Abstract
Measuring individual quality in vertebrates is difficult. Focusing on allostasis mechanisms may be useful because they are functionally involved in the ability of an individual to survive and reproduce in its environment. Thus, a rise in stress hormones levels (corticosterone) occurs when an organism has to cope with challenging environmental conditions. This has recently led to the proposal of the ‘cort–fitness hypothesis’, which suggests that elevated baseline corticosterone levels should be found in individuals of poor quality that have difficulty coping with their environment. We tested this hypothesis by comparing an integrative measure of individual quality to baseline corticosterone in black-browed albatrosses (Thalassarche melanophrys). We found that individual baseline corticosterone levels were related to individual quality and highly repeatable from one breeding season to the next. Importantly, this relationship was found in males, but not in females. Therefore, we suggest that the relationship between quality and baseline corticosterone levels may depend on the environmental and energetic constraints that individuals have to cope with.
Keywords: corticosterone, fitness, quality, albatross
1. Introduction
In vertebrates, a small proportion of individuals usually has a high fitness and produces the majority of surviving offspring. Most of the others survive less well and show poor reproductive performances (Wilson & Nussey 2010). Functionally, individual quality is related to the fitness of individuals and therefore, to their ability to cope with a changing environment. However, measuring such ability is difficult because it depends on many behavioural and physiological traits that covary in complex ways (Ricklefs & Wikelski 2002; Wilson & Nussey 2010). Allostasis, therefore, deserves specific attention because it is involved in the regulation of all these traits and, thus, is functionally involved in the ability of an organism to cope with its environment (McEwen & Wingfield 2003; Romero et al. 2009). Allostasis is defined as all the mechanisms that maintain homeostasis through changes in both predictable and unpredictable environments, and, in vertebrates, their main mediators are the glucocorticoid hormones (e.g. corticosterone, Landys et al. 2006). Glucocorticoid levels increase in response to environmental constraints and this rise acts on behaviour and physiology to adjust the phenotype of the organism to the challenging environmental conditions (Ricklefs & Wikelski 2002; Landys et al. 2006). Therefore, elevated glucocorticoid levels have been suggested as a reliable indicator of how an individual copes with its environment (i.e. allostatic load, McEwen & Wingfield 2003; Landys et al. 2006).
These findings have led to the proposal of the ‘cort–fitness hypothesis’, which suggests that, under identical environmental conditions, elevated baseline glucocorticoid levels should be found in individuals of low fitness and, therefore, of poor quality (Bonier et al. 2009). Several studies have tested this hypothesis by relating baseline corticosterone levels to specific fitness estimates, and surprisingly, they have found contrasting results (Bonier et al. 2009). However, all these studies have only used short-term proxies of fitness (yearly survival, annual reproductive success) and, moreover, not all individuals were sampled under identical environmental conditions. Therefore, there is undoubtedly a need to test the ‘cort–fitness hypothesis’ with an integrative measure of individual fitness (Bonier et al. 2009).
In this study, we tested this ‘cort–fitness hypothesis’ (Bonier et al. 2009) by examining for the first time the relationship that links baseline corticosterone levels to an integrative measure of individual quality in a long-lived species, the black-browed albatross (Thalassarche melanophrys). To reduce potential confounding factors, we tested this hypothesis by sampling albatrosses under comparable environmental conditions (at the colony, and immediately before a foraging trip). We predicted that elevated baseline corticosterone levels should be found in individuals of low quality (Bonier et al. 2009). In addition, we tested two underlying assumptions of this hypothesis. First, we examined whether the relationship linking baseline corticosterone levels to individual fitness is repeatable from one breeding season to another and consistent between sexes (assumption 1, ‘repeatability of corticosterone–fitness relationship’, Bonier et al. 2009). Second, we examined whether baseline corticosterone levels were repeatable, a condition necessary to be able to link baseline corticosterone levels to a fitness measurement (assumption 2, ‘repeatability of corticosterone measures’, Bonier et al. 2009).
