Abstract
Water impoundment imposes fundamental changes on natural landscapes by transforming rivers into reservoirs. The dramatic shift in physical conditions accompanying the loss of flow creates novel ecological and evolutionary challenges for native species. In this study, we compared the body shape of Cyprinella venusta collected from eight pairs of river and reservoir sites across the Mobile River Basin (USA). Geometric morphometric analysis of the body shape showed that river populations differ from reservoir populations. Individuals inhabiting reservoirs are deep-bodied and have a smaller head, a more anterior dorsal fin, a shorter dorsal fin base and a more ventral position of the eye than C. venusta in streams. The direction of shape divergence within reservoir–river pairs was consistent among pairs of sites, and the shape of C. venusta in reservoirs is strongly correlated with reservoir size. These findings show that physical characteristics of reservoirs drive changes in the morphological attributes of native fish populations, indicating that water impoundment may be an important, yet largely unrecognized, evolutionary driver acting on aquatic biodiversity.
Keywords: dam, flow, reservoirs, geometric morphometrics, selection, phenotypic plasticity
1. Introduction
Water impoundment represents one of the most widespread and disruptive anthropogenic modifications of ecosystems worldwide (Graf 1999). More than 79 000 dams are now operating in the United States alone, including 8100 major dams greater than 15 m in height with normal storage capacity of over six million cubic metres and a maximum storage capacity over 30 million cubic metres (The National Atlas; http://nationalatlas.gov). Water impoundment imposes fundamental changes to natural landscapes by transforming rivers into reservoirs. Dramatic shifts from lotic to lentic physical conditions resulting from the creation of artificial lakes can restructure communities (Lowe-McConnell 1987) and may create novel ecological and evolutionary challenges (Baxter 1977) for aquatic organisms capable of responding to the absence of flow.
Trade-offs involving body shape and swimming performance vary in relation to flow conditions (Vogel 1994). Deep-bodied fishes, for example, can perform sustained swimming more efficiently than burst swimming in low flow environments, and fishes with streamlined morphologies are better able to overcome hydrodynamic drag in high flow environments (Blake 1983). Body shape can also influence fitness by affecting foraging success, fecundity and predator avoidance (Langerhans & Reznick 2010). Such fitness trade-offs have been linked to habitat-associated morphological divergence in fishes and other aquatic organisms, including intraspecific differences attributable to lentic and lotic conditions (Robinson & Wilson 1994; Hendry et al. 2002; Langerhans et al. 2003; McGuigan et al. 2003).
Although relationships between body shape and flow regime have been well studied in naturally occurring environments (Langerhans 2008), we do not know whether body shape changes following anthropogenic modification of flow regime. In this study, we investigated whether impoundment affects the body shape of Cyprinella venusta, a widely distributed cyprinid in the southeastern United States. Fitness trade-offs in C. venusta vary in response to natural flow regimes (Machado et al. 2002). Here, we examine how C. venusta body shape responds to alterations in flow attributable to the installation of dams intended to increase water and energy availability.
2. Material and methods
We examined sites within the Mobile River Basin, which encompasses an area of 113 000 km2 in the southeastern United States. The basin contains 19 navigational locks and dams and 15 hydroelectric dams that together impound 44 per cent of mainstem length (Pringle et al. 2000). In June and July 2008, we collected C. venusta by seine from eight pairs of sites on tributaries in the Mobile River Basin (table 1 and electronic supplementary material, figure S1). For each pair, one site was within a reservoir and the other was in an adjoining river at the same longitudinal position as the reservoir site (electronic supplementary material, figure S1). All specimens were fixed and stored in 10 per cent buffered formalin.
Table 1.
Collection localities, numbers of specimens examined (n), canonical variate (CV) scores and standard errors, and reservoir characteristics.
