0.05; see the electronic supplementary material for individual p-values and also figure S2).
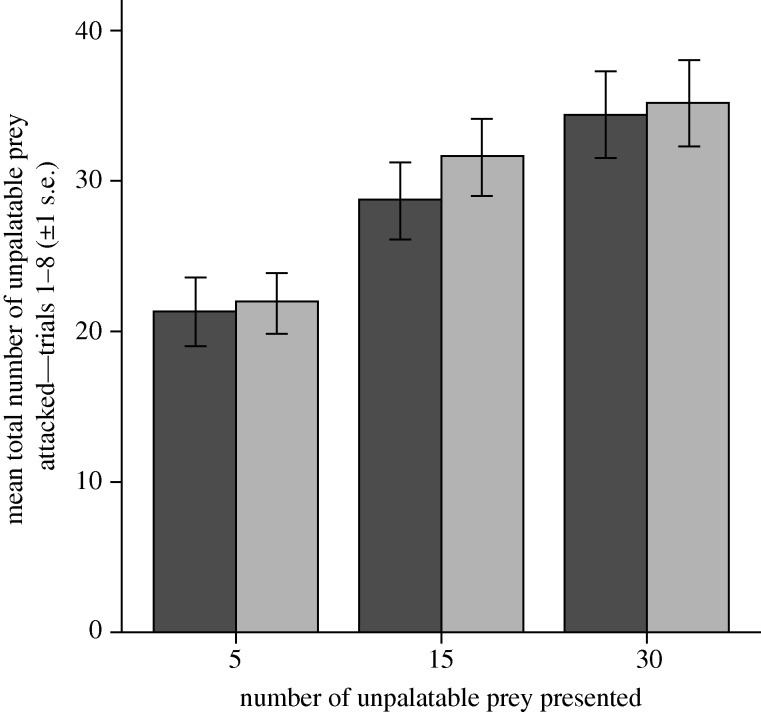
Chicks attacked significantly more unpalatable red prey as greater numbers were presented (F2,65 = 13.89, p < 0.001; figure 1), and this effect was independent of the number of edible prey available (F1,65 = 0.59, p = 0.444; the interaction term was also non-significant, F2,65 = 0.13, p = 0.878).
Figure 1.
The mean total number (+s.e.m.) of unpalatable red prey attacked, calculated as the sum of unpalatable prey attacked in trials 1–8. Dark grey bars, 20 edible alternative prey; light grey bars, 30 edible prey.
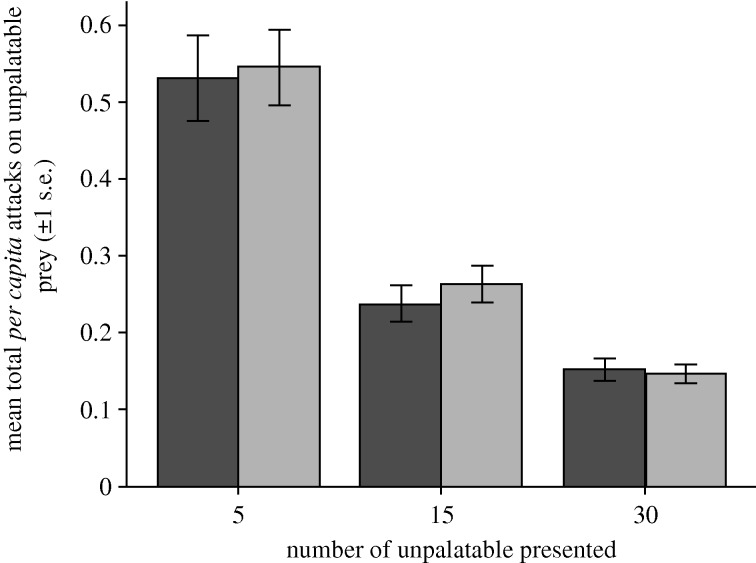
Mean per capita attack rates (total attacked/total presented) of unpalatable prey, decreased as the number of unpalatable prey in the population increased (F2,65 = 69.95, p < 0.001; see figure 2). This effect was independent of the number of edible prey available (F1,65 = 0.150, p = 0.70) and there was no interaction between the number of unpalatable and the number of edible prey on the per capita attack rate of unpalatable prey (F2,65 = 0.11, p = 0.896).
