Abstract
Mammalian tooth enamel is often chipped, providing clear evidence for localized contacts with large hard food objects. Here, we apply a simple fracture equation to estimate peak bite forces directly from chip size. Many fossil hominins exhibit antemortem chips on their posterior teeth, indicating their use of high bite forces. The inference that these species must have consumed large hard foods such as seeds is supported by the occurrence of similar chips among known modern-day seed predators such as orangutans and peccaries. The existence of tooth chip signatures also provides a way of identifying the consumption of rarely eaten foods that dental microwear and isotopic analysis are unlikely to detect.
Keywords: dietary reconstruction, hominid, dentition, fracture
1. Introduction
Diet is intricately tied to almost all aspects of an organism's lifestyle. Palaeontologists trying to reconstruct the behaviour of fossil species benefit greatly from knowing what the members of those species were eating. However, the tools currently available for dietary reconstruction are few, and all have limitations. Tooth chipping analysis is a new methodology that can provide immediate information on diet, both in the types of foods eaten and in the forces used to process those foods.
Tooth enamel is a brittle material that is easily chipped at high loads. Such chipping results when a large hard object forcefully contacts the tooth near an occlusal edge (Chai & Lawn 2007). Small hard objects do not cause chipping, instead inducing microscale plastic deformation in the enamel at the points of contact (Lucas et al. 2008). Neither do soft objects cause chipping because they deform around the tooth, smothering it within a compressive contact stress field. Accordingly, chipping instantly reveals a history of large hard food objects in the diet. Chipping can also provide quantitative information on bite forces from routine measurements of chip and tooth dimensions in conjunction with a simple fracture equation. Traditionally, bite forces for fossil species have been estimated from analyses of jaw musculature and lever arms (Demes & Creel 1988; Rayfield et al. 2001; Wroe et al. 2005). However, such analysis is feasible only for those rare fossils with reasonably well-preserved crania.
Studies going back over 50 years have noted a link between chipping and past oral activity among early hominin taxa (Robinson 1954; Tobias 1967; Wallace 1973). However, the precise link between foods and enamel chipping has remained unclear, and there has never been any attempt to use chipping to acquire a quantitative measure of bite force. In this study, we revisit fossil hominins in order to demonstrate the potential utility of chipping analysis. Comparative analysis on certain species of great apes, monkeys and peccaries—extant hard-food eaters with similar tooth morphology to hominins (Kiltie 1982)—highlights a certain universality of the concept.
2. Material and methods
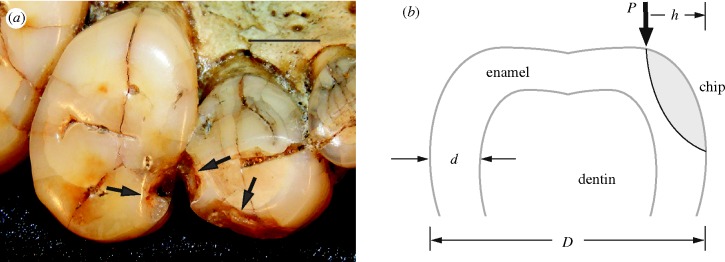
We obtained photographs of hominin, ape, monkey and peccary enamel chips from original specimens or casts of museum specimens (see the electronic supplementary material). Chip sizes h from the tooth side wall and tooth base diameters D were measured from these photographs (figure 1) to the nearest 0.03 mm. Consideration was given only to antemortem chips (i.e. those with smoothed edges, indicative of wear occurring subsequent to the fracture event). Chip size data are tabulated in the electronic supplementary material. Critical loads for chip formation were then estimated from the relation (Chai & Lawn 2007)
| 2.1 |
where T′ is a coefficient proportional to material ‘toughness’ (intrinsic resistance to crack growth). We used data from failure tests on modern human enamel to calibrate this relation via a linear least squares best fit of PF versus h data to obtain T′ = 9.3 MN m−3/2 (see the electronic supplementary material).
Figure 1.
(a) Photograph of antemortem chips on teeth of Paranthropus robustus. (b) Schematic of enamel chip geometry. Scale bar, (a) 4 mm.
For comparison, we turned to an earlier study by Demes & Creel (1988) of maximum possible bite forces Pjaw from jaw mechanics of great apes and fossil hominins. Their analysis produced ‘bite force equivalents’ from an analysis of jaw muscle sizes and lever arms. Following Demes & Creel, we converted these equivalents to absolute forces by calibrating proportionality factors from the average maximum bite forces for male and female Macaca fascicularis from Hylander (1979). This procedure yielded maximum estimated bite forces of 720 and 510 N for human males and females, a little below estimates for human first molars using ‘soft’ bite force gauges (Braun et al. 1995) but within a broad range of values from other methods of bite force estimation (Hylander 1977).
