Abstract
Fish act aggressively towards their mirror image suggesting that they consider it another individual, whereas in some mammals behavioural response to mirrors may be an evidence of self-recognition. Since fish cannot self-recognize, we asked whether they could distinguish between fighting a mirror image and fighting a real fish. We compared molecular, physiological and behavioural responses in each condition and found large differences in brain gene expression levels. Although neither levels of aggressive behaviour nor circulating androgens differed between these conditions, males fighting a mirror image had higher immediate early gene (IEG) expression in brain areas homologous to the amygdala and hippocampus than controls. Since amygdalar responses are associated with fear and fear conditioning in other species, higher levels of brain activation when fighting a mirror suggest fish experience fear in response to fights with a mirror image. Clearly, the fish recognize something unusual about the mirror image and the differential brain response may reflect a cognitive distinction.
Keywords: immediate early genes, hippocampus, amygdala, pre-optic area, egr-1, c-fos
1. Introduction
What do animals make of seeing their own image in a mirror? Gallup (1968) used mirrors to assess animals' ability to recognize themselves and based on such tests, most vertebrates do not have self-recognition (e.g. Anderson & Gallup 1999). In fish, observers noted that a mirror image would elicit apparently unconditioned aggressive display (e.g. Betta splendens, Lissmann 1932). Tinbergen (1951) observed that male three-spined sticklebacks displayed aggressive behaviour towards mirror images, suggesting that fish treat mirror images as an intruding individual.
To discover whether fighting an opponent is similar to fighting a mirror image, we measured differences in behavioural, hormonal and brain activity between conditions. We quantified localized differences in immediate early gene (IEG) expression levels as a proxy for neural activation (e.g. Clayton 2000) in context-relevant processing regions of the brain. Based on previous behavioural tests (Burmeister et al. 2005), we hypothesized that IEG measurements might reveal subtle differences between conditions since IEGs are known to play many roles in mediating neural plasticity, including the activation of signal transduction cascades through which neurons convert extracellular chemical or electrical signals into genomic activation (e.g. Morgan & Curran 1995). Moreover, many IEGs are activated by neuronal activity (Dragunow & Robertson 1987). Two of the most widely expressed are c-fos and egr-1 (Clayton 2000), which have been used widely as genomic markers for brain activity. c-fos is an indicator of immediate neural activity, while egr-1 is a transcription factor and is hypothesized to indicate upregulation of later acting genes, like GnRH, the primary signal for the reproductive axis (Burmeister et al. 2005).
We measured effects in an African cichlid fish, Astatotilapia burtoni, fighting with a conspecific male across a clear barrier, fighting with a mirror image or seeing no opponent (control). We measured IEGs in four brain regions: (i) Dm (dorsomedial telencephalon), the fish homologue of the amygdala; (ii) DI (dorsolateral telencephalon) the hippocampus homologue; (iii) the pre-optic area (POA); and (iv) the cerebellum (Cce). These brain loci have known roles in the control of fear response (Dm, Portavella et al. 2002), spatial learning (Dl, Rodriguez et al. 2002), reproduction (POA, Francis et al. 1993) and attention (Cce, Rodriguez et al. 2005).
2. Material and methods
Size-matched dominant males were in an 8-gallon tank subdivided into two equal, water-tight compartments separated by a clear barrier and a removable opaque barrier. The size difference between these animals ranged from 0 to 6 per cent in standard length, and from 0 to 9 per cent in body mass. For three groups of eight fish, the opaque barrier between the compartments was removed to reveal: (i) a mirror (mirror group); (ii) a fish opponent (opponent group); (iii) an empty chamber (control group). In all three treatments, fish were videotaped while exposed to the condition for 20 min. Observations scored for total aggression by an observer blinded to the condition. ‘Total aggression’ included the number of bites, rams and side body displays, all typical cichlid aggressive behaviours and the number of aggressive bouts and the duration of these bouts were counted.