Figure 2.
The mean total per capita attack rate (+s.e.m.) on unpalatable prey, calculated by dividing the total number of unpalatable prey attacked across all eight trials, by the total number presented during the eight trials. Dark grey bars, 20 edible alternative prey; light grey bars, 30 edible prey.
We also assessed the effect of differences in prey numbers on the birds' preferences for palatable over unpalatable prey by comparing predation relative to that which would be expected if birds were foraging randomly (see the electronic supplementary material and also Rowland et al. (2007)). We term this ‘relative predation risk’. We found that, for unpalatable prey, relative predation risk decreased with increasing abundance of unpalatable prey (the electronic supplementary material, figure S3; F2,65 = 30.04, p < 0.001). There was a significant main effect of the abundance of alternative edible prey (F1,65 = 16.38, p < 0.001), such that predation risk was higher, in all cases, when there were 30 alternative edible prey as opposed to 20 edible prey. There was no interaction between the number of unpalatable and number of edible prey on predation risk (F2,65 = 1.72, p = 0.188). Bonferroni corrected EMM pairwise comparisons showed that predation risk was higher when there were 30 alternative edible prey, than when there were 20 alternative edible prey, when there were five and 15 unpalatable prey (p < 0.001 and p = 0.031, respectively), but not when there were 30 unpalatable prey (p = 0.277).
(a). Implications for prey fitness
If the birds did not attack greater numbers of unpalatable prey when greater numbers were presented, the fitness of individual prey would be higher. We evaluated this by comparing the observed per capita attack rate to a ‘revised per capita attack rate’ based on the minimum number of prey required for learning (see the electronic supplementary material for detailed methods and also table 1). From this we determined how per capita mortality would be affected if the birds did not attack greater numbers of unpalatable prey when greater numbers were presented. The difference between the observed and the revised per capita mortality shows that the mortality costs to the prey of increased attacks when greater numbers are presented is substantial (e.g. if a bird attacked unpalatable prey according to the minimum number of prey required for learning i.e. 21.27, then the reduction in mortality for 15 and 30 unpalatable prey would be 0.061 and 0.053, respectively; column 8, table 1). We did find though, that as the unpalatable prey number doubled from 15 to 30 presented, the size of the increase in per capita attack was reduced.
4. Discussion
There are four clear results from our experiment. First, we found that during learning, chicks attacked a greater number of unpalatable prey as the number of unpalatable prey available increased. This finding does not support Müller's assumption of a fixed number (n) of attacks during learning. We kept the number of edible prey constant within each treatment, so we could be sure that the result was due to the increase in absolute number of unpalatable prey, and not because the edible prey became scarcer in absolute numbers (as happens in Lindström et al. (2001)). A possible explanation for this predatory behaviour is that, if the unpalatable prey form is numerous then the cost to a predator of excluding them from their diet is higher if some of them turn out to be palatable (Beatty et al. 2004; Ruxton et al. 2004).
Second, judged in terms of per capita attack rates, mortality declined as the number of unpalatable prey increased, even though the number of prey attacked increased (see also Greenwood et al. 1981, 1989; Lindström et al. 2001; Beatty et al. 2004). Because the total density of prey increased with the addition of unpalatable prey, the probability of attack for a member of the population of unpalatable prey decreased. Therefore this reduction in mortality can be explained by density-dependent dilution (see also Rowland et al. 2007).
Third, to our knowledge, this study provides the first evidence that availability of alternative edible prey may not modify the per capita mortality levels of the unpalatable prey (though see Lindström et al. 2004). Although per capita mortality was not affected, the relative preferences of the birds were affected by increases in numbers of prey. Relative predation risk for unpalatable prey increased with higher abundance of edible alternative prey, suggesting higher densities of alternative palatable prey slows aversion learning. This is possibly due to predators experiencing longer intervals between aversive contacts with unpalatable prey (see Matthews 1977). Overall, however, this effect was more than compensated for through density-dependent dilution, so the availability of alternative edible prey did not modify the per capita mortality levels of the unpalatable prey.
