Abstract
Fig wasps and fig trees are mutually dependent, with each of the 800 or so species of fig trees (Ficus, Moraceae) typically pollinated by a single species of fig wasp (Hymenoptera: Agaonidae). Molecular evidence suggests that the relationship existed over 65 Ma, during the Cretaceous. Here, we record the discovery of the oldest known fossil fig wasps, from England, dated at 34 Ma. They possess pollen pockets that contain fossil Ficus pollen. The length of their ovipositors indicates that their host trees had a dioecious breeding system. Confocal microscopy and scanning electron microscopy reveal that the fossil female fig wasps, and more recent species from Miocene Dominican amber, display the same suite of anatomical characters associated with fig entry and pollen-carrying as modern species. The pollen is also typical of modern Ficus. No innovations in the relationship are discernible for the last tens of millions of years.
Keywords: Agaonidae; amber; coevolution; Ficus, mutualism; pollination
1. Introduction
Fig trees are keystone elements of many tropical forests, where more species of mammals and birds feed on figs than any other fruits (Shanahan et al. 2001). This reflects their abundance, diversity and an unusual phenology that supports their host specific pollinator fig wasps. Ficus have a unique inflorescence, the fig, formed like a hollow ball lined by numerous tiny flowers. Pollination is reliant on female fig wasps which crawl through a narrow bract-lined ostiole to lay their eggs in the ovules, which they gall and pollinate. Female fig wasps display morphological adaptations for fig entry that include mandibular appendages and robust legs to push them through the ostiole, a flattened head and hooked antennae (Frank 1984). Ovipositor lengths reflect host style lengths (Nefdt & Compton 1996).
Pollination is either passive or active, whereby pollen is loaded and transported in mesothoracic pollen baskets (Kjellberg et al. 2001). Active pollination involves the collection, storage and subsequent dispersal of pollen, and its improved efficiency means that host plants need to produce much less pollen (Jousselin & Kjellberg 2001). In monoecious fig trees, wasps and seeds develop in the same figs, whereas in more derived, functionally dioecious species, male trees produce only wasps, female trees only seeds.
Molecular analyses (Rønsted et al. 2005; Lopez-Vaamonde et al. 2009) suggest that the trees and wasps have co-radiated since the late Cretaceous (100–65 Myr ago). Fossil fig wasps are known previously only from Dominican amber (early Miocene, 23–16 Myr ago; Peñalver et al. 2006) and belong to the modern genera Pegoscapus and Tetrapus. An earlier putative fig wasp from Colorado (Tetrapus mayri, Brues 1910), does not display any agaonid characteristics (Lopez-Vaamonde et al. 2009).
Ficus fruits are known from the Eocene and Oligocene of Europe, but all records of putative fossil Ficus leaves or figs have now been rejected (Collinson 1989; Givulescu 1994; Mai & Walther 2000). This includes one leaf from the Isle of Wight Insect Limestone that we examined and consider incertae sedis. Individual Ficus pollen is severely under-represented even in modern dispersed pollen assemblages (Bush & Riviera 2001), reflecting the specialized pollination mechanism. Its small size (typically ca 9–15 µm by 6–9 µm) also makes recognition difficult.
2. Material and methods
An INTAS-funded project to survey the fauna and flora of the Bembridge Marls, Isle of Wight, UK, led to the late Dr Mikhail Kozlov discovering that the holotype of the ‘ant’ ‘Ponera’ minuta (Donisthorpe 1920) in the Natural History Museum, London (NHM I.9734) was a possible fig wasp (figure 1a). Two additional specimens from the same locality were found by A.J.R. in the Sedgwick Museum, Cambridge (X50140.47a and X.50140.97c). Examination has confirmed that they are typical agaonids, with flattened heads, mandibular appendages and characteristic legs. They constitute one species of a new genus to be described elsewhere. The fig wasps come from the Insect Limestone bed, which is late Eocene (34 Ma) in age (Hooker et al. 2009), a period when climates were warmer than today. They are the oldest known representatives of the Agaonidae. Typically for this bed, they are preserved in three dimensions in extremely fine-grained limestone (McCobb et al. 1998). Organic cuticle is well preserved, showing original structures at the micron scale.