| site name | pair no. | latitude | longitude | n | average CV1 | standard error CV1 | average CV2 | standard error CV2 | distance to river site (km) | age (years) | surface area (hectares) | volume (m3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allatoona Lake | 1 | 34.15001 | −84.71608 | 50 | −0.00463 | 0.00062 | −0.00251 | 0.00072 | 17.17 | 59 | 4860 | 4.5 × 108 |
| Pumpkinvine Creek | 1 | 34.11121 | −84.74976 | 50 | 0.00066 | 0.00059 | 0.00656 | 0.00088 | ||||
| Carters Lake | 2 | 34.61138 | −84.63608 | 50 | −0.00133 | 0.00067 | −0.00313 | 0.00061 | 94.148 | 32 | 1303 | 4.7 × 108 |
| Conasauga River | 2 | 34.78284 | −84.87241 | 50 | 0.00506 | 0.00075 | 0.00585 | 0.00084 | ||||
| H. Neely Henry Lake | 3 | 33.94295 | −86.02836 | 50 | −0.00056 | 0.00074 | −0.00042 | 0.00078 | 12.737 | 43 | 4532 | 1.5 × 108 |
| Tallasseehatchie Creek | 3 | 33.79630 | −85.96252 | 50 | 0.00210 | 0.00074 | 0.00394 | 0.00081 | ||||
| Jordan Lake | 4 | 32.62893 | −86.26440 | 50 | −0.00371 | 0.00071 | −0.00332 | 0.00052 | 44.637 | 81 | 2751 | 2.9 × 108 |
| Tallapoosa River | 4 | 32.43352 | −86.12518 | 50 | 0.00130 | 0.00065 | −0.00072 | 0.00059 | ||||
| Lake Purdy | 5 | 33.46740 | −86.64118 | 50 | −0.00399 | 0.00053 | −0.00120 | 0.00071 | 26.629 | 86 | 424 | 1.9 × 107 |
| Cahaba River | 5 | 33.51150 | −86.65275 | 50 | −0.00255 | 0.00055 | 0.00048 | 0.00087 | ||||
| Lewis Smith Lake | 6 | 34.08263 | −86.95404 | 50 | −0.00031 | 0.00071 | −0.00527 | 0.00075 | 72.199 | 48 | 8579 | 1.7 × 109 |
| Mulberry Fork | 6 | 33.99774 | −86.75114 | 50 | 0.00443 | 0.00062 | −0.00324 | 0.00055 | ||||
| Logan Martin Lake | 7 | 33.42615 | −86.32645 | 50 | −0.00060 | 0.00073 | −0.00253 | 0.00070 | 11.192 | 45 | 6176 | 3.4 × 108 |
| Kelley Creek | 7 | 33.44732 | −86.38732 | 48 | −0.00345 | 0.00074 | 0.00569 | 0.00079 | ||||
| Martin Lake | 8 | 32.85765 | −85.91925 | 51 | −0.00374 | 0.00056 | −0.00583 | 0.00052 | 22.156 | 83 | 17 806 | 2.0 × 109 |
| Uphappee Creek | 8 | 32.49016 | −85.74246 | 50 | 0.01126 | 0.00068 | 0.00573 | 0.00081 |
Geometric morphometric methods were used to quantify body shape (Zelditch et al. 2004). Photographs of the right side of each specimen were taken with a Sony DSC-H2 digital camera and then analysed using the tps software package (http://life.bio.sunysb.edu/morph/). The thin plate spline (tps) functions implemented by the software describe statistical variation in deformation of a thin sheet. After first concatenating all photographs into a single file, we placed landmarks on 11 morphological features in each image and computed scale factors. We then placed five additional landmarks along the midline of each specimen to implement the ‘Unbend specimens’ feature in tpsUtil for removing the effects of any bending of specimens owing to preservation. After also removing confounding effects of rotation, translation and scaling via generalized least-squares Procrustes superimposition, we computed partial warps, uniform components and centroid size for each specimen (Zelditch et al. 2004). Partial warps describe localized shape variation and uniform components describe shape variation owing to compression, dilation and shear across the entire form (Zelditch et al. 2004). We used tpsSplin to visualize morphological differences between reservoir and river populations. To do so, we created Thompson transformation grids from comparisons of (i) reservoir individuals and the average shape of all reservoir + all river individuals, and (ii) river individuals and the average shape of all reservoir+all river individuals.
To test the hypothesis that significant shifts in body shape are associated with water impoundment, we conducted a multivariate analysis of covariance (MANCOVA) in which impoundment status (reservoir versus river), location in the drainage basin (comparison among river–reservoir pairs), and the interaction between impoundment status and location within the drainage basin served as fixed effects. Partial warp and uniform component scores were used as dependent variables, and centroid size was a covariate to control for size allometry.
We also used canonical correlation analysis to reduce 18 geometric morphometric variables to three sets of canonical variates (CVs) describing body shape. Partial warps and uniform components were used as dependent variables in the analysis; impoundment status (reservoir versus river), centroid size and the interaction between impoundment status and centroid size served as independent variables. We assessed pairwise and basin-wide patterns of morphological divergence based upon calculated differences in CV values for each pair of reservoir and river sites.
We used linear regression to evaluate the strength of pairwise relationships between reservoir–river morphological divergence (based on the difference in CV values) and landscape and reservoir characteristics. We examined both distance between paired sites and reservoir age as potential explanatory variables. We also used linear regression to examine whether variability in body shape among reservoir populations corresponded to reservoir surface area and volume. SPSS v.16 and SYSTAT v.10 were used for all statistical analyses except when otherwise noted.
