Abstract
We examined cell size correlations between tissues, and cell size to body mass relationships in passerine birds, amphibians and mammals. The size correlated highly between all cell types in birds and amphibians; mammalian tissues clustered by size correlation in three tissue groups. Erythrocyte size correlated well with the volume of other cell types in birds and amphibians, but poorly in mammals. In birds, body mass correlated positively with the size of all cell types including erythrocytes, and in mammals only with the sizes of some cell types. Size of mammalian erythrocytes correlated with body mass only within the most taxonomically uniform group of species (rodents and lagomorphs). Cell volume increased with body mass of birds and mammals to less than 0.3 power, indicating that body size evolved mostly by changes in cell number. Our evidence suggests that epigenetic mechanisms determining cell size relationships in tissues are conservative in birds and amphibians, but less stringent in mammals. The patterns of cell size to body mass relationships we obtained challenge some key assumptions of fractal and cellular models used by allometric theory to explain mass-scaling of metabolism. We suggest that the assumptions in both models are not universal, and that such models need reformulation.
Keywords: erythrocytes, cellular architecture, metabolic rate, scaling
1. Introduction
Any substantial change of body mass in a lineage must be caused by alteration of cell size, cell number or a combination of the two. Because cell size differs between tissues in the same organism, we have to ask whether the sizes of different cell types evolve independently or in concert. If it is the latter, evolution is more constrained in changing body size through alterations of cell size. Animal bodies would change by roughly rescaling all types of cells, which creates a size correlation between different cell types.
Evolutionary coupling between cell size and body mass is postulated to play an important role in shaping the mass-scaling of metabolism (Davison 1955; Szarski 1983; Kozłowski et al. 2003). With increasing cell size, the cell surface area/volume ratio decreases and a smaller fraction of metabolism is spent on maintaining ionic gradients across cell membranes. In a group of closely related organisms with differences in body mass attributed entirely to cell size change, the metabolic rate should scale with body mass with the exponent b = 0.67 dictated by the changing surface area/volume ratio of the cells. The exponent should become 1.0 if cell size remains unaltered and body mass varies entirely owing to differences in cell numbers. Then the question is whether the component of body size change realized through the change in cell sizes is sufficient to explain the usually negative allometries of metabolism in nature (0.6 < b < 0.8; Glazier 2005)?
A concurrent model (West et al. 1997) predicts the unique metabolic scaling exponent 0.75 from the fractal nature of the supplying system. One of its key assumptions is body mass-invariance of capillary length and diameter. Given that adequate gas exchange requires the short axis of the erythrocyte to be about 25 per cent more than capillary diameter (Snyder & Sheafor 1999), West et al. implicitly assume mass-invariance of erythrocyte size. Consequently, if organism-wide rescaling of cell sizes exists in nature, other cell types should not change their size with the organisms' mass. Savage et al. (2007) attempt to link fractal and cellular models by considering either body mass-scaling or mass-invariance of cell sizes in mammals, and show that both patterns are present depending on cell type. This result requires verification for other taxa, and testing whether mass-scaling of cell size supports a cellular model and to what extent it challenges the assumptions of a fractal model.
Measuring cell size is extremely laborious, which explains the paucity of available data and the lack of conclusive evidence for the arguments. Here, we analyse the sparse data on cell size in tissues of passerine birds, amphibians and mammals to test whether cell size for different cell types is intercorrelated and related to body mass. In particular, we examine whether erythrocyte size correlates with the size of other cells and with body mass. The existence of these intercorrelations in different taxa would indicate the need to reconcile some key assumptions of the fractal model. To test the cellular model, we calculate exponents of mass-scaling of cell sizes, assess the corresponding exponents of metabolism predicted by the model and compare the predicted exponents with their actual values from the literature.