3. Results
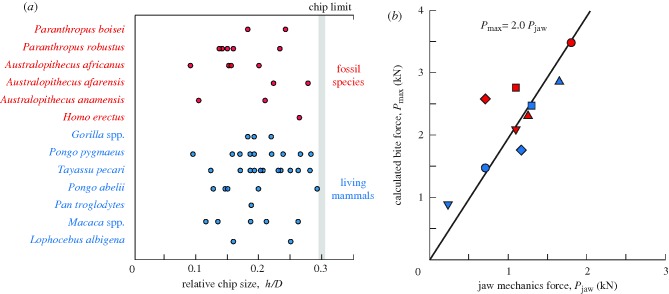
Individual chip/tooth sizes h/D for the various living and fossil species are plotted in figure 2a (see also table S1). Note the wide range of values for each species. Clearly, the chip location must lie within the range 0 < h/D < 0.5. Our data lie within h/D < 0.3, indicating a practical chip limit in normal dental function. Higher h/D would cause the starter cracks to arrest at the dentine and, at ultra-high loads, to split the tooth. Substitution of h/D = 0.3 into equation (2.1) provides a corresponding limiting bite force
| 3.1 |
Figure 2.
(a) Plot of chip dimension h relative to tooth diameter D for a variety of living species and fossil hominins. Values of h and D are from individual chips (excluding small chips, h < 0.1 mm). Note upper limit h/D ≈ 0.30. (b) Plot of maximum chipping force Pmax from equation (3.1) using averaged molar diameter D versus Pjaw from jaw mechanics. Solid line is a least squares best fit (red circle, P. boisei; red square, P. robustus; red triangle, A. afarensis; red diamond, A. africanus; inverted triangle, Homo erectus; blue circle, Homo sapiens; blue triangle, Gorilla spp.; blue square, Pongo spp.; blue diamond, Pan troglodytes; blue inverted triangle, Macaca spp.).
Table 1 compares bite forces Pmax calculated from equation (3.1) for fossil hominins as well as for extant primates and peccaries, using averaged molar sizes D (Demes & Creel 1988), with PF calculated from equation (2.1), using the highest measured h (see table S1).
Table 1.
Maximum bite forces for fossil hominins and select extant mammals.
| taxon | maximum bite force (N) |
|
|---|---|---|
| equation (2.1) | equation (3.1) | |
| fossil hominins | ||
| Paranthropus boisei | 1751 | 3471 |
| Paranthropus robustus | 1742 | 2769 |
| Australopithecus africanus | 1253 | 2598 |
| Australopithecus afarensis | 1860 | 2305 |
| Australopithecus anamensis | 1671 | 2120 |
| Homo erectus | 1085 | 2075 |
| extant mammals | ||
| Gorilla spp. | 1693 | 2865 |
| Tayassu pecari | 2024 | 2280 |
| Pongo spp. | 2036 | 2460 |
| Pan troglodytes | 1086 | 1766 |
| Homo sapiens | 970 | 1470 |
| Macaca spp. | 624 | 875 |
| Lophocebus albigena | 601 | 660 |
Values of Pmax calculated from equation (3.1) are plotted in figure 2b versus estimates of maximum bite force Pjaw from the Demes & Creel (1988) study of jaw mechanics. The straight line through the data is a least squares best fit, yielding Pmax = 2.0Pjaw, so the value from chip size is somewhat higher than that from jaw mechanics. Notwithstanding this difference in values, the linear correlation confirms the usefulness of equation (3.1) in providing relative estimates of maximum bite forces in fossil species from measured tooth sizes.
4. Discussion
The analysis of enamel chips adds a simple but powerful new tool for evaluating jaw performance and reconstructing diet. The technique is valid for any dentate fossil or living vertebrate (see the electronic supplementary material) and provides estimates of bite forces commensurate with other techniques. In this context, it may be noted that values from the jaw mechanics analysis of Demes & Creel may be underestimates because of simplifications in the underlying two-dimensional lever mechanics (Thomason 1991; Ellis et al. 2008). Independent food fracture experiments suggest that orangutans are capable of cracking open macadamia nuts at forces over 2 kN (Lucas et al. 1994, 2009), which compares with Pmax = 2.5 kN calculated from equation (3.1) for a mean molar size of 14.0 mm for this animal. Similarly, peccaries are capable of fracturing Iriartea ventricosa palm nuts at Pmax = 3.4 kN (Kiltie 1982), which compares with a value 2.3 kN calculated for a measured mean molar size 13.3 mm. The relatively high incidence of chipping on the teeth of orangutans and peccaries (table 2) and many fossil hominins visually confirms the consumption of large hard foods and the use of high bite forces to process these foods. A lower incidence of chipping on gorilla and chimpanzee teeth suggests that these animals process such foods less frequently. Our method also circumvents the requirement of a nearly complete fossil cranium to obtain an estimate of bite force. All that is needed is a few teeth with well-formed chips.