Following behavioural trials, blood samples were collected and plasma testosterone and 11-ketotestosterone were measured using commercially available kits (Parikh et al. 2006). Brains were removed and frozen in a mounting medium (Tissue Tek) on dry ice. Brains were sliced coronally in 300 µm sections using a cryostat and mounted on glass slides. A frozen stage (Physitemp) viewed through a dissection microscope was used for microdissection, performed with a modified 27G needle with an internal diameter of 190 µm following an established protocol (Korzan et al. 2000). Brain atlases from A. burtoni (Fernald & Shelton 1985; Burmeister et al. 2009) and from other fish species (e.g. Reiner & Northcutt 1992) were used to target the Cce, Dl, Dm and POA and for all of these brain areas, the entire nucleus was collected and treated identically (e.g. multiple subdivisions of each nucleus are included). Micro-dissected tissue was collected in a lysis buffer (RNeasy, Qiagen Inc.).
We used qRT-PCR to measure mRNA expression in each region of the brain. Primers for the A. burtoni target genes, egr-1 and c-fos and for control genes, 18s rRNA and actin were designed according to published sequences (Burmeister et al. 2005). The qRT-PCR was performed using 30 µl duplicate reactions (SYBR Green; Bio-Rad) and performed on a real-time PCR system (Bio-Rad). Original fluorescence readings were analysed using a curve-fitting real-time PCR algorithm (Zhao & Fernald 2005). Computed cDNA concentrations of the two housekeeping genes (18s and actin) were not significantly different from each other, so we used the geometric mean of these as a normalized standard for each tissue sample. The relative mRNA levels of the target genes (c-fos and egr-1) were calculated as the percentage of the geometric mean of the housekeeping genes.
One-way ANOVAs were used (JMP) to test hormone levels (T, 11KT) and RT-PCR data for statistical significance. Tukey's HSD post hoc tests were conducted to detect pairwise differences between treatments with the overall alpha level at 0.05. A t-test was conducted on total aggression, all components of aggression as well as number of aggressive bouts and aggressive bout time between fish that were aggressive towards a mirror and fish that were not. To test for any relationships between size of the opponent and behaviour, hormone and gene expression, a composite of size difference was calculated as the sum of difference in standard length and difference in body mass. Means for groups and overall test statistics are in appendix A, electronic supplementary material.
3. Results
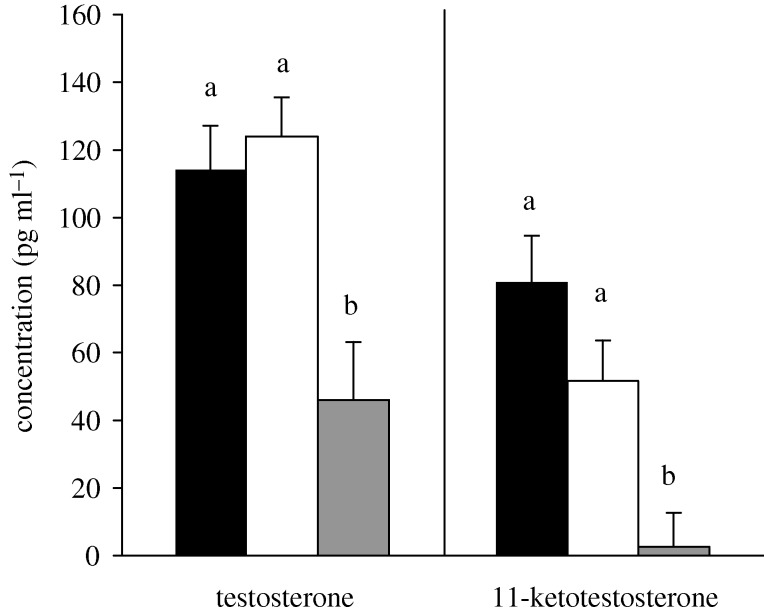
A. burtoni males fight vigorously to establish and defend territories (Fernald 1977) and will fight with other males when separated by a clear barrier (e.g. Burmeister et al. 2005). In our experiments, there was no difference in total aggression or the components of total aggression between ‘opponent’ and ‘mirror’ males (all t < 0.992 and all p > 0.34; appendix A, electronic supplementary material). Among individuals that fought an opponent, there were no statistical relationships between total aggression and any of the components of total aggression or size of the opponent (all p > 0.15; appendix B, electronic supplementary material). While androgens did not differ between males ‘mirror’ or ‘opponent’ males (T: p = 0.59; 11KT: p = 0.157) animals in these groups had higher androgen levels than control males (figure 1, T: p = 0.002; 11KT: p = 0.006).
