Abstract
Selection imposed through sperm competition is commonly thought to promote the evolution of longer sperm, since sperm length is assumed to be positively associated with sperm swimming velocity. Yet, the basis for this assumption remains controversial, and there is surprisingly little intraspecific evidence demonstrating such a link between sperm form and function. Here, we show that sperm length and velocity are highly correlated in the sea urchin Heliocidaris erythrogramma, but importantly we report that failure to account for within-male variation in these sperm traits can obscure this relationship. These findings, in conjunction with the mounting evidence for extremely high levels of intra-specific variance in sperm traits, suggest that a functional link between sperm morphology and velocity may be more prevalent than what current evidence suggests. Our findings also suggest that selection for faster swimming sperm may promote the evolution of longer sperm, thereby supporting recent findings from macroevolutionary studies.
Keywords: sperm competition, sexual selection, sperm swimming speed, sperm length, sperm morphology, free-spawning
1. Introduction
When sperm from multiple males compete to fertilize ova, selection is expected to favour sperm traits that enhance a male's competitive fertilization success (Snook 2005). A common view in the sexual selection literature is that sperm flagellum length influences sperm swimming velocity, and therefore that selection imposed through sperm competition will favour males with longer, faster sperm that are capable of reaching ova more quickly than shorter sperm from rivals (Gomendio & Roldan 1991). Interspecific studies generally support this view, demonstrating that longer sperm swim faster than shorter sperm across species (mammals: Gomendio & Roldan 1991, 2008; fishes: Fitzpatrick et al. 2009; birds: Lüpold et al. 2009, but see Kleven et al. 2009) and that sperm size and velocity are typically greater in species experiencing an increased risk of sperm competition (Gomendio & Roldan 2008; Fitzpatrick et al. 2009; Kleven et al. 2009). However, the relationship between sperm length and velocity is far less clear at the intraspecific level. While there is some evidence of a relationship between certain measures of sperm length and velocity (e.g. flagellum, Mossman et al. 2009; midpiece, Malo et al. 2006; Firman & Simmons in press; head : flagellum ratio, Helfenstein et al. 2010), in a recent review of nine intraspecific studies, Humphries et al. (2008) highlighted that most studies have failed to detect such relationships.
Here, we evaluated the relationship between sperm length and velocity in the broadcast spawning sea urchin, Heliocidaris erythrogramma. Externally fertilizing marine invertebrates are ideal for studying such relationships as they produce copious gametes and it is straightforward to mimick their natural fertilization environment in a laboratory setting, allowing sperm motility to be assessed under semi-natural conditions. Moreover, like other sea urchins, H. erythrogramma is likely to be subject to strong selection on sperm performance because sperm from multiple males often compete to fertilize eggs, and the first sperm that reaches an egg (i.e. faster swimming sperm) experiences a fertilization advantage and sires higher quality offspring (Marshall et al. 2004). To assess the relationship between sperm length and velocity, we develop a novel method that measures both variables in individual sperm cells. We show that intramale variance in sperm traits needs to be considered when assessing sperm length–velocity relationships.
2. Material and methods
H. erythrogramma is a coastal sea urchin found in southern Australia (Keesing 2001). Reproductively mature urchins were collected from South Mole Jetty in Fremantle, Western Australia, in late April 2009 and transported to the laboratory. Urchins were placed in individual containers with approximately 500 ml sea water at 22°C and induced to spawn with a non-lethal intracoelomic injection of 5 ml of 3 per cent KCl. To standardize the time of sample collection and to allow an appropriate concentration of sperm for our analyses (sperm are released within a few minutes of KCl injections), we collected a sperm/sea water sample from a male's container 10 min after the induction of spawning and immediately recorded sperm velocity (urchin sperm velocity remains constant for approx. 45 min, Levitan 2000). We obtained sperm samples from 18 males using this approach.