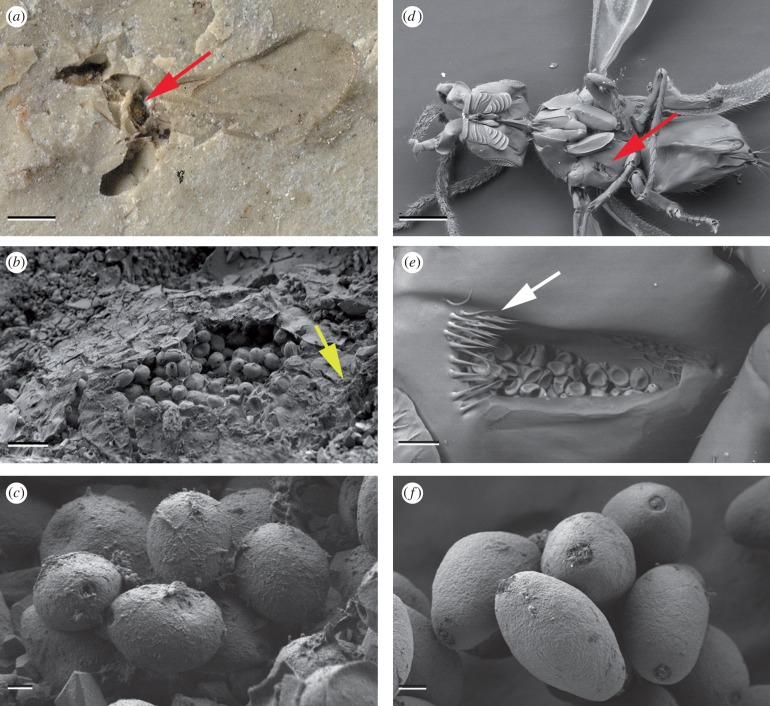
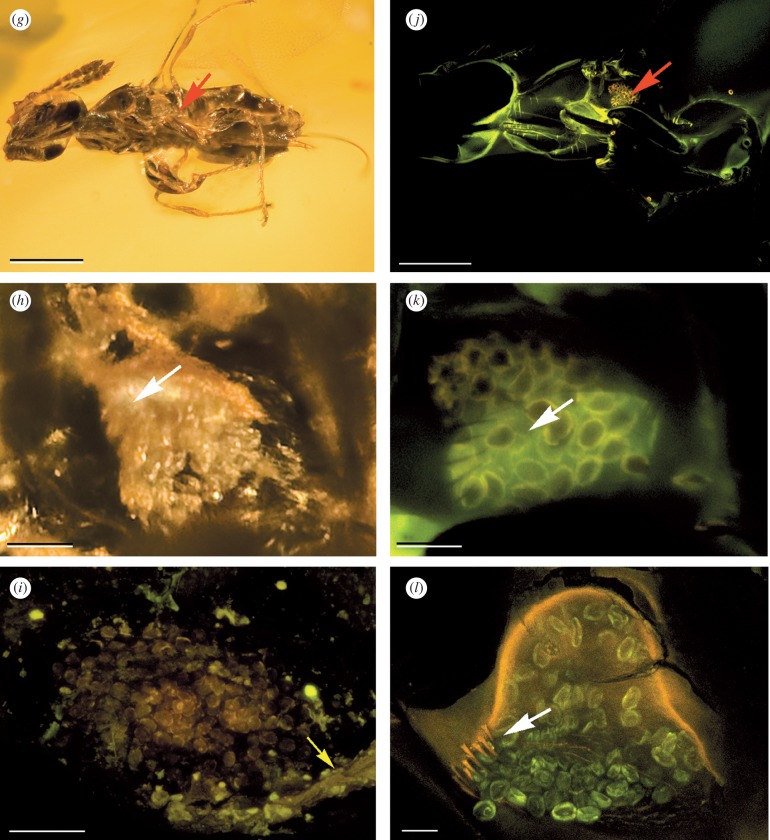
Figure 1.