Figure 1.
Plasma testosterone and 11-ketotestosterone levels (mean + s.e.) between males aggressing towards a mirror (mirror, black bars), males aggressing towards a conspecific opponent across a clear barrier (opponent, open bars) and males who had not been in an aggressive encounter (control, grey bars). Different letters above the bars indicate a significant difference between groups with overall α = 0.05.
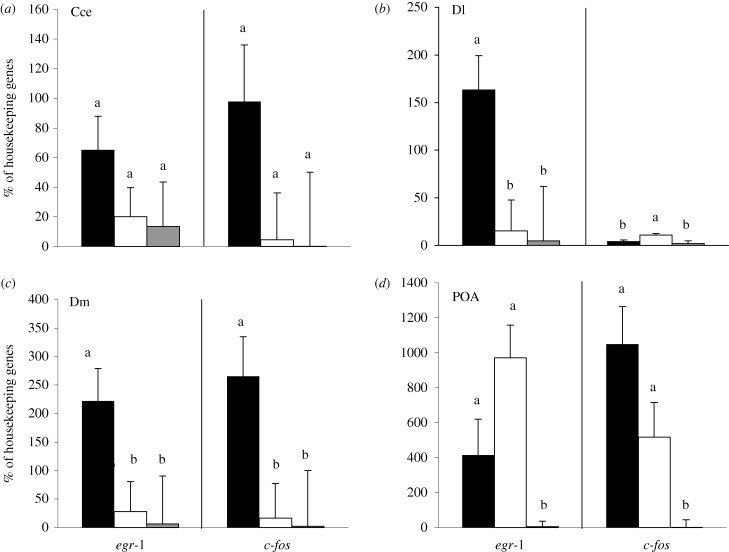
Surprisingly, mirror and regular fights had strikingly different effects on the brain. ‘Mirror’ males had higher levels of egr-1 expression in the homologue of the hippocampus, Dl, than ‘opponent’ males or controls (p = 0.008). By contrast, c-fos expression in Dl was significantly higher in ‘opponent’ males, when compared with ‘mirror’ and control males (p = 0.006). Males who fought a mirror image had much higher egr-1 and c-fos expression in Dm than males who fought an opponent or controls (egr-1: p = 0.03; c-fos: p = 0.02). In the Cce, there were no differences in egr-1 or c-fos expression among any of the males (figure 2: egr-1: p = 0.252; c-fos: p = 0.14). In the POA, egr-1 and c-fos males who fought a mirror or an opponent had higher expression than controls (egr-1: p = 0.02; c-fos: p = 0.05), however, there was no difference between the fighting males (mirror and opponent, egr-1: p = 0.295; c-fos: p = 0.203). Opponent size did not influence egr-1 or c-fos expression in any brain areas (all p > 0.21; appendix B, electronic supplementary material).
Figure 2.
Levels (mean + s.e.) of immediate early genes (IEGs) egr-1 and c-fos as a percentage of the geometric mean of two housekeeping genes (actin, 18s) in four target brain areas: (a) the cerebellum (Cce), (b) dorsolateral telencephalon (Dl), (c) dorsomedial telencephalon (Dm) and (d) the preoptic area (POA). Black bars are males who were aggressive towards a mirror, open bars are males who were aggressive towards an opponent across a clear barrier and grey bars are males who were not involved in an aggressive encounter. Different letters above the bars indicate a significant difference between groups with overall α = 0.05.
4. Discussion
Males fighting an opponent through a clear barrier or fighting their mirror image showed similar behaviour, circulating androgens and similar gene expression in the POA and the Cce but vastly different gene expression in Dl (hippocampus) and Dm (amygdala) Both of these nuclei receive multimodal sensory inputs (Northcutt 2006). When males were aggressive towards their mirror images, egr-1 mRNA was higher in Dl, while both egr-1 and c-fos mRNA was higher in Dm. Increases in both c-fos and egr-1 reflect higher immediate and long-term neural activity possibly including an increase in the transcription of late-acting genes and neuronal firing in the amygdala, as suggested by egr-1 and c-fos. In Dl, the mirror possibly elicits an increase only in transcription of later acting genes associated only with egr-1. Interestingly, c-fos expression was higher in Dl of males who had interacted with a true opponent. The significant differences in brain activity show that males recognize and respond to something about the mirror, but what?