The sperm/sea water sample was placed in isolated wells of a glass 12-cell multitest slide (MP Biomedicals, Aurora, OH, USA), previously coated with 1 per cent polyvinyl alcohol to prevent sperm from sticking to the slide (Wilson-Leedy & Ingermann 2007). Sperm motility was recorded for 1 s (97 frames s−1, shutter 0.01 s) at 400× magnification using a Prosilica EC-650 digital camera (resolution 640 × 480) and Norpix StreamPix 3.4 image capture software. Sperm velocity was analysed using NIH ImageJ v. 1.37 computer-assisted sperm analysis (CASA) software (http://rsb.info.nih.gov/ij/plugins/casa.html, Wilson-Leedy & Ingermann 2007). For each 1 s recording, we used the ‘threshold’ and ‘find edges’ functions prior to analyses. We isolated a single randomly chosen spermatozoon, clearing all other cells from the image sequence and recorded sperm velocity of the isolated spermatozoon using CASA. We focused our analyses on average path velocity (VAP) and curvilinear velocity (VCL), as these measures are positively correlated with fertilization success in many external fertilizers (e.g. Au et al. 2002; Casselman et al. 2006). We measured sperm traits for each spermatozoon from which we determined sperm velocity, thereby linking sperm length and velocity at the individual cell level. We took three images of each spermatozoon (at the beginning, middle and at the end of the recording) from the original recording for length measures. We measured various sperm traits but focused on flagellum length, as it was the best predictor of sperm velocity (electronic supplementary material, table S1). For each individual sperm we used the mean flagellum length from the three images for analyses, as within-sample repeatability among sperm measures/male was high (intraclass correlation coefficients = 0.881 ± 0.01; Lessells & Boag 1987). For each male, we measured sperm length and velocity from a mean of 17.7 ± 0.92 s.e. (range 10–21) individual progressively motile sperm.
(a). Statistical analyses
Sperm length–velocity correlations are usually assessed among males using mean sperm length and velocity measures for each male (see studies in table 1 of Humphries et al. 2008). However, this traditional technique removes potentially important within-male variation in sperm traits. Here, we use within-subject centering (van de Pol & Wright 2009) in a linear mixed model with male identity as a random factor, to separate within-male from among-male effects, when assessing sperm length–velocity correlations. This method is preferable to the traditional technique, because it does not assume that a relationship, or lack thereof, among males is reflected within males and even allows for the detection of within and among-male effects that have opposite signs (see van de Pol & Wright 2009).
To further explore how intramale sperm traits variation influences sperm length–velocity correlations, we used a resampling approach: five pairs of sperm length–velocity measures from the same ejaculate that were either from the same subset of five sperm (matched) or from different subsets of five sperm (mismatched) were randomly selected from each male (n = 18) and the correlation coefficient was calculated across males (see electronic supplementary material, figure S1 for an outline of the procedure). This process was iterated 10 000 times to obtain distributions of sperm length–velocity correlation coefficients, when sperm measurements were matched or mismatched. Data analyses were performed using JMP v. 7.0.1 (SAS Institute Inc. 2007) and simulations were carried out using PopTools 3.0.6 (Hood 2008).
