Abstract
The exoskeleton of arthropods forms an efficient protection against pathogens, but this first line of defence is periodically weakened during ecdysis, increasing the opportunity for surrounding pathogens to invade the body cavity. Since the richness of pathogens in the environment can be spatially and temporally variable, arthropods may have a fitness advantage in moulting in a place and time of low infection risk. Consistent with this hypothesis, we found that the amphipod crustacean, Gammarus pulex, exhibits temporal adjustment of the moult cycle in response to elevated risks of infection. Interestingly, this phenomenon is variable between two populations and independent of levels of immune defences. These results suggest that plasticity of the moult cycle in response to elevated risks of infection is adaptive and may result from adaptation to local variations in the risk of infection.
Keywords: condition-dependent ecdysis, innate immunity, invertebrates
1. Introduction
In arthropods, ecdysis is a critical period of vulnerability to pathogens. The resulting softness of the cuticle favours the entrance of opportunistic pathogens into the haemocoel leading to dramatic survival consequences (Le Moullac et al. 1997; Morado et al. 1999). Many environmental factors affect the duration of the moult cycle (Kuballa & Elizur 2007). The presence of pathogens in the environment is temporally and spatially variable, resulting in periods or sites of variable risk of infection to which arthropods may be exposed during moult. Therefore, arthropods may gain a survival advantage by adjusting moult timing to correspond with the lowest risks of infection.
In contrast to the costs of ecdysis, moulting may serve as a defensive mechanism reducing the negative effects of wounding or pathogens from the shell. In nature, wounds can be extremely prevalent and form the main points of entry for pathogens (Plaistow et al. 2003). Wounds are rapidly healed by melanotic plugs, but these plugs may be less protective than a new cuticle. Furthermore, wounds favour the development of shell disease, resulting from the spread of chitinolytic microbes in the cuticle (Vogan et al. 2008). Recent data suggest that shell disease induces precocious moulting, which could help to recover a new and solid cuticle and allows the shedding of shell disease agents (Laufer et al. 2005). Therefore, arthropods may benefit from advancing ecdysis to recover the full protective ability of their cuticle when under a high risk of infection caused by wounding.
The arthropod immune system may also play a key role in determining temporal adjustments of ecdysis. The prophenoloxidase system is involved in wound-healing, cuticle sclerotization and forms an important component of the arthropod immune system (Cerenius & Söderhäll 2004). Therefore, depending on levels of the prophenoloxidase system, arthropods may not necessarily need to adjust the timing of ecdysis in response to elevated risks of infection.
In this study, we experimentally mimicked various risks of wounding and microbial infection to test for temporal changes in moulting events in the amphipod crustacean Gammarus pulex. Natural populations of G. pulex are likely to face variable water conditions with temporal changes in flow rate, and in the concentration of organic materials including bacteria and fungi, which determine temporally variable risks of wounding and microbial infection. Since adjustments of moulting might also result from local adaptation to different river conditions, resulting from selective responses imposed by local variation in the risks of wounding and microbial infection, we used gammarids from two natural populations that are characterized by different water conditions. Changes in moulting were examined in relation to the general maintenance and use of the prophenoloxidase system to test whether plastic changes in time to ecdysis would depend on individual immune function. If wounding enhances the vulnerability of the gammarid exoskeleton, it is expected to induce precocious moult. However, if moult enhances susceptibility to infection, micro-organism-enriched water is expected to postpone ecdysis to a time of better water conditions.
2. Material and methods
Gammarids were collected in July 2009 in the river Ouche at Dijon and at the source of a tributary of the river Suzon at Val-Suzon (eastern France). The quality of the water at this latter site is very good and stable across time. In contrast, the river Ouche crosses the city of Dijon downstream from a dam. Water quality there is highly variable with a lot of organic material and micro-organisms in suspension, depending on water releases at the dam (see the appendix in the electronic supplementary material). Hence, the locations represent contrasting water conditions that reflect either invariable low risks of microbial infections in the river Suzon or variable, unpredictable and higher risks of microbial infections in the river Ouche. About 200 males from each location were collected and kept in plastic containers (40 × 25 × 20 cm) under standard laboratory conditions (Cornet et al. 2009).