Table 2.
Relative chip frequencies in living taxa.
| taxon | % individuals with one or more chips |
|---|---|
| Pongo abelii | 16.0 |
| Tayassu pecari | 10.8 |
| Lophocebus albigena | 10.0 |
| Pongo pygmaeus | 7.1 |
| Macaca spp. | 7.1 |
| Gorilla spp. | 3.9 |
| Pan troglodytes | 2.2 |
Tooth chipping can provide information about diet that other methods tend to miss. For example, the hard objects that cause chipping are orders of magnitude larger than objects recordable in dental microwear (Lucas 2004; Lucas et al. 2008). Also, because chipping patterns are permanent, they can reveal the practice of hard-object feeding even if it was a rare event, as is the case with many fallback foods (Marshall & Wrangham 2007; Constantino & Wright 2009). Such incidences may be missed by microwear analysis, in which wear features are rapidly replaced (Grine & Kay 1988; Teaford & Oyen 1989), or by isotopic analysis where the signal from rarely eaten foods can be masked by that from more commonly eaten items (Sponheimer & Lee-Thorp 1999; Yeakel et al. 2007). Interestingly, postcanines of the robust australopith Paranthropus boisei show enamel chips but no evidence of hard objects in their dental microwear (Ungar et al. 2008).
We can go further and analyse the intrinsic resistance to fracture of mammalian dentition in terms of tooth morphology. Almost certainly, such resistance is subject to selective pressure, with tooth dimensions optimized to enable maximum efficiency in food processing (Lucas 2004). We have already indicated how the maximum sustainable bite force Pmax scales with tooth size D in equation (3.1). Another important tooth dimension is enamel thickness d. Teeth with thin enamel may fracture by a mode other than chip spallation, e.g. by longitudinal propagation of ribbon-like radial and margin cracks around the side walls from the occlusal surface to the margin (or vice versa) (Chai et al. 2009; Lawn & Lee 2009; Lee et al. 2009). Such modes are favoured at contact locations h in excess of 0.30D. Longitudinal cracks of this kind are less likely to leave a visible imprint on the tooth surface. Nevertheless, they weaken the tooth structure, and in extreme cases can lead to penetration through the dentine with consequent tooth splitting. Tooth size and enamel thickness may therefore have evolved in part to afford protection against potentially catastrophic effects of fracture in the eating of hard food objects.
Acknowledgements
We thank Bernard Wood and Fred Grine for access to their hominin cast collections, Linda Gordon, Colin Menter, and Stephany Potze for access to specimens in their care, and Ashley Hammond and Carol Ward for providing images of Australopithecus anamensis teeth. Human teeth were supplied by the Paffenbarger Research Center. This work was supported by the National Science Foundation (grant 0851351 to PWL, PJC, JJWL, and BRL; and 0725122 to PWL), the George Washington University Research Enhancement Fund (to PJC), the Israeli Science Foundation (to HC), and the Palaeontological Scientific Trust (to BZ).
References
- Braun S., Bantleon H. P., Hnat W. P., Freudenthaler J. W., Marcotte M. R., Johnson B. E.1995A study of bite force, part 1: relationship to various physical characteristics. Angle Orthod. 65, 367–372 [DOI] [PubMed] [Google Scholar]
- Chai H., Lawn B. R.2007A universal relation for edge chipping from sharp contacts in brittle materials and its use as a simple means of toughness evaluation. Acta Mater. 55, 2555–2561 (doi:10.1016/j.actamat.2006.10.061) [Google Scholar]
- Chai H., Lee J. J.-W., Kwon J.-Y., Lucas P. W., Lawn B. R.2009A simple model for enamel fracture from margin cracks. Acta Biomater. 5, 1663–1667 (doi:10.1016/j.actbio.2008.11.007) [DOI] [PubMed] [Google Scholar]
- Constantino P. J., Wright B. W.2009The importance of fallback foods in primate ecology and evolution. Am. J. Phys. Anthropol. 140, 599–602 (doi:10.1002/ajpa.20978) [DOI] [PubMed] [Google Scholar]
- Demes B., Creel N.1988Bite force, diet, and cranial morphology of fossil hominids. J. Hum. Evol. 17, 657–670 (doi:10.1016/0047-2484(88)90023-1) [Google Scholar]