3. Results
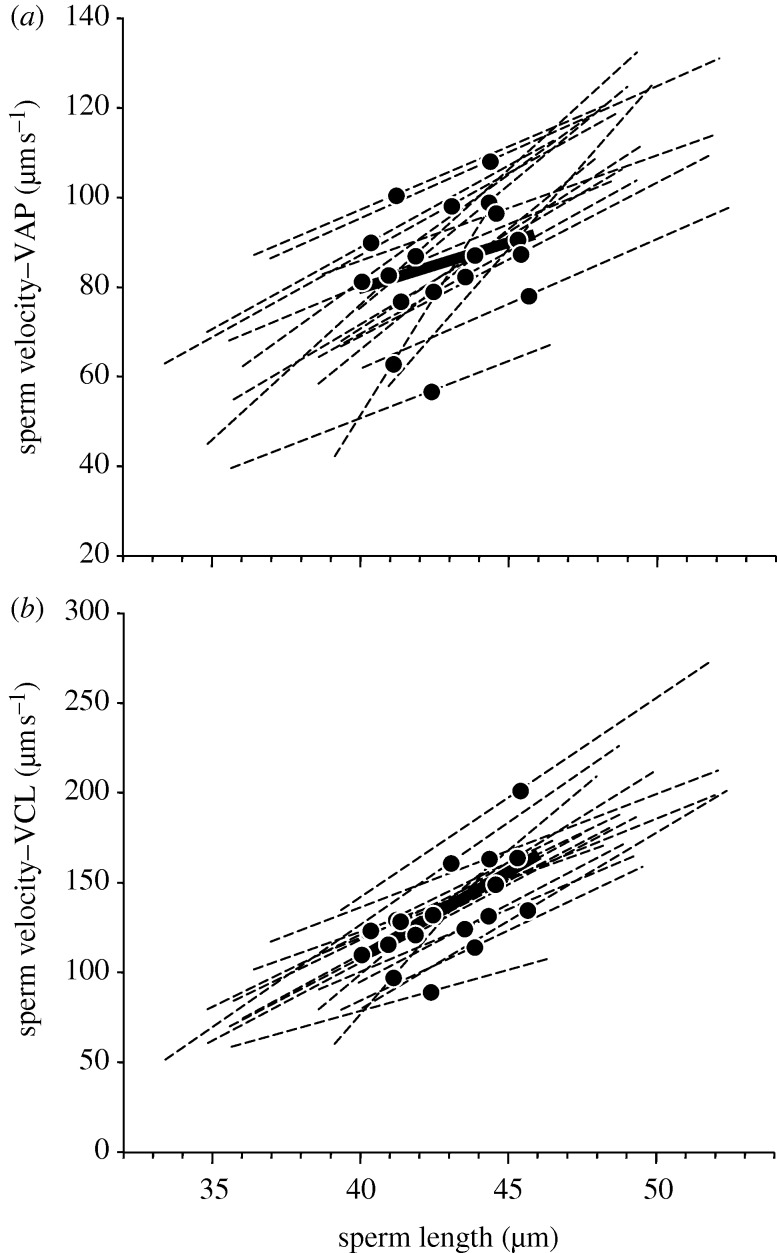
Our mixed model analyses revealed that flagellum length was significantly positively correlated with VAP within each male's ejaculate, but among males, mean VAP was not significantly correlated with mean flagellum length (within-subject effect: F1,300 = 285.4, p < 0.0001, r = 0.70; among-subject effect: F1,16 = 1.53, p = 0.24, r = 0.30, figure 1a). Likewise, there was a significant positive relationship between flagellum length and VCL within males (F1,300 = 421.40, p < 0.0001, r = 0.76); although unlike VAP, among males the relationship between mean VCL and flagellum length from each male was also significant, albeit weaker than the within-subject effect (F1,16 = 11.13, p = 0.004, r = 0.64, figure 1b).
Figure 1.
The relationship between sperm flagellum length and velocity within- and among-males for (a) VAP and (b) VCL. Data points represent the mean sperm flagellum length and velocity value for each male. The slopes of the regression lines between these variables are depicted for the within-male (dashed lines) and among-males (solid line) data.
Both measures of sperm velocity were significantly correlated with flagellum length within each male's ejaculate for the 18 males examined (electronic supplementary material, table S2), further supporting the within-subjects effects in our mixed models (above). Intramale analyses also revealed that correlation coefficients were significantly higher for VCL (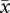 r = 0.80 ± 0.02 s.e.) than for VAP (
r = 0.80 ± 0.02 s.e.) than for VAP (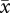 r = 0.72 ± 0.02 s.e.; paired t-test: t = 2.66, n = 18, p = 0.02).
r = 0.72 ± 0.02 s.e.; paired t-test: t = 2.66, n = 18, p = 0.02).