On the day after field collection, 120 healthy males from each location were measured for size (Plaistow et al. 2003) and then assigned to the wound treatment (wounded or control) and the water treatment (micro-organism-enriched water or clean water). Wounding was performed by laterally piercing the third dorsal segment using a fine sterile needle under a stereoscopic microscope. Control individuals were similarly manipulated but not pierced. Gammarids were then individually maintained in glass vials with 20 ml of either clean or micro-organism-enriched water under standard laboratory conditions, supplied with a piece (1 cm2) of dead elm leaf per week. To obtain micro-organism-enriched water, we aged 40 l of oxygenated dechlorinated UV-treated tap water for one week inoculated with organic material from an aquarium that housed local gammarids and decaying material. This approach allowed us to mimic a rather realistic enrichment of micro-organisms in the water to which gammarids might be exposed in natural conditions. Indeed, while the microbial communities in Burgundy's rivers are not known, some remain dominant at the regional scale (Ostman et al. 2010). Clean water was obtained in the same way but without inoculation. Concentration of micro-organisms in clean and enriched water was measured using a Neubauer-improved haemocytometer under a phase contrast microscope. For the duration of the experiment, water in the glass vials was renewed twice a week to provide enough oxygen to the gammarids.
Survival and ecdysis (the presence of the shaded cuticle) were recorded daily for the duration of the experiment. After ecdysis, gammarids were kept in experimental conditions for two more days, allowing the cuticle to harden, and then used for haemolymph extraction. Each individual provided 2 µl of haemolymph that was diluted in 20 µl of phosphate buffer saline (PBS: 8.74 g NaCl; 1.78 g Na2HPO4, 2H2O; 1000 ml of distilled water; pH 6.5), frozen in liquid nitrogen within 3 s and then stored at –80°C until later measurement of the prophenoloxidase system. Gammarids were then freeze-killed and removed from the experiment. For each individual haemolymph extract, the activity of naturally activated phenoloxidase (PO) enzymes only (hereafter PO activity) and the activity of the proenzymes (ProPO) in addition to that of the PO (total activity) were measured using a spectrophotometric assay following the method described in Cornet et al. (2009) (see the appendix in the electronic supplementary material). Enzyme activity (Vmax: change in absorbance unit min−1) was reported as the activity for 1 µl of pure haemolymph.
Pre-ecdysis survival and time to ecdysis were analysed using Cox regression analyses with the population of origin of the gammarids, wounding and water treatments and their interactions as explanatory variables. For the survival analysis, gammarids that had moulted were censored in the statistical model because the follow-up of their survival was interrupted by haemolymph collection. Similarly, to analyse time to ecdysis, gammarids that died before moulting were censored as well (see full models in the appendix in the electronic supplementary material). Parameter estimates produced by these statistical models were used to plot probability functions over time for both pre-ecdysis survival and occurrence of ecdysis (see details in the appendix in the electronic supplementary material) as described in Moret & Schmid-Hempel (2004).