2. Material and methods
Our study was carried out in January 2004 and 2005 at Kerguelen Island (50°S, 70°E) during the early brooding period. Black-browed albatrosses are philopatric birds with a high survival probability and a low fecundity (one egg per year, Warham 1990). On Kerguelen, the population of albatrosses has been monitored every year since 1976. So, it is possible to follow the breeding performances and return rates of individuals across their entire lives (Rolland et al. 2009).
Birds were captured at the colony by hand off the nest just after their mate relieved them from their brooding duties. We collected blood from the tarsus vein with a syringe within 3 min (n = 73) to measure baseline corticosterone levels. Eight albatrosses were captured in both 2004 and 2005 in order to test the repeatability of baseline corticosterone levels from one breeding season to the next (hypothesis 3). Blood samples were centrifuged, the plasma was decanted, and blood and plasma were stored at −20°C until analyses. Sex and plasma concentrations of corticosterone were determined as previously described (Angelier et al. 2007a).
We estimated individual quality by how many fledged chicks were produced by a given albatross over five breeding seasons (2004–2008, hereafter called ‘quality’). This integrative measure takes into account several components that collectively determine the quality of an albatross: (i) the ability of albatrosses to survive over 5 years; (ii) their ability to attempt breeding in a given year (albatrosses can skip breeding); (iii) their ability to successfully rear a chick. This measure of quality was not correlated with age in our sub-sample of known-aged albatrosses (p > 0.50).
We only focused on experienced breeders (albatrosses that had already bred at least once before 2004) because it is known that inexperienced breeders are less able to survive and to reproduce (Angelier et al. 2007a; Rolland et al. 2009). Including inexperienced breeders could therefore lead to biased estimates of quality if some of the birds monitored, but not all, were inexperienced. We also measured chick productivity over the same period for all individuals (2004–2008), therefore avoiding the bias of monitoring chick productivity over different periods, which are characterized by contrasting environmental conditions.