Perhaps the responses reflect fear associated with a mirror image. In the hippocampus (Dl), egr-1 may be acting as a transcription factor for later-acting genes coding for stress responses including mineralocorticoid, glucocorticoid and NMDA ligands and receptors (Bannerman et al. 1995). In mice, learning a spatial task associated with mild stress activates physical activity-related genes differentially in the hippocampus (Cavallaro et al. 2002). It seems plausible that increased egr-1 activity in Dl may signal the encoding of stress-related spatial information in animals fighting their mirror image. However, the increased c-fos expression in Dl, exclusively in the males who had interacted with a true opponent does not support this hypothesis. An alternative hypothesis is that the mirror image represents a perfectly size-matched opponent. Theoretical models and behavioural evidence in a number of taxa suggest that aggressive behaviours between territory holders increase with decreasing size difference between combatants (e.g. Maynard Smith & Price 1973). If true, we would expect an inverse relationship between opponent size difference, aggression and IEG expression in the amygdala, which we did not. Fear induces associative long-term potentiation in the amygdala (Rogan et al. 1997), a nucleus that contains a large population of corticotropin-releasing hormone containing neurons (Schulkin et al. 1997) known to be active when animals face a fearful stimulus.
The mirror image presentation may induce fear in A. burtoni males because it is a completely novel stimulus, not interpretable based on past experience because the mirror ‘opponent’ does not react in familiar ways. Alternatively, the opponent and the mirror conditions may be seen as two different stimuli resulting in two different brain gene expression patterns and neither induces fear. However, since the central difference between these conditions is in the amygdalar response, this probably reflects fear in these animals.
The differential increase of IEGs in the homologues of the hippocampus and amygdala of fish fighting their own image shows that IEGs can provide important information not found in behavioural responses or hormone levels. These brain activity measurements show that the animal considers fighting a mirror image different from fighting a conspecific suggesting that these fish may have cognitive capacities that go beyond Tinbergen's (1951) suggestion that they were limited to fixed action patterns. While using mirrors to stimulate and test cognitive abilities in vertebrates is widespread, caution should be used when interpreting a response towards a mirror as identical to that towards a conspecific opponent.
Acknowledgements
All experimental procedures have been approved by Stanford University and APLAC (IACUC assurance number: A3213–01).
We thank H. Baier, K. Maruska, R. Carpenter, J. Fitzpatrick, B. Grone and 3 anonymous reviewers for comments on this MS, H. Cooper and K. Eaton for technical help. J.K.D. was supported by an NSERC PDF and R.D.F. by a Jacob Javits Award from NIH NS034 950.
References
- Anderson J. R., Gallup G. G., Jr1999Animal models of human emotion and cognition. (eds Haug M., Whalen R. E.), pp. 175–194 Washington, DC: American psychological association [Google Scholar]
- Bannerman D. M., Good M. A., Butcher S. P., Ramsay M., Morris R. G. M.1995Distinct components of spatial learning revealed by prior training and NMDA receptor blockage. Nature 378, 182–186 (doi:10.1038/378182a0) [DOI] [PubMed] [Google Scholar]
- Burmeister S. S., Jarvis E. D., Fernald R. D.2005Rapid behavioral and genomic responses to social opportunity. PLoS biol. 3, e363 (doi:10.1371/journal.pbio.0030363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister S. S., Munshi R. G., Fernald R. D.2009Cytoarchitecture of a cichlid fish telencephalon. Brain Behav. Evol. 74, 110–120 (doi:10.1159/000235613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallaro S., D'Agata V., Manickam P., Dufour F., Alkon D. L.2002Memory-specific temporal profiles of gene expression in the hippocampus. Proc. Natl Acad. Sci. USA 99, 16 279–16 284 (doi:10.1073/pnas.242597199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F.2000The genomic action potential. Neurobiol. Learn. Mem. 74, 185–216 (doi:10.1006/nlme.2000.3967) [DOI] [PubMed] [Google Scholar]