PO activities were square root transformed and analysed using a full-factorial MANOVA with the population of origin of the gammarids, wounding and water treatments as fixed factors and with body size and time to ecdysis as covariates. Data were analysed using SPSS11 for Macintosh.
3. Results
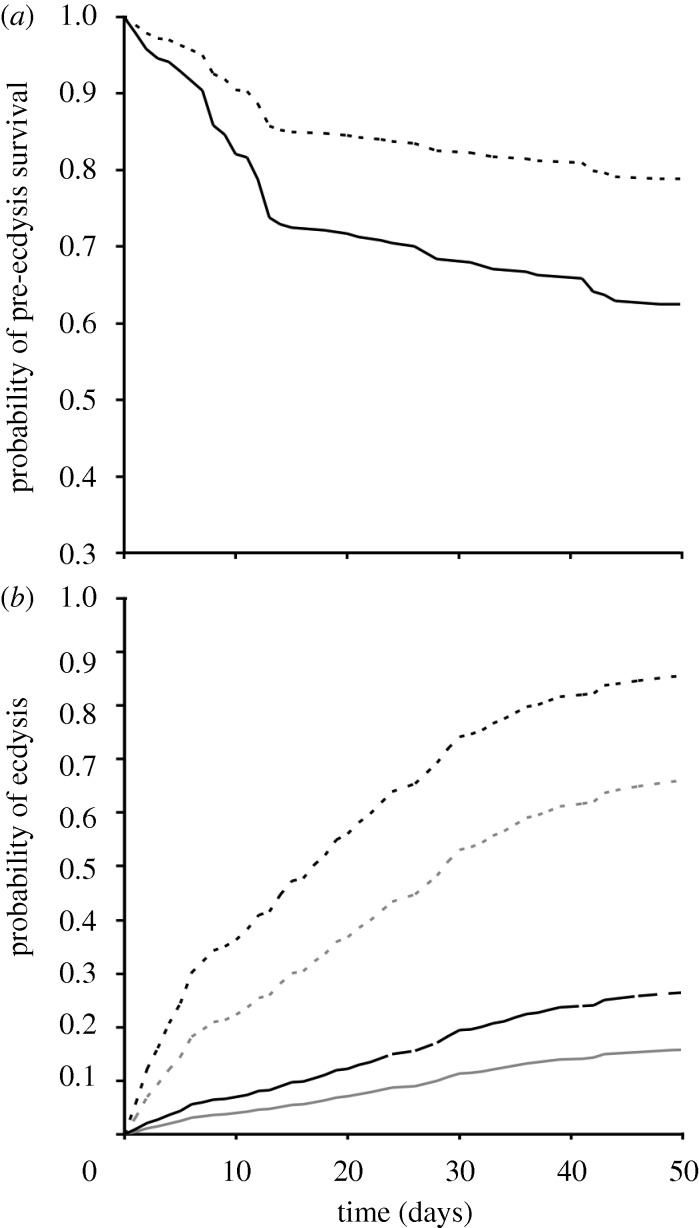
As expected, micro-organism enriched water contained, more bacteria and fungi (mean ± s.e. 38.5 ± 2.6 104 cells ml−1) than clean water (11.7 ± 1.3 104 cells ml−1) after one week of ageing (see the appendix in the electronic supplementary material). Only the water treatment explained survival changes, with micro-organism-enriched water inducing a 1.98-fold reduction in survival (figure 1a; Wald = 27.35, d.f. = 1, p < 0.001).
Figure 1.
Probability functions over time generated by Cox regression statistical models for (a) pre-ecdysis survival and (b) occurrence of ecdysis in gammarids exposed to micro-organism enriched (solid lines) and clean water (dashed lines). In (a), there is no distinction between populations, whereas light and dark lines in (b) refer to gammarids from Val-Suzon and Dijon, respectively. Details for the calculation of these probability functions are given in the appendix in the electronic supplementary material.
Overall, gammarids from Dijon moulted earlier than those from Val-Suzon (figure 1b; Wald = 5.02, d.f. = 1, p = 0.025). Gammarids in micro-organism-enriched water moulted significantly later than those in clean water (figure 1b; Wald = 24.31, d.f. = 1, p < 0.001). The magnitude of the water effect was slightly larger for gammarids from Dijon than from Val-Suzon, as suggested by the near significant interaction between water and population (Wald = 3.78, d.f. = 1, p = 0.052).
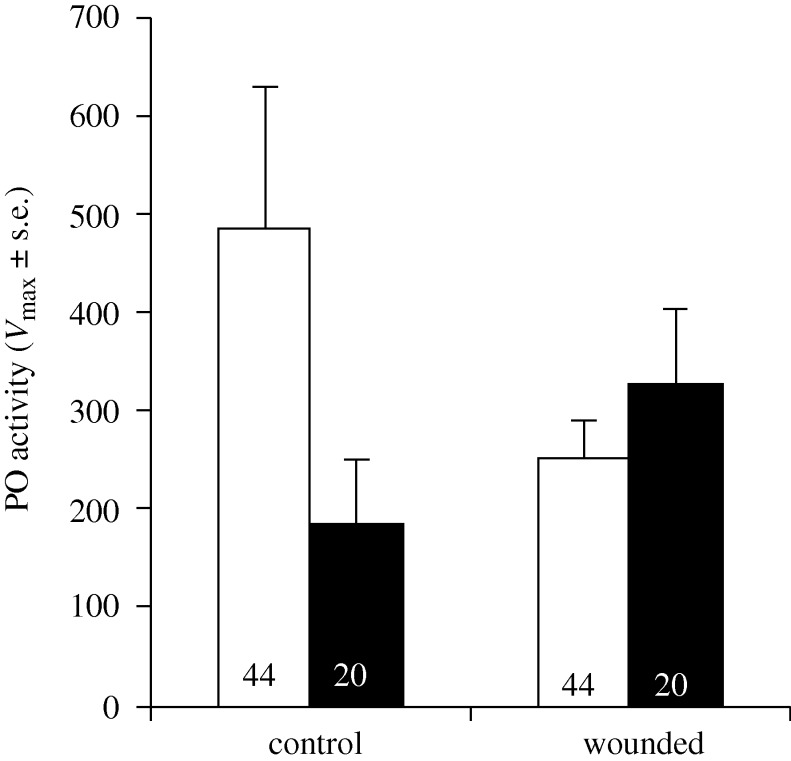
Small gammarids had significantly more active PO enzymes than large ones, despite having a similar total concentration of enzymes in their haemolymph (table 1). Among gammarids that survived until ecdysis, those from Val-Suzon had a higher PO activity than those from Dijon (mean ± s.e., Val-Suzon: 15.02 ± 1.03; Dijon: 14.89 ± 1.50), while the total activity was similar for gammarids of both populations (table 1). Wound and water treatments only affected the PO activity through their interaction (table 1). Micro-organism-enriched water significantly decreased the PO activity of control gammarids (F1,61 = 4.39, p = 0.040), but had no significant effect in wounded ones (F1,62 = 1.38, p = 0.244; figure 2). Interestingly, gammarids that moulted earlier exhibited higher levels of both PO and total activities (table 1 and the appendix in the electronic supplementary material).