The importance of accounting for intramale variance in sperm traits was evident in our resampling approach, where a positive relationship between flagellum length and velocity was apparent when assessing matched sperm values (VAP: 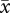 r = 0.41, CL = 0.12–0.64; VCL:
r = 0.41, CL = 0.12–0.64; VCL: 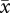 r = 0.67, CL = 0.46–0.82). In contrast, when mismatched values were analysed, the strength of the sperm length–velocity correlation was no longer significant for VAP (
r = 0.67, CL = 0.46–0.82). In contrast, when mismatched values were analysed, the strength of the sperm length–velocity correlation was no longer significant for VAP (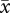 r = 0.12, CL =− 0.19–0.45) and was reduced for VCL (
r = 0.12, CL =− 0.19–0.45) and was reduced for VCL (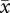 r = 0.37, CL = 0.04–0.66).
r = 0.37, CL = 0.04–0.66).
4. Discussion
We found that sperm flagellum length and velocity (VAP and VCL) are highly correlated within each male urchin's ejaculate, but among male sperm length–velocity correlations were either weaker (VCL) or absent (VAP). These results highlight that a positive relationship between sperm length and velocity may be evident within a male's ejaculate even when no such relationship is found among males within the same species. Thus, covariance between sperm length and velocity may be more prevalent than what current evidence suggests. Our approaches may therefore allow researchers working on other species to explore sperm length–velocity associations with greater resolution than what current methods allow, even in species where intermale variance in sperm traits is reduced (e.g. where selection through sperm competition erodes variation in sperm phenotype; Immler et al. 2008).
Recent studies in birds and mammals have reported relationships between sperm length and velocity (Malo et al. 2006; Firman & Simmons in press; Mossman et al. 2009; Helfenstein et al. 2010). However, most studies have failed to reveal such patterns (Humphries et al. 2008), possibly because the traditional approach of analysing sperm length and velocity using different samples of sperm underestimates the covariance between sperm length and velocity at the within- and among-male levels. Our resampling approach supports this idea, as the strength of the correlation between sperm length and velocity among males was greater when sperm traits were taken from the same sperm sample (versus different sperm samples) within a male's ejaculate. Therefore, our results highlight the importance of accounting for all sources of variance in sperm traits when assessing sperm length–velocity correlations, particularly as developmental noise during spermatogenesis means that many species exhibit substantial within- and among-male variance in these traits (Pitnick et al. 2009).
By demonstrating a link between sperm length and velocity, our results suggest that selection for faster swimming sperm may promote the evolution of longer sperm. This helps to explain the results from macroevolutionary studies, which show that males typically have longer sperm in species where females are polyandrous (reviewed by Gomendio & Roldan 2008). However, fully appreciating how selection acts on sperm traits at the intra- and interspecific level will require additional insights into the maintenance of variation in sperm traits in the light of selection arising from sperm competition. Here, we focused on an externally fertilizing marine invertebrate where it is relatively straightforward to observe sperm under semi-natural conditions. A crucial next step is to examine the importance of intramale variation in sperm traits in other species, including internal fertilizers, to test the generality of these findings.
Acknowledgements
We thank Cameron Shamone Duggin and John Trainer for assistance with data collection, Ted Morrow, Joe Tomkins, Dale Roberts, Stuart Humphries and Leigh Simmons for helpful comments/discussion and the Australian Research Council, the Natural Sciences and Engineering Research Council of Canada and the University of Western Australia for financial support.