Analyses were performed with SAS. We used generalized linear models (GLMs) with ‘baseline corticosterone level’ as our dependent variable to test the ‘cort–fitness hypothesis’. We randomly selected one corticosterone value for the birds that were sampled in both 2004 and 2005 to avoid pseudo-replication (final sample size: 65). We tested whether (i) baseline corticosterone levels were explained by individual quality (independent variable: ‘quality’); (ii) the relationship linking individual quality and baseline corticosterone levels differed between years of sampling and sexes (independent variables: ‘year’, ‘sex’; interactions: ‘quality × year’, ‘quality × sex’); (iii) baseline corticosterone levels were repeatable between seasons (n = 8—four males and four females). The repeatability of baseline corticosterone levels was calculated from the variance components derived from a one-way ANOVA (Lessels & Boag 1987).
3. Results
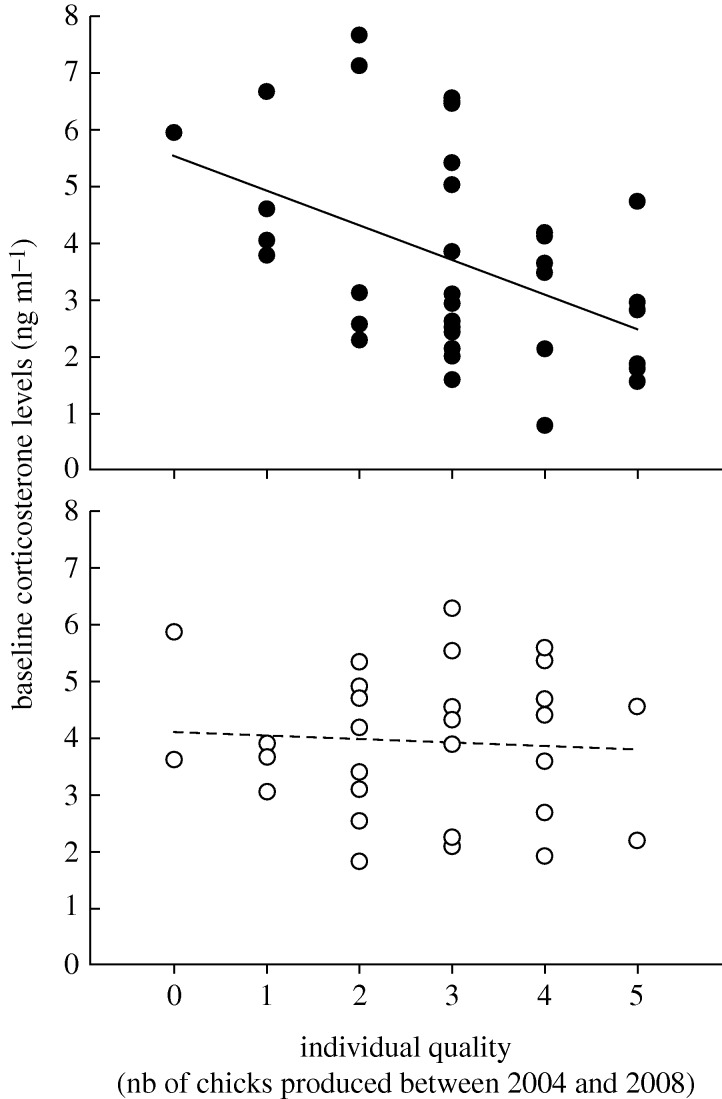
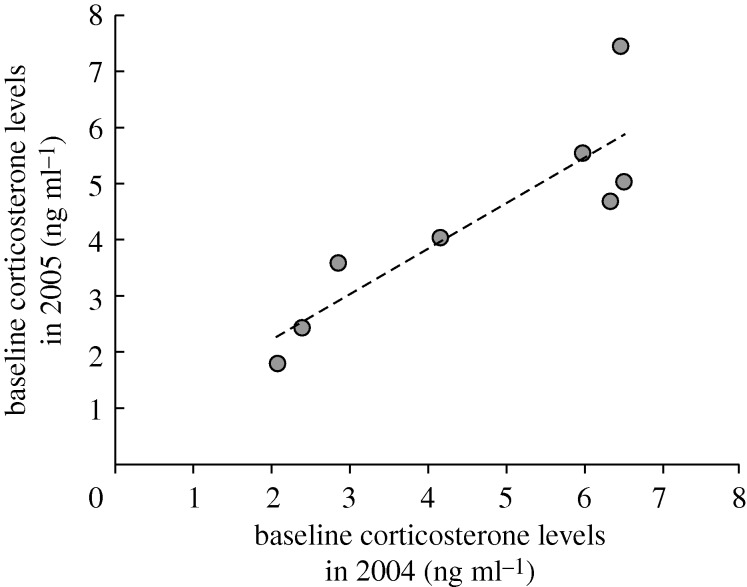
Baseline corticosterone levels did not vary between years (F1,59 = 3.02, p = 0.087) and sexes (F1,59 = 0.12, p = 0.731) but, were highly correlated with individual quality (F1,62 = 7.32, p = 0.009). Moreover, we found a significant effect of the interaction ‘quality × sex’ on baseline corticosterone levels (F1,59 = 4.18, p = 0.045), demonstrating that the relationship linking baseline corticosterone levels and quality differed between sexes (figure 1). Specifically, we found a negative relationship between baseline corticosterone levels and quality in males (χ2 = 10.41, p = 0.001, figure 1), but not in females (χ2 = 0.07, p = 0.791, figure 1). We did not find any significant effect of the interaction ‘quality × year’ on baseline corticosterone levels (GLM, F1,59 = 2.44, p = 0.124), demonstrating that the significant relationship between baseline corticosterone levels and quality did not differ between breeding seasons (figure 1). Baseline corticosterone levels were repeatable from one year to the next (n = 8, r = 0.878; Lessels & Boag 1987). An albatross with a relatively low baseline corticosterone level in 2004 had a similarly low baseline corticosterone level in 2005 (figure 2).
Figure 1.