Table 1.
Results of the MANOVA analysis for the activity of naturally activated PO enzymes and those of the proenzymes in addition to that of the PO (total activity), with population, wound treatment, water treatment, size and time to ecdysis as explanatory variables. Values p ≤ 0.05 are given in bold.
| source | Pillai's trace | PO activity | total activity |
|---|---|---|---|
| global models | F6,122 = 3.99, p = 0.001 | F6,122 = 6.71, p < 0.001 | |
| population | F2,121 = 2.13, p = 0.123 | F1,122 = 4.25, p = 0.041 | F1,122 = 0.67, p = 0.415 |
| wound | F2,121 = 0.49, p = 0.613 | F1,122 = 0.14, p = 0.907 | F1,122 = 0.83, p = 0.363 |
| water | F2,121 = 1.54, p = 0.219 | F1,122 = 1.67, p = 0.199 | F1,122 = 2.30, p = 0.132 |
| size | F2,121 = 4.31, p = 0.016 | F1,122 = 7.49, p = 0.007 | F1,122 = 0.07, p = 0.798 |
| time to ecdysis | F2,121 = 16.29, p < 0.001 | F2,122 = 9.52, p = 0.003 | F2,122 = 30.41, p < 0.001 |
| wound * water | F2,121 = 2.80, p = 0.065 | F1,122 = 5.39, p = 0.022 | F1,122 = 1.34, p = 0.250 |
Figure 2.
Mean PO activity (Vmax: change in absorbance unit min−1) of G. pulex when exposed to wounding and micro-organism-enriched water. Numbers in the bottom of bars are sample size. Open bar, clean water; filled bar, enriched water.
4. Discussion
Increasing the concentration of micro-organisms in the water significantly increased the mortality of G. pulex, but wounding did not further enhance the mortality. This demonstrates that micro-organisms in suspension in water are, on their own, an important threat to gammarids.
As expected from the hypothesis that time to ecdysis could be plastically adjusted in response to infection risk, we found that micro-organism-enriched water significantly postponed ecdysis. Such plasticity in the timing of ecdysis may allow gammarids to respond to water conditions in a way that would minimize infection probability. In this experiment, we could not test for the potential benefit of postponing ecdysis in micro-organism-enriched water since gammarids that had moulted were used for haemolymph extraction. Further studies will compare the survival of gammarids that have moulted and those that did not.
Moult timing differed between populations and difference in the magnitude of the induced temporal shift of ecdysis caused by the water treatment in each population was close to be significant. These differences may reflect a local adaptation to temporal variation in water conditions at each site. However, this requires additional examination.
PO activities and time to ecdysis negatively covaried independently of water conditions, suggesting that levels of immune defence did not condition the magnitude of the temporal shift of ecdysis. Such covariation between PO activities and time to ecdysis could either reflect a genetic relationship between these parameters or result from inter-individual variation in body conditions, where gammarids of poor body condition grow slowly, exhibit long inter-moult periods, and have low levels of immune defences.
While having a similar total concentration of PO enzymes, unwounded gammarids exposed to micro-organism-enriched water had less active PO than those in clean water. This phenomenon was absent in wounded gammarids. This may suggest that an additional, unmeasured immune pathway, which is probably functionally antagonist to PO activation, is dealing with the microbial infection. While PO provides rapid protection against a wide range of pathogens during wound healing, it does not fully predict resistance against bacterial infections (Adamo 2004) and is often traded-off against inducible antimicrobial immune pathways (Moret & Schmid-Hempel 2009). Furthermore, PO activation in response to injuries has limited ability to cause cuticular disinfection (Vogan et al. 2008). Antimicrobial compounds similar to those extractable from the exoskeletons of marine crustaceans (Stagner & Redmond 1975; Haug et al. 2002) might be produced antagonistically to PO activity in G. pulex.