2. Material and methods
We used published and unpublished data on birds, amphibians and mammals collected by the group founded by Henryk Szarski, mostly at Nicolaus Copernicus University (Toruń, Poland, TG hereafter), and two datasets for mammals extracted from Morgado et al. (1990) by Savage et al. (2007) and from Levi (1925) by Szarski (1985). The data on six species of passerine birds were published by Nitecki (1972). They were collected by one person, and the tissues were taken from the same individuals, increasing the accuracy of data on birds. TG's data on amphibians and mammals (restricted to closely related groups, rodents and lagomorphs) were compiled from different papers and MSc theses. Not all same-species measurements were taken from the same individuals or made by the same person. Thus, the amphibian and mammalian data are less precise. Original data are given in the electronic supplementary material.
All analyses used original data and phylogenetically independent contrasts. Contrasts were calculated following the method in Phenotypic Diversity Analysis Programmes (Garland et al. 1999) with Nee's transformation (Purvis 1995) using the trees in the electronic supplementary material. We calculated correlations between cell size and body mass, and size correlations between cell types (see the electronic supplementary material). Mass-scaling exponents of cell size for different tissues were calculated from slopes of log cell volume – log body mass least-squares regressions. Mean exponents in each animal group were calculated from exponent values in different tissues. The means were tested against zero with the t-test. To examine intercorrelations between cell sizes and to reduce the problem of multiple comparisons, we performed principal component analysis (PCA) on the most complete data subsets on cell size. Phylogenetically controlled PCA was carried out on correlation coefficients (calculated through origin) of standardized contrasts from cell volumes (Garland et al. 1992). Our principal components (PCs) identify tissue associations with highly correlated cell sizes. PC scores measure the combined cell sizes in such groups. Loadings indicate the strength and direction of tissue contributions to PCs. Negative loadings indicate an inverse relationship between PC scores and the cell size of contributing tissues. PC scores were used to examine correlations of cell size with body size and erythrocyte size.
3. Results
Birds and amphibians differed from mammals in the structure of intercorrelations between cell types (table 1). Birds and amphibian tissues formed a single major PC with high loadings of the same sign, revealing positive cell size correlations between tissues. Mammalian tissues clustered in three PCs (tissue groups), indicating that not all cell types had correlated sizes. Within each group, cell size usually correlated positively between tissues (high loadings with the same sign), with a few exceptions. Some tissues contributed simultaneously to two PCs. PCA on contrasts produced similar results, but some tissues, especially in mammals, changed their quantitative (loading value) and qualitative (loading sign) impacts on PCs. In mammals (Morgado), PCs on contrasts changed in relative importance (cPC1 corresponds to PC2, cPC2 to PC3, cPC3 to PC1).
Table 1.