Individual quality and baseline corticosterone levels in albatrosses. Black and white dots, respectively, indicate males and females. The relationship is highly significant for males, but not for females.
Figure 2.
Repeatability of baseline corticosterone levels from one breeding season to the next.
4. Discussion
Our results support the ‘cort–fitness hypothesis’. We showed for the first time that baseline corticosterone levels are related to an integrative measurement of individual quality in free-living birds (Bonier et al. 2009). Importantly, our protocol avoids several differences in conditions between individuals that could potentially confound individual quality (all birds were experienced, sampled at the colony, immediately before a foraging trip). How can we explain such a result at the functional level? In vertebrates, the maintenance of elevated baseline corticosterone levels over a prolonged period can have deleterious effects on immunity, cognitive abilities and can even induce important metabolic changes (Sapolsky et al. 2000; Landys et al. 2006). In addition, elevated corticosterone levels disrupt the hypothalamo–pituitary–gonadal axis and reduce reproductive effort (Wingfield & Sapolsky 2003). Overall, baseline corticosterone levels can reflect how challenging it is for an organism to deal with its environment: baseline corticosterone levels are elevated when an individual is energetically challenged, and has a high allostatic load (McEwen & Wingfield 2003; Romero et al. 2009). For example, baseline corticosterone levels are elevated when albatrosses fast during a prolonged period or are unable to acquire food at sea (Hector & Harvey 1986; Angelier et al. 2007b). This can explain why elevated baseline corticosterone levels could functionally be associated with high mortality, low breeding frequency, low breeding success and overall poor fitness (Bonier et al. 2009).
Importantly, we found that individual baseline corticosterone levels were highly repeatable from one year to the next. This repeatability of corticosterone levels suggests that the ability of an albatross to cope with the energetic constraints of the brooding period is similar among breeding seasons and baseline corticosterone levels of some individuals therefore appear consistently higher than those of others. This repeatability of baseline corticosterone is a major underlying assumption of the ‘cort–fitness hypothesis’ (Bonier et al. 2009) and it probably explains why we found that the relationship between quality and corticosterone levels did not differ between 2004 and 2005. However, baseline corticosterone levels are not always repeatable in vertebrates (Cockrem et al. 2009), which suggests that it may not always be possible to relate corticosterone levels with individual quality (Bonier et al. 2009).
Supporting a context-dependent relationship between quality and corticosterone (Bonier et al. 2009), baseline corticosterone levels were linked with quality in males, but not in females. The relationship between quality and baseline corticosterone levels is complex and may depend on the environmental and energetic situations with which individuals have to cope (Bonier et al. 2009). Thus, the negative correlation between corticosterone levels and quality is probably most obvious under constraints in which some, but not all, individuals have difficulties coping with their environment. Similarly, this relationship should be least apparent under optimal conditions (high food availability, low energetic demands) because baseline corticosterone levels of most individuals stay low. In that respect, it is essential to note that we sampled albatrosses during the most constraining phase of their breeding cycle (brooding period, Warham 1990) and during two comparable years in terms of environmental conditions (Rolland et al. 2009). Differences between male and female albatrosses could be explained by differential breeding investment, males investing more in chick provisioning than females (Weimerskirch et al. 2000). Although elevated corticosterone levels mirror energetic constraints in vertebrates, corticosterone can also sustain reproductive effort under some circumstances and thus, benefits to fitness of individuals (the ‘cort–adaptation hypothesis’, Bonier et al. 2009). In addition, corticosterone levels do not always increase in response to energetic challenges in vertebrates. All this complexity may also explain why the corticosterone–fitness hypothesis is not supported in female albatrosses (Bonier et al. 2009; Dingemanse et al. 2010). To conclude, future studies should experimentally investigate what energetic factors can affect the ability of corticosterone to predict fitness in wild vertebrates.
Acknowledgements
The present research project (109) was supported by the French Polar Institute (IPEV). F.A. was supported by a Marie-Curie fellowship. We thank F. Le Bouard, L. Denonfoux and D. Hyrenbach for fieldwork assistance.