To conclude, our study demonstrates the existence of a temporal adjustment of the moult cycle in response to elevated risks of infection in G. pulex. This phenomenon is probably adaptive by avoiding exposure of the gammarids to pathogens during ecdysis, a period of high susceptibility to infection (Le Moullac et al. 1997; Morado et al. 1999). This plastic change in time to ecdysis in response to water quality is independent of levels of immune defences. The mechanisms through which moulting is temporally regulated according to the pathogenic threat in the water are yet not known. However, we may speculate about a potential interaction between effectors of the gammarid immune system and the regulation of the synthesis and storage of both ecdysteroids and moult-inhibiting hormones (Kuballa & Elizur 2007).
Acknowledgements
This study was supported by the CNRS and a grant ANR-08-JCJC-0006 to Y.M. Thanks to M. J. F. Brown for comments on the manuscript.
References
- Adamo S. A.2004How should behavioural ecologists interpret measurements of immunity? Anim. Behav. 68, 1443–1449 (doi:10.1016/j.anbehav.2004.05.005) [Google Scholar]
- Cerenius L., Söderhäll K.2004The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 199, 116–126 [DOI] [PubMed] [Google Scholar]
- Cornet S., Biard C., Moret Y.2009Variation in immune defence among populations of Gammarus pulex (Crustacea: Amphipoda). Oecologia 159, 257–269 (doi:10.1007/s00442-008-1211-y) [DOI] [PubMed] [Google Scholar]
- Haug T., Kjuul A. K., Stensvåg K., Sandsdalen E., Styrvold O. B.2002Antibacterial activity in four marine crustacean decapods. Fish Shellfish Immunol. 12, 371–385 (doi:10.1006/fsim.2001.0378) [DOI] [PubMed] [Google Scholar]
- Kuballa A., Elizur A.2007Novel molecular approach to study moulting in crustaceans. Bull. Fish. Res. Agen. 20, 53–57 [Google Scholar]
- Laufer H., Demir N., Biggers W. J.2005Response of the American lobster to the stress of shell disease. J. Shellfish Res. 24, 757–760 [Google Scholar]
- Le Moullac G., Le Groumellec M., Ansquer D., Froissard S., Levy P. & Aquacop 1997Haematological and phenoloxidase activity changes in the shrimp Penaeus stylirostris in relation with the moult cycle: protection against vibriosis. Fish Shellfish Immunol. 7, 227–234 (doi:10.1006/fsim.1996.0077) [Google Scholar]
- Morado J. F., Giesecke R. H., Syrjala S. E.1999Molt related mortalities of the Dungeness crab Cancer magister by a marine facultative ciliate Mesanophrys pugettensis. Dis. Aquat. Organ 38, 143–150 (doi:10.3354/dao038143) [Google Scholar]
- Moret Y., Schmid-Hempel P.2004Social life-history response to individual immune challenge of workers of Bombus terrestris L.: a possible new cooperative phenomenon. Ecol. Lett. 7, 146–152 (doi:10.1046/j.1461-0248.2003.00561.x) [Google Scholar]
- Moret Y., Schmid-Hempel P.2009Immune responses of bumblebee workers as a function of individual and colony age: senescence versus plastic adjustment of the immune function. Oikos 118, 371–378 (doi:10.1111/j.1600-0706.2008.17187.x) [Google Scholar]
- Ostman O., Drakare S., Kritzberg E. S., Langenheder S., Logue J. B., Lindstrom E. S.2010Regional invariance among microbial communities. Ecol. Lett. 13, 118–127 (doi:10.1111/j.1461-0248.2009.01413.x) [DOI] [PubMed] [Google Scholar]
- Plaistow S. J., Outreman Y., Moret Y., Rigaud T.2003Variation in the risk of being wounded: an overlooked factor in studies of invertebrate immune function? Ecol. Lett. 6, 489–494 (doi:10.1046/j.1461-0248.2003.00455.x) [Google Scholar]
- Stagner H., Redmond J.1975The immunological mechanisms of the horseshoe crab, Limulus polyphemus. Mar. Fish. Rev. 37, 11–19 [Google Scholar]
- Vogan C. L., Powell A., Rowley A. F.2008Shell disease in crustaceans—just chitin recycling gone wrong? Env. Microbiol 10, 826–835 (doi:10.1111/j.1462-2920.2007.01514.x) [DOI] [PubMed] [Google Scholar]