3. Results
Individuals from reservoir sites are deep-bodied and have a smaller head, a more anterior dorsal fin, a shorter dorsal fin base and a more ventral position of the eye than individuals inhabiting streams (figure 1). MANCOVA revealed that the observed shape differences among reservoir and river populations, as quantified by partial warps and uniform components, are statistically significant. After controlling for allometry—which explains 41 per cent of partial variance in shape (F = 29.935, d.f. = 18, p < 0.0001)—43 per cent of partial variance is attributable to flow regime (F = 31.931, d.f. = 18, p < 0.001), 17 per cent corresponds to the location of paired sites within the drainage basin (F = 8.531, d.f. = 126, p < 0.001) and 16 per cent of partial variance reflects the interaction between hydrology and location (F = 7.909, d.f. = 126, p < 0.001).
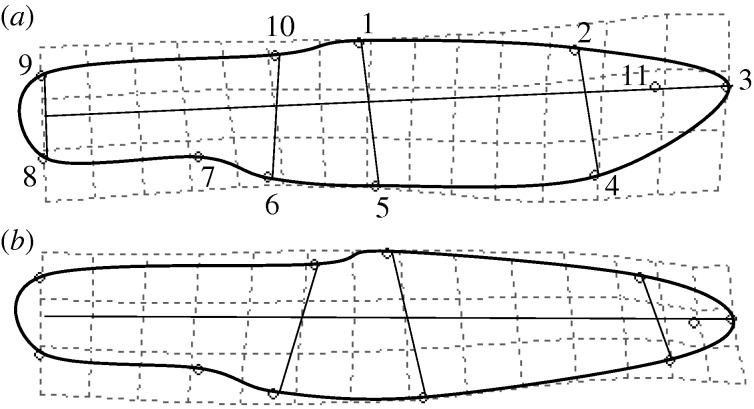
Figure 1.
Thompson transformation grids depicting statistically significant morphological differences (magnified 10×) in C. venusta between (a) stream and (b) reservoir habitats. Right side of the fish is shown. Visualizations describe five major shifts in body shape in reservoir populations: deeper body despite changes in head configuration (landmarks 1–5), smaller head (landmarks 2–4), more ventrally placed eye (landmark 11), more anterior dorsal fin (landmarks 10 and 1), and a shorter dorsal fin base (landmarks 10 and 1). Numbering on the upper image indicates the order in which landmarks were digitized.
Our analyses revealed three canonical correlations and a set of three CVs. CV1, corresponding to eye position, size of head and length of anal fin base, accounts for 52.9 per cent of observed variance; CV2, corresponding to length of dorsal fin base, position of dorsal fin and body depth, accounts for 31 per cent of observed variance. CV3 accounted for only 2.9 per cent of observed variance, and therefore was not used. CV1 and CV2 values of all individuals from a site were then averaged to create two summary measures describing shape for each population.
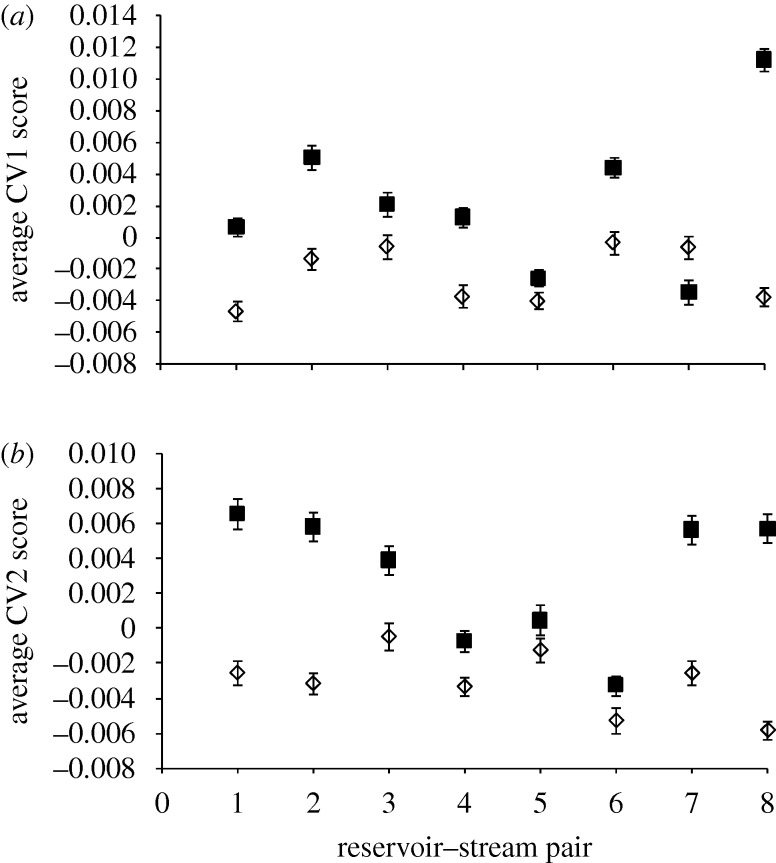
Canonical correlation analysis and estimates of morphological divergence from average CVs revealed that the direction of shape divergence within reservoir–river pairs was consistent among locations (figure 2). The CV value for each river population was larger than the CV value of the corresponding reservoir population in seven of eight pairs (figure 2). We also found that the variability in body shape among river populations was roughly twice that among reservoir populations (s.d. of CV1river = 0.005, s.d. of CV1reservoir = 0.002; s.d. of CV2river = 0.004, s.d. of CV2reservoir = 0.002).