References
- Angelier F., Weimerskirch H., Dano S., Chastel O.2007aAge, experience and reproductive performance in a long-lived bird: a hormonal perspective. Behav. Ecol. Sociobiol. 61, 611–621 (doi:10.1007/s00265-006-0290-1) [Google Scholar]
- Angelier F., Shaffer S. A., Weimerskirch H., Chastel O.2007bCorticosterone and foraging behavior in a pelagic seabird. Physiol. Biochem. Zool. 80, 283–292 (doi:10.1086/512585) [DOI] [PubMed] [Google Scholar]
- Bonier F., Martin P. R., Moore I. T., Wingfield J. C.2009Do baseline glucocorticoid predict fitness? Trends Ecol. Evol. 24, 634–642 (doi:10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- Cockrem J. F., Barette D. P., Candy E. J., Potter M. A.2009Corticosterone responses in birds: individual variation and repeatability in Adelie penguins (Pygoscelis adeliae) and other species, and the use of power analysis to determine sample sizes. Gen. Comp. Endocrinol. 163, 158–168 (doi:10.1016/j.ygcen.2009.03.029) [DOI] [PubMed] [Google Scholar]
- Dingemanse N. J., Edelaar P., Kempenaers B.2010Why is there variation in baseline glucocorticoid levels? Trends Ecol. Evol. 25, 261–262 (doi:10.1016/j.tree.2010.01.008) [DOI] [PubMed] [Google Scholar]
- Hector J. A. L., Harvey S.1986Corticosterone secretion through long incubation shifts in Diomedea albatrosses. Gen. Comp. Endocrinol. 62, 349–352 (doi:10.1016/0016-6480(86)90043-2) [DOI] [PubMed] [Google Scholar]
- Landys M. M., Ramenofsky M., Wingfield J. C.2006Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 148, 132–149 (doi:10.1016/j.ygcen.2006.02.013) [DOI] [PubMed] [Google Scholar]
- Lessels C. M., Boag P. T.1987Unrepeatable repeatabilities—a common mistake. Auk 104, 116–121 [Google Scholar]
- McEwen B. S., Wingfield J. C.2003The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15 (doi:10.1016/S0018-506X(02)00024-7) [DOI] [PubMed] [Google Scholar]
- Ricklefs R., Wikelski M.2002The physiology/life history nexus. Trends Ecol. Evol. 17, 462–468 (doi:10.1016/S0169-5347(02)02578-8) [Google Scholar]
- Rolland V., Nevoux M., Barbraud C., Weimerskirch H.2009Respective impact of climate and fisheries on the growth of an albatross population. Ecol. Appl. 19, 1336–1346 (doi:10.1890/08-1060.1) [DOI] [PubMed] [Google Scholar]
- Romero L. M., Dickens M. J., Cyr N. E.2009The reactive scope model—a new model integrating homeostasis, allostasis and stress. Horm. Behav. 55, 375–389 (doi:10.1016/j.yhbeh.2008.12.009) [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Romero L. M., Munck A. U.2000How do glucocorticoids influence stress responses: integrating permissive, suppressive, stimulatory, and preparative actions. Endocrinol. Rev. 21, 55–89 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- Warham J.1990The petrels: their ecology and breeding systems. San Diego, CA: Academic Press [Google Scholar]
- Weimerskirch H., Barbraud C., Lys P.2000Sex differences in parental investment and chick growth in wandering albatrosses: fitness consequences. Ecology 81, 309–318 (doi:10.1890/0012-9658(2000)081[0309:SDIPIA]2.0.CO;2) [Google Scholar]
- Wilson A. J., Nussey D. H.2010What is individual quality? An evolutionary perspective. Trends Ecol. Evol. 25, 207–214 (doi:10.1016/j.tree.2009.10.002) [DOI] [PubMed] [Google Scholar]
- Wingfield J. C., Sapolsky R. M.2003Reproduction and resistance to stress: when and how? J. Neuroendocrinol. 15, 711–724 [DOI] [PubMed] [Google Scholar]