Results of PCA of cell size in amphibian, bird and mammalian tissues (on raw data and phylogenetic contrasts). (Loadings in bold are greater than 0.5. n is the number of species used.)
| group | cell type | raw data |
phylogenetic contrasts |
||||
|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | cPC1 | cPC2 | cPC3 | ||
| birds (Nitecki) n = 6 | hepatocytes | 0.990 | 0.123 | −0.001 | 0.977 | 0.179 | −0.007 |
| proximal kidney tubules | 0.997 | −0.028 | 0.058 | 0.989 | −0.085 | −0.100 | |
| duodenal epithelium | 0.988 | 0.139 | 0.067 | 0.976 | 0.187 | −0.108 | |
| skin epithelium | 0.991 | 0.005 | −0.132 | 0.976 | 0.051 | 0.210 | |
| tracheal chondrocytes | 0.957 | −0.290 | 0.022 | 0.898 | −0.438 | −0.004 | |
| thyroid follicles | 0.997 | 0.042 | −0.013 | 0.992 | 0.072 | 0.010 | |
| explained proportion of variation | 0.97 | 0.02 | 0.004 | 0.94 | 0.05 | 0.01 | |
| amphibians (TG) n = 8 | kidney (nephron) | 0.974 | −0.118 | −0.193 | 0.911 | −0.266 | 0.315 |
| intestinal epithelium | 0.966 | −0.200 | 0.164 | 0.912 | −0.262 | −0.316 | |
| epidermis | 0.945 | 0.325 | 0.031 | 0.798 | 0.602 | 0.002 | |
| explained proportion of variation | 0.93 | 0.05 | 0.02 | 0.77 | 0.17 | 0.07 | |
| mammals (Morgado) n = 9 | adipocytes (skin) | 0.729 | −0.064 | −0.580 | 0.013 | −0.650 | 0.723 |
| cerebellar granule neurons | 0.925 | −0.173 | 0.283 | −0.676 | 0.482 | 0.533 | |
| cerebellar Purkinje neurons | 0.932 | −0.054 | 0.338 | −0.430 | 0.651 | 0.573 | |
| fibroblasts | −0.256 | −0.809 | 0.496 | −0.789 | 0.228 | −0.513 | |
| fibrocytes | 0.285 | −0.636 | −0.682 | −0.566 | −0.697 | 0.298 | |
| glomerular epithelium | −0.085 | −0.946 | 0.236 | −0.917 | −0.201 | −0.223 | |
| goblet cells | 0.800 | 0.319 | −0.118 | 0.468 | −0.246 | 0.705 | |
| Henle-loop cells | −0.225 | −0.560 | −0.216 | −0.002 | −0.718 | −0.164 | |
| hepatocytes | −0.400 | 0.162 | 0.483 | 0.320 | −0.008 | −0.228 | |
| proximal convoluted tubules | 0.586 | −0.780 | 0.173 | −0.966 | −0.080 | 0.159 | |
| sebaceous gland cells (skin) | 0.699 | 0.416 | 0.466 | 0.211 | 0.870 | 0.216 | |
| explained proportion of variation | 0.37 | 0.29 | 0.17 | 0.34 | 0.27 | 0.20 | |
| mammals (TG) n = 8 | hepatocytes | 0.343 | −0.914 | 0.206 | 0.128 | −0.972 | −0.164 |
| proximal kidney tubules | −0.065 | 0.418 | 0.903 | 0.056 | −0.117 | 0.989 | |
| duodenal epithelium | 0.800 | 0.542 | −0.002 | 0.883 | 0.358 | 0.119 | |
| skin epithelium | 0.878 | 0.328 | −0.248 | 0.891 | 0.329 | −0.191 | |
| thyroid follicles | 0.861 | −0.442 | 0.242 | 0.780 | −0.614 | 0.039 | |
| explained proportion of variation | 0.46 | 0.32 | 0.20 | 0.44 | 0.31 | 0.21 | |
| mammals (Levi) n = 8 | Purkinje cells (cerebellum) | 0.843 | 0.445 | −0.076 | 0.917 | −0.205 | −0.001 |
| neuron (small ganglion) | 0.829 | 0.311 | −0.372 | 0.807 | −0.207 | −0.466 | |
| hepatocytes | −0.354 | 0.628 | 0.551 | 0.622 | 0.544 | 0.413 | |
| adrenals fascicular zone | −0.313 | 0.710 | −0.546 | 0.316 | 0.749 | −0.460 | |
| pancreas | −0.705 | 0.593 | −0.133 | 0.012 | 0.838 | 0.142 | |
| tongue epithelium | 0.507 | 0.454 | 0.595 | 0.794 | −0.290 | 0.332 | |
| explained proportion of variation | 0.40 | 0.29 | 0.19 | 0.44 | 0.29 | 0.12 | |
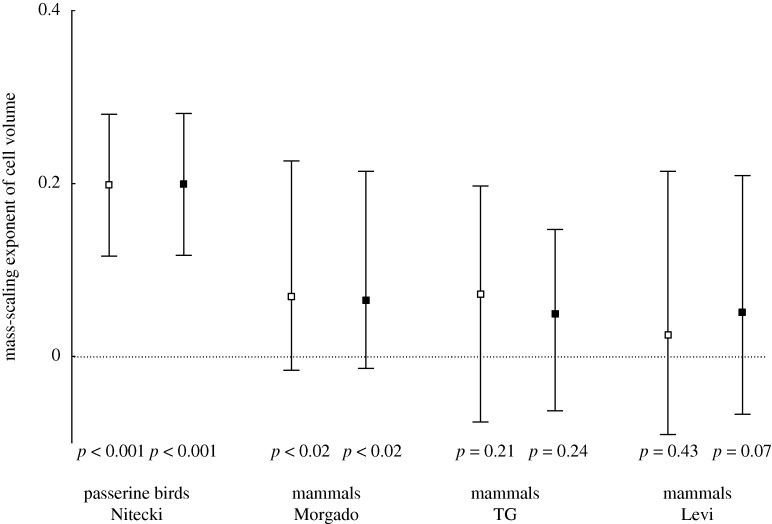