References
- Au D. W. T., Chiang M. W. L., Tang J. Y. M., Yuen B. B. H., Wang Y. L., Wu R. S. S.2002Impairment of sea urchin sperm quality by UV-B radiation: predicting fertilization success from sperm motility. Mar. Pollut. Bull. 44, 583–589 (doi:10.1016/S0025-326X(01)00288-0) [DOI] [PubMed] [Google Scholar]
- Casselman S. J., Schulte-Hostedde A. I., Montgomerie R.2006Sperm quality influences male fertilization success in walleye (Sander vitreus). Can. J. Fish. Aquat. Sci. 63, 2119–2125 (doi:10.1139/F06-108) [Google Scholar]
- Firman R. C., Simmons L. W.In press Sperm midpiece length predicts sperm swimming velocity in house mice. Biol. Lett. (doi:10.1098/rsbl.2009.1027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick J. L., Montgomerie R., Desjardins J. K., Stiver K. A., Kolm N., Balshine S.2009Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proc. Natl Acad. Sci. USA 106, 1128–1132 (doi:10.1073/pnas.0809990106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomendio M., Roldan E. R. S.1991Sperm competition influences sperm size in mammals. Proc. R. Soc. Lond. B 243, 181–185 (doi:10.1098/rspb.1991.0029) [DOI] [PubMed] [Google Scholar]
- Gomendio M., Roldan E. R. S.2008Implications of diversity in sperm size and function for sperm competition and fertility. Int. J. Dev. Biol. 52, 439–447 (doi:10.1387/ijdb.082595mg) [DOI] [PubMed] [Google Scholar]
- Helfenstein F., Podevin M., Richner H.2010Sperm morphology, swimming velocity, and longevity in the house sparrow Passer domesticus. Behav. Ecol. Sociobiol. 64, 557–565 (doi:10.1007/s00265-009-0871-x) [Google Scholar]
- Hood G. M.2008. PopTools version 3.0.6. See http://www.cse.csiro.au/poptools
- Humphries S., Evans J. P., Simmons L. W.2008Sperm competition: linking form to function. BMC Evol. Biol. 8, 319 (doi:10.1186/1471-2148-8-319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immler S., Calhim S., Birkhead T. R.2008Increased postcopulatory sexual selection reduces the intramale variation in sperm design. Evolution 62, 1538–1543 (doi:10.1111/j.1558-5646.2008.00393.x) [DOI] [PubMed] [Google Scholar]
- Keesing J. K.2001The ecology of Heliocidaris erythrogramma. In Edible sea urchins: biology and ecology (ed. Lawrence J. M.), pp. 261–270 Berlin, Germany: Elsevier Science [Google Scholar]
- Kleven O., Fossøy F., Laskemoen T., Robertson R. J., Rudolfsen G., Lifjeld J. T.2009Comparative evidence for the evolution of sperm swimming speed by sperm competition and female sperm storage duration in passerine birds. Evolution 63, 2466–2473 (doi:10.1111/j.1558-5646.2009.00725.x) [DOI] [PubMed] [Google Scholar]
- Lessells C. M., Boag P. T.1987Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121 [Google Scholar]
- Levitan D. R.2000Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proc. R. Soc. Lond. B 267, 531–534 (doi:10.1098/rspb.2000.1032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüpold S., Calhim S., Immler S., Birkhead T. R.2009Sperm morphology and sperm velocity in passerine birds. Proc. R. Soc. B 276, 1175–1181 (doi:10.1098/rspb.2008.1645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo A. F., Gomendio M., Garde J., Lang-Lenton B., Soler A. J., Roldan E. R. S.2006Sperm design and sperm function. Biol. Lett. 2, 246–249 (doi:10.1098/rsbl.2006.0449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall D. J., Steinberg P. D., Evans J. P.2004The early sperm gets the good egg: mating order effects in free spawners. Proc. R. Soc. Lond. B 271, 1585–1589 (doi:10.1098/rspb.2004.2790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman J., Slate J., Humphries S., Birkhead T.2009Sperm morphology and velocity are genetically co-determined in the zebra finch. Evolution 63, 2730–2737 (doi:10.1111/j.1558-5646.2009.00753.x) [DOI] [PubMed] [Google Scholar]
- Snook R. R.2005Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 20, 46–53 (doi:10.1016/j.tree.2004.10.011) [DOI] [PubMed] [Google Scholar]
- Pitnick S., Hosken D. J., Birkhead T. R.2009Sperm morphological diversity. In Sperm biology: an evolutionary perspective (eds Birkhead T. R., Hosken D. J., Pitnick S.), pp. 69–149 London, UK: Elsevier [Google Scholar]
- van de Pol M. M., Wright J.2009A simple method for distinguishing within- versus between-subject effects using mixed models. Anim. Behav. 77, 753–758 (doi:10.1016/j.anbehav.2008.11.006) [Google Scholar]
- Wilson-Leedy J. G., Ingermann R. L.2007Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology 67, 661–672 [DOI] [PubMed] [Google Scholar]