Fossil and recent fig wasps, their pollen pockets and Ficus pollen. (a) Macrophotograph of the holotype of ‘Ponera’ minuta, NHM I.9734, Eocene, Insect Limestone, Isle of Wight, England. The specimen is viewed from the left side, the pollen pocket is indicated by the red arrow (scale bar, 500 µm). (b) SEM of pollen pocket from (a). Yellow arrow is for orientation with (i) (scale bar, 20 µm). (c) SEM of fossil pollen within pollen pocket in (b)—small, isopolar, psilate, oblate, diporate, with no annulus or operculum to pores, like modern Ficus (scale bar, 2 µm). (d) SEM ventral view of a modern fig wasp (Liporrhopalum tentacularis (Grandi)) showing the position of the pollen pocket (red arrow; scale bar, 200 µm). (e) SEM of the right pollen pocket from (d) showing the guard hairs (arrowed) and pollen grains (scale bar, 20 µm). (f) SEM of pollen grains isolated from a modern Ficus species (F. montana Burm. f.) grown at the Leeds University Experimental Gardens, sourced from Bogor, Indonesia, showing that they are essentially identical to the pollen grains from the Eocene Insect Limestone specimen (scale bar, 2 µm). (g) Macrophotograph of Pegoscapus cf. peritus, NMH Pal.II.3039, Miocene Dominican amber. The specimen is viewed from the lower left, showing the left pollen pocket (arrowed; scale bar, 250 µm). (h) Macrophotograph of the left pollen pocket from (g). The arrow indicates the position of the guard hairs (scale bar, 50 µm). (i) CLSM image of the pollen pocket from (b) ‘Ponera’ minuta, Eocene Insect Limestone. The arrow is for orientation and points to the same ridge indicated in (b) (scale bar, 50 µm). (j) CLSM image taken from the same orientation as (g), Pegoscapus cf. peritus, Miocene Dominican amber. Note the clearly visible pollen grains in the pollen pocket (arrowed; scale bar, 200 µm). (k) CLSM image detail from (j) showing the sub-cuticular pollen pocket, pollen grains and guard hairs (arrowed; scale bar, 25 µm). (l) CLSM image of a modern fig wasp, Liporrhopalum tentacularis showing the subcuticular pollen pocket, pollen grains and guard hairs (arrowed; scale bar, 25 µm).
Light microscopy, scanning electron microscopy (SEM) and confocal microscopy (CLSM) were employed to examine their anatomy and to compare it with Miocene fig wasps preserved in Dominican amber (housed in the NHM) and modern species.
3. Results
Two specimens from the Insect Limestone have golden-yellow tetragonal regions visible on the mesothorax, the area where pollen pockets of Recent agaonids are located. SEM of the regions revealed numerous organic oblate grains with a pore at either end (figure 1b). Their size and morphology (compare figure 1c and f) identifies them as Ficus pollen (small, isopolar, psilate, oblate, diporate, with no annulus or operculum to pores (Burn & Mayle 2008); terminology sensu, Punt et al. 2007). This confirms that these ancient fig wasps had already evolved pollen pockets by 34 Ma and were actively pollinating their host Ficus.
Examination of Miocene amber specimen NHM Pal.II.3039 (Pegoscapus cf. peritus; Peñalver et al. 2006) confirmed that, like its modern congeners, it possesses pollen pockets (figure 1g,h). CLSM (figure 1j) shows that its pollen pocket anatomy (figure 1k) is functionally identical to those of an example modern species, Liporrhopalum tentacularis (Grandi) (figure 1d,e,l). The pockets of both comprise a relatively narrow open section guarded by a row of stout setae, where pollen is inserted using the front legs, and a lateral compartment extending underneath the cuticle (figure 1e,h,k,l—arrowed). CLSM of the pollen pocket of the English fossil I.9734 (figure 1i) shows it is comparable, though no setae are visible since the pollen pocket is viewed from the interior of the wasp in this specimen.
One of the English Insect Limestone fossils has the protruding section of the ovipositor (the sheaths or third valvulae) clearly visible and undamaged. The exserted ovipositor to gaster length ratio is 0.4, which is typical of modern fig wasps associated with dioecious figs, but outside the range for monoecious figs (table 1). Flowers in monoecious figs have longer styles than those in male figs of dioecious species and in response the pollinators of monoecious species have relatively longer ovipositors (Nefdt & Compton 1996). The shorter ovipositor in the English fossils shows that the host of ‘Ponera’ minuta was dioecious, a major innovation in Ficus evolution.
Table 1.