An index of cell sizes based on raw PC1 was positively correlated with body mass in birds and mammals (table 2). It was impossible to define a single size in indeterminately growing amphibians, and this precluded such an analysis. Note that in mammals (Levi), the increase in the PC1 index with body mass corresponded to an increase in the size of tongue epithelium, Purkinje and neuronal cells, but a decrease in pancreatic cell size (table 1). The correlation between erythrocyte size and body mass (table 2) was positive in birds and only in the taxonomically most uniform mammalian dataset (rodents and lagomorphs, TG); it became nearly significant in mammals (Levi) after phylogenetic corrections (p = 0.071). Erythrocyte size appeared to be a reliable predictor of PC1 only in the birds (p < 0.001) and in amphibians (p = 0.036), but not in mammals. Contrasts for the examined variables correlated similarly (table 2), except for erythrocyte size versus PC1 (amphibians), and body mass versus PC1 (mammals, TG). In mammals (Morgado), contrasts for erythrocyte size correlated with cPC2, and a positive correlation between cPC3 and mass contrasts corresponded to the correlation between PC1 and mass on raw data. Cell volume in tissues scaled with body mass, with exponents ranging from −0.09 to 0.280 (−0.067 to 0.281 for contrasts). Mean exponent values were significantly higher than zero in birds and mammals (Morgado) (figure 1).
Table 2.
Correlation matrix of erythrocyte size, body mass and scores of PCs: conventional analysis (normal font) and with phylogenetically independent contrasts (in italics). (Significant correlations are in bold. PCs define groups of tissues with correlated cell sizes (table 1). PC scores measure cell size in these groups. Only PCs explaining greater than 10% variance were used. n reports the number of species used.)
| group | trait | log body mass | erythrocyte | PC1 | PC2 | PC3 |
|---|---|---|---|---|---|---|
| birds (Nitecki) n = 6 | log body mass | 0.987 | 0.986 | |||
| 0.969 | 0.965 | |||||
| erythrocyte | p < 0.001 | 0.995 | ||||
| p < 0.002 | 0.988 | |||||
| PC1 | p < 0.001 | p < 0.001 | ||||
| p < 0.002 | p < 0.001 | |||||
| amphibians (TG) n = 8 | erythrocyte | 0.739 | 0.147 | |||
| 0.342 | 0.480 | |||||
| PC1 | p = 0.036 | |||||
| p = 0.41 | ||||||
| PC2 | p = 0.73 | |||||
| p = 0.23 | ||||||
| mammals (Morgado) n = 9 | log body mass | −0.243 | 0.876 | 0.244 | −0.025 | |
| 0.245 | −0.262 | −0.317 | 0.729 | |||
| erythrocyte | p = 0.53 | 0.528 | 0.064 | −0.558 | ||
| p = 0.53 | 0.304 | −0.774 | −0.065 | |||
| PC1 | p < 0.002 | p = 0.14 | ||||
| p = 0.50 | p = 0.43 | |||||
| PC2 | p = 0.53 | p = 0.87 | ||||
| p = 0.41 | p = 0.014 | |||||
| PC3 | p = 0.95 | p = 0.12 | ||||
| p = 0.026 | p = 0.87 | |||||
| mammals (TG) n = 8 | log body mass | 0.854 | 0.876 | 0.244 | 0.025 | |
| 0.834 | 0.396 | −0.554 | −0.286 | |||
| erythrocyte | p = 0.007 | 0.612 | 0.529 | −0.280 | ||
| p = 0.010 | 0.474 | −0.517 | −0.014 | |||
| PC1 | p = 0.002 | p = 0.11 | ||||
| p = 0.33 | p = 0.24 | |||||
| PC2 | p = 0.53 | p = 0.18 | ||||
| p = 0.15 | p = 0.19 | |||||
| PC3 | p = 0.95 | p = 0.50 | ||||
| p = 0.49 | p = 0.97 | |||||
| mammals (Levi) n = 8 | log body mass | −0.016 | 0.807 | 0.160 | −0.255 | |
| 0.667 | 0.674 | −0.293 | −0.196 | |||
| erythrocyte | p = 0.97 | −0.191 | 0.308 | 0.490 | ||
| p = 0.071 | 0.549 | −0.016 | 0.471 | |||
| PC1 | p = 0.016 | p = 0.65 | ||||
| p = 0.067 | p = 0.16 | |||||
| PC2 | p = 0.70 | p = 0.46 | ||||
| p = 0.48 | p = 0.97 | |||||
| PC3 | p = 0.54 | p = 0.22 | ||||
| p = 0.64 | p = 0.24 |
Figure 1.