- Dragunow M., Robertson H. A.1987Kindling stimulation induces c-fos protein(s) in granule cells of the rat dentate gyrus. Nature 329, 441–442 (doi:10.1038/329441a0) [DOI] [PubMed] [Google Scholar]
- Fernald R. D.1977Quantitative behavioural observations of Haplochromis burtoni under semi-natural conditions. Anim. Behav. 25, 643–653 (doi:10.1016/0003-3472(77)90115-4) [Google Scholar]
- Fernald R. D., Shelton L. C.1985The organization of the diencephalons and the pretectum in the cichlid fish, Haplochromis burtoni. J. Comp. Neurol. 238, 202–217 (doi:10.1002/cne.902380207) [DOI] [PubMed] [Google Scholar]
- Francis R. C., Soma K. K., Fernald R. D.1993Social regulation of the brain-pituitary-gonadal axis. Proc. Natl Acad. Sci. USA 90, 7794–7798 (doi:10.1073/pnas.90.16.7794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallup G. G., Jr1968Mirror-image stimulation. Psychol. bull. 70, 782–793 (doi:10.1037/h0026777) [DOI] [PubMed] [Google Scholar]
- Korzan W. J., Summers T. R., Summers C. H.2000Monoaminergic activities of limbic regions are elevated during aggression: influence of sympathetic social signaling. Brain Res. 870, 170–178 (doi:10.1016/S0006-8993(00)02420-3) [DOI] [PubMed] [Google Scholar]
- Lissmann H. W.1932Die Umwelt des Kampffisches (Betta splendens regan). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Phsyiol. 18, 65–111 [Google Scholar]
- Maynard Smith J., Price G. R.1973The logic of animal conflict. Nature 246, 15–18 [Google Scholar]
- Morgan J. I., Curran T.1995The immediate early gene response and neuronal death and regeneration. Neuroscientist 1, 68–75 (doi:10.1177/107385849500100203) [Google Scholar]
- Northcutt R. G.2006Connections of the lateral and medial divisions of the goldifhs telencephalic pallium. J. Comp. Neurol. 494, 903–943 (doi:10.1002/cne.20853) [DOI] [PubMed] [Google Scholar]
- Parikh V. N., Clement T. S., Fernald R. D.2006Androgen level and male social status in the African cichlid, Astatotilapia burtoni. Behav. Brain Res. 166, 291–295 (doi:10.1016/j.bbr.2005.07.011) [DOI] [PubMed] [Google Scholar]
- Portavella M., Vargas J. P., Torres B., Salas C.2002The effects of telencephalic pallial lesions on spatial, temporal, and emotional learning in goldfish. Brain Res. Bull. 57, 3–4 [DOI] [PubMed] [Google Scholar]
- Reiner A., Northcutt R. G.1992An immunohistochemical study of the telencephalon of the Senegal bichir (Polypterus senegalus). J. Comp. Neurol. 319, 359–386 (doi:10.1002/cne.903190305) [DOI] [PubMed] [Google Scholar]
- Rodriguez F., Lopez J. C., Vargas J. P., Broglio C., Gomez Y., Salas C.2002Spatial memory and hippocampal pallium through vertebrate evolution: insights from reptiles and fish. Brain Res. Bull. 57, 499–503 (doi:10.1016/S0361-9230(01)00682-7) [DOI] [PubMed] [Google Scholar]
- Rodriguez F., Duran E., Gomez A., Ocana F. M., Alvarez E., Jimenez-Moya F., Broglio C., Salas C.2005Cognitive and emotional functions of the teleost fish cerebellum. Brain Res. Bull. 66, 365–370 (doi:10.1016/j.brainresbull.2004.11.026) [DOI] [PubMed] [Google Scholar]
- Rogan M. T., Staubli U., Ledoux J.1997Fear conditioning induces long term potentiation in the amygdala. Nature 456, 604–607 [DOI] [PubMed] [Google Scholar]
- Schulkin J., Gold P. W., McEwen B. S.1997Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinol. 23, 219–243 (doi:10.1016/S0306-4530(97)00099-1) [DOI] [PubMed] [Google Scholar]
- Tinbergen N.1951The study of instinct. Oxford, UK: Clarendon Press [Google Scholar]
- Zhao S., Fernald R. D.2005Comprehensive algorithm for quantitative real-time polymerase chain reaction. J. Comput. Biol. 12, 1047–1064 (doi:10.1089/cmb.2005.12.1047) [DOI] [PMC free article] [PubMed] [Google Scholar]