The lengths of exserted ovipositor sheaths (third valvulae) in relation to gaster (metasoma) length in modern species of fig wasps (Agaonidae). (Pollinators of monoecious species have relatively longer ovipositors, as is particularly evident in Ceratosolen subgenus Ceratosolen, where species pollinate hosts with both breeding systems. Based on published measurements (Grandi 1925; Berg & Wiebes 1992; Wiebes 1994, 2005).)
| fig wasp genera (and subgenera) | n (species) | ratios of exserted ovipositor : gaster length |
||
|---|---|---|---|---|
| minimum | maximum | mean | ||
| monoecious hosts | ||||
| Agaon | 8 | 1.0 | 1.4 | 1.19 |
| Alfonsiella | 6 | 0.9 | 1.1 | 1.00 |
| Courtella | 12 | 0.85 | 1.75 | 1.06 |
| Deilagaon | 4 | 1.10 | 4.0 | 2.15 |
| Dolichoris | 8 | 1.25 | 2.0 | 1.61 |
| Elisabethiella | 12 | 1.0 | 2.0 | 1.40 |
| Eupristina | 9 | 1.25 | 2.0 | 1.64 |
| Nigeriella | 4 | 1.0 | 2.0 | 1.17 |
| Paragaon | 2 | 1.5 | 1.5 | 1.50 |
| Pegoscapus | 25 | 0.7 | 1.75 | 1.21 |
| Platyscapa | 4 | 0.9 | 1.5 | 1.15 |
| Pleistodontes | 15 | 0.6 | 2.0 | 1.12 |
| Tetrapus | 1 | — | — | 1.14 |
| Waterstoniella | 15 | 1.0 | 3.5 | 1.92 |
| Ceratosolen (Ceratosolen) | 9 | 0.7 | 1.5 | 1.12 |
| dioecious hosts | ||||
| Blastophaga | 1 | — | — | 0.25 |
| Kradibia | 17 | 0.17 | 0.67 | 0.41 |
| Liporrhopalum | 10 | 0.20 | 0.40 | 0.28 |
| Wiebesia | 13 | 0.05 | 0.40 | 0.20 |
| Ceratosolen (Rothropus) | 12 | 0.05 | 0.23 | 0.08 |
| Ceratosolen (Strepitus) | 3 | 0.40 | 0.75 | 0.63 |
| Ceratosolen (Ceratosolen) | 16 | 0.05 | 0.60 | 0.29 |
4. Discussion
The 34 Ma fig wasps are almost indistinguishable from modern species. They display the same range of adaptations for fig entry, they possess pollen pockets loaded with pollen, confirming that they were actively collecting and storing Ficus pollen, and their short ovipositors show that they pollinated dioecious hosts. We conclude that the key co-adaptive features of the fig tree and fig wasp mutualism were already in place 34 Ma, and perhaps much earlier. The extreme specificity of the Ficus pollination system may have facilitated the speciation evidenced by the hundreds of modern species (Schiestl & Schlüter 2009), but this mutualism displays no recent innovations. Other selective pressures, most obviously dramatic changes over time in the vertebrates that disperse figs, may have driven the diversity among Ficus species that we see today (Herrera 1985).
Acknowledgements
We thank P. Crabb (NHM) and H. Taylor (NHM) for photography and C. Mellish (NHM) for general help. A.J.R., A.P.R., M.E.C. and P.H. acknowledge INTAS for financial support.
References
- Berg C. C., Wiebes J. T.1992African fig trees and fig wasps. Amsterdam, The Netherlands: Koninklijke Nederlandse Akademie Van Wetenschappen [Google Scholar]
- Brues C. T.1910The parasitic Hymenoptera of the tertiary of Florissant, Colorado. Bull. Mus. Comp. Zoo. Harvard 54, 1–12 [Google Scholar]
- Burn M. J., Mayle F. E.2008Palynological differentiation between genera of the Moraceae family and implications for Amazonian palaeoecology. Rev. Palaeobot. Palynol. 149, 187–201 (doi:10.1016/j.revpalbo.2007.12.003) [Google Scholar]
- Bush M. B., Riviera R.2001Reproductive ecology and pollen representation among neotropical trees. Global Ecol. Biogeog. 10, 359–367 (doi:10.1046/j.1466-822X.2001.00247.x) [Google Scholar]
- Collinson M. E.1989The fossil history of the Moraceae, Urticaceae (including Cecropiaceae) and Cannabaceae. In Evolution, systematics and fossil history of the Hamamelidae, volume 2 ‘Higher Hamamelidae’ Systematics association special volume (eds Crane P. R., Blackmore S.), pp. 319–339 Oxford, UK: Oxford University Press [Google Scholar]