Mean mass-scaling exponents of cell volumes in different tissues of birds and mammals; whiskers show minimum and maximum values. Means were tested against zero with a t-test. Open squares, raw; filled squares, contrasts.
4. Discussion
We demonstrated that cell sizes in different tissues, including erythrocytes, are highly intercorrelated in passerine birds and amphibians, which indicates that some insufficiently recognized epigenetic mechanisms determining cell size relationships are very conservative in these groups. The correlation of erythrocyte size with the size of other cell types in birds and amphibians also has practical implications. Blood samples can be taken in the field without killing, and erythrocytes are easy to measure. The cellular architecture of mammals seems different. Variation of their cell sizes is not entirely captured by a single PC, but splits between three PCs (table 1), and erythrocyte size is usually unrelated to the size of other cell types (table 2). In birds, our measure of cell sizes across tissues (PC1) and erythrocyte area correlates strongly with body mass. A similar increase in erythrocyte size with body mass was earlier reported by Starostová et al. (2009) in geckos. In these groups, at least, this evidence invalidates the assumption of mass-invariance of erythrocyte size in West et al.'s (1997) fractal model. A positive correlation between erythrocyte size and body mass was also reported in mammals when phylogeny was considered (Promislow 1991), but it was not detected when the phylogenetic context was ignored (Savage et al. 2007). According to our results, erythrocyte size increases with body mass only in the phylogenetically uniform dataset on rodents and lagomorphs (TG), and the increase becomes nearly significant in mammals (Levi) after phylogenetic corrections. Interestingly, only the size of cell types forming PC1 correlates with body mass in mammals; the cells involved in the remaining PCs stay mass-invariant (in mammals (Morgado), body mass correlates with the PC1 on raw data, and with the PC3 in contrasts, but similar tissues contribute to both PCs). Thus, the size of erythrocytes and of other cell types may depend on body mass in mammals, but it is not a norm.
Cell size seems to play a different role in the body mass evolution of the studied animals. The exponent of mass-scaling of cell volumes in different tissues is on average positive in all groups, but only in passerine birds and in Morgado's mammals is the mean exponent value significantly larger than zero (figure 1). Mass-scaling exponents of cell size did not exceed 0.3 in individual tissues, which means that changes in cell number also contributed to evolutionary differentiation of body masses, especially in mammals. According to the cellular model (Davison 1955; Kozłowski et al. 2003), an increase in cell volume with body mass to the power 0.3 should be associated with a metabolic scaling exponent b equal to 0.9. Apparently, this value of the metabolic exponent is much higher than the values actually observed in nature (b usually ranges from 0.6 to 0.8 in birds and mammals; Glazier 2005). Low exponents of the mass-scaling of erythrocytes and metabolism were also reported in a group of gecko species (Starostová et al. 2009). These pieces of evidence suggest that metabolic differences between species may be only partly determined by the cell surface/volume ratio, and mostly by other body size-related factors such as membrane permeability and the density and activity of mitochondria.