- Ellis J. L., Thomason J. J., Kebreab E., France J.2008Calibration of estimated biting forces in domestic canids: comparison of post-mortem and in vivo measurements. J. Anat. 212, 769–780 (doi:10.1111/j.1469-7580.2008.00911.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grine F. E., Kay R. F.1988Early hominid diets from quantitative image analysis of dental microwear. Nature 333, 765–768 (doi:10.1038/333765a0) [DOI] [PubMed] [Google Scholar]
- Hylander W. L.1977. In The adaptive significance of Eskimo craniofacial morphology (eds Dahlberg A., Graber T. M.), pp. 129–169 Paris, France: Orofacial Growth and Development. Mouton Publishers [Google Scholar]
- Hylander W. L.1979Mandibular function in Galago crassicaudatus and Macaca fascicularis: an in vivo approach to stress analysis of the mandible. J. Morphol. 159, 253–296 (doi:10.1002/jmor.1051590208) [DOI] [PubMed] [Google Scholar]
- Kiltie R. A.1982Bite force as a basis of niche differentiation between rain forest peccaries (Tayassu tajacu and T. pecari). Biotropica 14, 188–195 (doi:10.2307/2388025) [Google Scholar]
- Lawn B. R., Lee W.2009Analysis of fracture and deformation modes in teeth subjected to occlusal loading. Acta Biomater. 5, 2213–2221 (doi:10.1016/j.actbio.2009.02.001) [DOI] [PubMed] [Google Scholar]
- Lee J. J.-W., Kwon J.-Y., Chai H., Lucas P. W., Thompson V. P., Lawn B. R.2009Fracture modes in human teeth. J. Dent. Res. 88, 224–228 [DOI] [PubMed] [Google Scholar]
- Lucas P. W.2004Dental functional morphology: how teeth work. Cambridge, UK: Cambridge University Press [Google Scholar]
- Lucas P. W., Peters C. R., Arrandale S. R.1994Seed-breaking forces exerted by orang-utans with their teeth in captivity and a new technique for estimating forces produced in the wild. Am. J. Phys. Anthropol. 94, 365–378 (doi:10.1002/ajpa.1330940306) [DOI] [PubMed] [Google Scholar]
- Lucas P., Constantino P., Wood B., Lawn B.2008Dental enamel as a dietary indicator in mammals. Bioessays 30, 374–385 (doi:10.1002/bies.20729) [DOI] [PubMed] [Google Scholar]
- Lucas P. W., et al. 2009Indentation as a technique to assess the mechanical properties of fallback foods. Am. J. Phys. Anthropol. 140, 643–652 (doi:10.1002/ajpa.21026) [DOI] [PubMed] [Google Scholar]
- Marshall A. J., Wrangham R. W.2007Evolutionary consequences of fallback foods. Int. J. Primatol. 28, 1219–1235 (doi:10.1007/s10764-007-9218-5) [Google Scholar]
- Rayfield E. J., Norman D. B., Horner C. C., Horner J. R., Smith P. M., Thomason J. J., Upchurch P.2001Cranial design and function in a large theropod dinosaur. Nature 409, 1033–1037 (doi:10.1038/35059070) [DOI] [PubMed] [Google Scholar]
- Robinson J. T.1954Prehominid dentition and hominid evolution. Evolution 8, 324–334 (doi:10.2307/2405779) [Google Scholar]
- Sponheimer M., Lee-Thorp J. A.1999Isotopic evidence for the diet of an early hominid, Australopithecus africanus. Science 283, 368–370 (doi:10.1126/science.283.5400.368) [DOI] [PubMed] [Google Scholar]
- Teaford M. F., Oyen O. J.1989In vivo and in vitro turnover in dental microwear. Am. J. Phys. Anthropol. 80, 447–460 (doi:10.1002/ajpa.1330800405) [DOI] [PubMed] [Google Scholar]
- Thomason J. J.1991Cranial strength in relation to estimated biting forces in some mammals. Can. J. Zool. 69, 2326–2333 (doi:10.1139/z91-327) [Google Scholar]
- Tobias P. V.1967The cranium and maxillary dentition of Australopithecus (Zinjanthropus) boisei. Olduvai Gorge. Cambridge, UK: Cambridge University Press [Google Scholar]
- Ungar P. S., Grine F. E., Teaford M. F.2008Dental microwear and diet of the Plio-Pleistocene hominin Paranthropus boisei. PLoS ONE 3, e2044 (doi:10.1371/journal.pone.0002044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. A.1973Tooth chipping in the australopithecines. Nature 244, 117–118 (doi:10.1038/244117a0) [DOI] [PubMed] [Google Scholar]
- Wroe S., McHenry C., Thomason J.2005Bite club: comparative bite force in big biting mammals and the prediction of predatory behaviour in fossil taxa. Proc. R. Soc. B 272, 619–625 (doi:10.1098/rspb.2004.2986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeakel J. D., Bennett N. C., Koch P. L., Dominy N. J.2007The isotopic ecology of African mole rats informs hypotheses on the evolution of human diet. Proc. R. Soc. B 274, 1723–1730 (doi:10.1098/rspb.2007.0330) [DOI] [PMC free article] [PubMed] [Google Scholar]