- Donisthorpe H. S. J. K.1920British Oligocene ants. Ann. Mag. Nat. Hist. Ser. 9 6, 81–94 [Google Scholar]
- Frank S. A.1984The behavior and morphology of the fig wasps Pegoscapus assuetus and P. jimenezi: descriptions and suggested behavioral characters for phylogenetic studies. Psyche 91, 289–308 (doi:10.1155/1984/35653) [Google Scholar]
- Givulescu R.1994Einige Bemerlungen zum Auftreten der Gattungen Ficus L. in tertiären Floren von Europa. Feddes Repertorium 105, 1–6 [Google Scholar]
- Grandi G.1925Morfologia del gen. Tetrapus Mayr e descrizione di una nuova species della Costa Rica. Boll. Soc. Ent. Ital. 57, 1–13 [Google Scholar]
- Herrera C. M.1985Determinants of plant-animal coevolution: the case of mutualistic dispersal of seeds by vertebrates. Oikos 44, 132–141 (doi:10.2307/3544054) [Google Scholar]
- Hooker J., Grimes S. T., Mattey D. P., Collinson M. E., Sheldon N. D.2009Refined correlation of the UK late Eocene–Early Oligocene Solent Group and timing of its climate history. Geol. Soc. Am. Spec. Pap. 452, 179–195 [Google Scholar]
- Jousselin E., Kjellberg F.2001The functional implications of active and passive pollination in dioecious figs. Ecol. Lett. 4, 151–158 (doi:10.1046/j.1461-0248.2001.00209.x) [Google Scholar]
- Kjellberg F., Jousselin E., Bronstein J. L., Patel A., Yokoyama J., Rasplus J. Y.2001Pollination mode in fig wasps: the predictive power of correlated traits. Proc. R. Soc. Lond. B 268, 1113–1121 (doi:10.1098/rspb.2001.1633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vaamonde C., Wikstrom N., Weiblen G. D., Rasplus J.-Y., Machado C., Cook J. M.2009Molecular dating and biogeography of fig-pollinating wasps. Mol. Phyl. Evol. 52, 715–726 (doi:10.1016/j.ympev.2009.05.028) [DOI] [PubMed] [Google Scholar]
- Mai D. H., Walther H.2000Die fundstellen eozäner Floren des Weisselstyer-beckens und seiner Radgebiete. Altenburger Naturwiss. Forsch. 13, 1–59 [Google Scholar]
- McCobb L. M. E., Duncan I. J., Jarzembowski E. A., Stankiewicz B. A., Wills M. A., Briggs D. E. G.1998Taphonomy of the insects from the Insect Bed (Bembridge Marls), late Eocene, Isle of Wight, England. Geol. Mag. 135, 553–563 (doi:10.1017/S0016756898001204) [Google Scholar]
- Nefdt R. J. C., Compton S. G.1996Regulation of seed and pollinator production in the fig–fig wasp mutualism. J. Anim. Ecol. 65, 170–182 [Google Scholar]
- Peñalver E., Engel M. S., Grimaldi D. A.2006Fig wasps in Dominican amber (Hymenoptera: Agaonidae). Am. Mus. Novit. 3541, 1–16 (doi:10.1206/0003-0082(2006)3541[1:FWIDAH]2.0.CO;2) [Google Scholar]
- Punt W., Hoen P. P., Blackmore S., Nilsson S., Le Thomas A.2007Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 143, 1–81 (doi:10.1016/j.revpalbo.2006.06.008) [Google Scholar]
- Rønsted N., Weiblen G. D., Cook J. M., Salamin N., Machado C. A., Savolainen V.200560 million years of codivergence in the fig-wasp symbiosis. Proc. R. Soc. B 272, 2593–2599 (doi:10.1111/j.1096-0031.2009.00291.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl F. P., Schlüter P. M.2009Floral isolation, specialized pollination, and pollinator behavior in orchids. Annu. Rev. Entomol. 54, 425–446 (doi:10.1146/annurev.ento.54.110807.090603) [DOI] [PubMed] [Google Scholar]
- Shanahan M., So S., Compton S. G., Corlett R.2001Fig-eating by vertebrate frugivores: a global review. Biol. Rev. 76, 529–572 (doi:10.1017/S1464793101005760) [DOI] [PubMed] [Google Scholar]
- Wiebes J. T.1994The Indo-Australian Agaoninae (pollinators of figs). Amsterdam, The Netherlands: North-Holland [Google Scholar]
- Wiebes J. T.2005The New World Agaoninae (pollinators of figs). Amsterdam, The Netherlands: North-Holland [Google Scholar]