The empirical data reveal that the patterns of mass dependence of cell sizes in different animal groups are inconsistent with the assumptions of fractal and cellular models. This calls for revision of the theories. Qualitative differences in the cellular architecture of amphibians and birds versus mammals point to the importance of the phylogenetic context in future metabolic studies, and warn against formulating universal explanations. We believe that further studies integrating the levels of cell and organism are required if we are to untangle the knot of causality in the relationships between body size, metabolism and cell traits.
Acknowledgements
We thank M. Ejsmond, M. Konarzewski, T. Müller, D. Zook and two anonymous reviewers for helpful comments, and T. Garland, P. Koteja and L. J. Revell for statistical advice. The work was supported by grant NN304172036 from the Polish Ministry of Science and Higher Education. M. Jacobs helped edit the manuscript.
References
- Davison J.1955Body weight, cell surface and metabolic rate in anuran Amphibia. Biol. Bull. 109, 407–419 (doi:10.2307/1539173) [Google Scholar]
- Garland T., Jr, Harvey P. H., Ives A. R.1992Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 41, 18–32 [Google Scholar]
- Garland T., Jr, Midford P. E., Ives A. R.1999An introduction to phylogenetically based statistical methods, with a new method for confidence intervals on ancestral states. Am. Zool. 39, 374–388 (doi:10.1093/icb/39.2.374) [Google Scholar]
- Glazier D. S.2005Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol. Rev. 80, 611–662 (doi:10.1017/S1464793105006834) [DOI] [PubMed] [Google Scholar]
- Kozłowski J., Konarzewski M., Gawelczyk A. T.2003Cell size as a link between noncoding DNA and metabolic rate scaling. Proc. Natl Acad. Sci. USA 100, 14 080–14 085 (doi:101073/pnas.2334605100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G.1925Wachstum und Körpergrösse bei vollausgebildeten und im Wachstum begriffenen Tieren. Erg. Anat. Entwickl.-Gesch 26, 86–352 [Google Scholar]
- Morgado E., Ocqueteau C., Cury M., Becker L., Gonzales U., Muxica L., Gunther B.1990Three-dimensional morphometry of mammalian cells. II. Areas, volumes, and area–volume ratios. Arch. Biol. Med. Exp. 23, 21–27 [PubMed] [Google Scholar]
- Nitecki Cz.1972Rozmiary komórek kilku narządów u wybranych gatunków z rzędu Passeriformes. Studia Soc. Sci. Torun. Zool. 9, 143–230 [Google Scholar]
- Promislow D. E.1991The evolution of mammalian blood parameters: patterns and their interpretation. Physiol. Zool. 64, 393–431 [Google Scholar]
- Purvis A.1995A composite estimate of primate phylogeny. Phil. Trans. R. Soc. Lond. B 348, 405–421 (doi:10.1098/rstb.1995.0078) [DOI] [PubMed] [Google Scholar]
- Savage V. M., Allen A. P., Brown J. H., Gillooly J. F., Herman A. B., Woodruff W. H., West G. B.2007Scaling of number, size, and metabolic rate of cells with body size in mammals. Proc. Natl Acad. Sci. USA 104, 4718–4723 (doi:10.1073/pnas.0611235104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder G. K., Sheafor B. A.1999Red blood cells: centerpiece in the evolution of the vertebrate circulatory system. Am. Zool. 39, 189–198 (doi:10.1093/icb/39.2.189) [Google Scholar]
- Starostová Z., Kubička L., Konarzewski M., Kozłowski J., Kratochvíl L.2009Cell size but not genome size affects scaling of metabolic rate in eyelid geckos. Am. Nat. 107, E–article (doi:10.1086/603610) [DOI] [PubMed] [Google Scholar]
- Szarski H.1983Cell size and the concept of wasteful and frugal evolutionary strategies. J. Theor. Biol. 105, 201–209 (doi:10.1016/S0022-5193(83)80002-2) [DOI] [PubMed] [Google Scholar]
- Szarski H.1985Cell size in various vertebrate tissues. Fortsch. Zool. 30, 313–316 [Google Scholar]
- West G. B., Brown J. H., Enquist B. J.1997A general model for the origin of allometric scaling laws in biology. Science 276, 122–126 (doi:10.1126/science.276.5309.122) [DOI] [PubMed] [Google Scholar]