Fourth, we were able to estimate the additional per capita mortality cost incurred because predators attacked greater numbers of unpalatable prey when greater numbers were presented. This cost was large in our experiment (table 1). Crucially though, the size of this cost diminished as the prey population size increased, so the cost to prey fitness is likely to be less affected as population sizes increase.
5. Conclusions
Our findings do not support Müller's assumption of a fixed number (n) of attacks during learning. Rather, predators attack more unpalatable prey when more are presented. A consequence is that the benefits of mimicry are probably smaller than those estimated by/using Müller's theory. As abundances become large, however, increased attacks become relatively less important, and the data will probably tend to converge towards Müller's predictions.
Acknowledgements
We thank four anonymous reviewers for their comments. HMR, MPS, JM & GDR were funded by NERC grant NE/D010 667/1.
References
- Beatty C. D., Beirinckx K., Sherratt T. N.2004The evolution of Müllerian mimicry in multispecies communities. Nature 431, 63–67 (doi:10.1038/nature02818) [DOI] [PubMed] [Google Scholar]
- Greenwood J. J. D., Wood E. M., Batchelor S.1981Apostatic selection of distasteful prey. Heredity 47, 27–34 (doi:10.1038/hdy.1981.56) [Google Scholar]
- Greenwood J. J. D., Cotton P. A., Wilson D. M.1989Frequency-dependent selection on aposematic prey: some experiments. Biol. J. Linn. Soc. 36, 213–226 (doi:10.1111/j.1095-8312.1989.tb00491.x) [Google Scholar]
- Kakade S., Dayan P.2002Acquisition and extinction in autoshaping. Psychol. Rev. 109, 533–544 (doi:10.1037/0033-295X.109.3.533) [DOI] [PubMed] [Google Scholar]
- Kokko H., Mappes J., Lindström L.2003Alternative prey can change model–mimic dynamics between parasitism and mutualism. Ecol. Lett. 6, 1068–1076 (doi:10.1046/j.1461-0248.2003.00532.x) [Google Scholar]
- Lindström L., Alatalo R. V., Lyytinen A., Mappes J.2001Strong antiapostatic selection against novel rare aposematic prey. Proc. Natl Acad. Sci. USA 98, 9181–9184 (doi:10.1073/pnas.161071598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström L., Alatalo R. V., Lyytinen A., Mappes J.2004The effect of alternative prey on the dynamics of imperfect Bayesian and Müllerian mimicries. Evolution 58, 1294–1302 (doi:10.1554/03-271) [DOI] [PubMed] [Google Scholar]
- Matthews E. G.1977Signal-based frequency-dependent defense strategies and the evolution of mimicry. Am. Nat. 111, 213–222 (doi:10.1086/283156) [Google Scholar]
- Müller F.1879Ituna and Thyridia; a remarkable case of mimicry in butterflies. Trans. Entomol. Soc. Lond. MayXX–XXIX [Google Scholar]
- Rowe C., Lindström L., Lyytinen A.2004The importance of pattern similarity between Müllerian mimics in predator avoidance learning. Proc. R. Soc. Lond. B 271, 407–413 (doi:10.1098/rspb.2003.2615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland H. M., Ihalainen E., Lindström L., Mappes J., Speed M. P.2007Co-mimics have a mutualistic relationship despite unequal defences. Nature 448, 64–67 (doi:10.1038/nature05899) [DOI] [PubMed] [Google Scholar]
- Ruxton G. D., Sherratt T. N., Speed M. P.2004Avoiding attack—the evolutionary ecology of crypsis, warning signals and mimicry. Oxford, UK: Oxford University Press [Google Scholar]
- Sherratt T. N.2008The evolution of Müllerian mimicry. Naturwissenschaften 95, 681–695 (doi:10.1007/s00114-008-0403-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelhorn J., Griksaitis D., Rowe C.2008Colour biases are more than a question of taste. Anim. Behav. 75, 827–835 (doi:10.1016/j.anbehav.2007.07.003) [Google Scholar]