Figure 2.
Body shape divergence within stream–reservoir pairs according to canonical variate (CV) scores (±1 s.e.) for each reservoir and river population. (a) CV1 scores correspond to eye position, size of head, length of anal fin base; (b) CV2 scores correspond to length of dorsal fin base, position of dorsal fin and body depth. Diamonds, reservoir; squares, stream.
Body shape of C. venusta also varies with reservoir size. Significant positive relationships (electronic supplementary material, figure S2) were found between CV2 values of reservoir populations, reservoir volume (r2 = 0.835, p = 0.001) and reservoir surface area (r2 = 0.537, p = 0.039). No significant relationships were found in regressions of CV2 values of reservoir populations against reservoir age (r2 = 0.028, p = 0.692) or in regressions of morphological divergence among reservoir–river pairs against reservoir surface area (CV1: r2 = 0.451, p = 0.068; CV2: r2 = 0.263, p = 0.193), reservoir age (CV1: r2 = 0.119, p = 0.403, CV2: r2 = 0.031, p = 0.674), and distance between paired sites (CV1: r2 = 0.039, p = 0.641; CV2: r2 = 0.019, p = 0.747).
4. Discussion
Here, we provide, to our knowledge, the first evidence demonstrating that anthropogenic transformation of rivers into reservoirs influences body shape in an aquatic organism. We found that C. venusta in reservoirs exhibits differences at five major morphological attributes compared with C. venusta inhabiting adjoining rivers (figure 1).
Habitat-associated morphological divergence is widespread in fishes, including intraspecific differences attributable to lentic and lotic conditions (Robinson & Wilson 1994; Hendry et al. 2002; Langerhans et al. 2003; McGuigan et al. 2003). The morphological differences that occur between reservoir and river populations of C. venusta, such as a deepening of the body in reservoir populations, parallel differences observed in populations of other species occupying naturally contrasting flow habitats (Langerhans 2008; but see Hendry et al. 2002; Krabbenhoft et al. 2009).
As with other studies of contrasting flow environments (McGuigan et al. 2003), we found that the direction of body shape divergence is consistent among reservoir–river pairs (figure 2). These results suggest that reservoir populations exhibit similar responses to novel environments created by dam closure. We also found that the average shape of reservoir populations varied in a predictable manner with reservoir size, suggesting that physical characteristics of reservoirs may determine evolutionary and ecological conditions driving changes in the morphological characteristics of resident fish populations.
Unlike prior studies (Langerhans et al. 2003), we did not find a relationship between the geographical distance and the degree of morphological divergence between reservoir and river populations. Variability in body depth among river populations may account for this inconsistency. Among riverine populations of C. venusta body shape varies with mean annual runoff (T. C. Haas, D. C. Heins & C. S. Hood 1997, unpublished data), suggesting that the degree of morphological divergence among reservoir and river populations of C. venusta may correspond more to patterns of stream size or river discharge than to geographical distance among longitudinally paired study sites.
The morphological differences occurring among reservoir and river populations of C. venusta correspond to traits that can have a direct impact on fitness (Langerhans & Reznick 2010). Intraspecific variation can correspond to habitat-associated divergent selection (Robinson & Wilson 1994; McGuigan et al. 2003). Adaptive responses to divergent selection, however, may correspond to genetic differentiation and phenotypic plasticity alone or in concert (Levins 1968; West-Eberhard 1989). Flow regime differences between reservoirs and streams can result in divergent selection in fishes (Langerhans 2008). We have observed morphological shifts (i.e. a deepening of the body) that may influence locomotive performance. Additionally, shifts in prey type and availability may give rise to differences in morphological features related to feeding performance, such as the observed changes in head size and eye position (Hendry et al. 2002). Further studies, such as raising progeny from controlled crosses under common laboratory conditions, will be necessary to identify whether the observed changes are attributable to selection on heritable traits or phenotypic plasticity (McGuigan et al. 2003). If patterns of divergence reflect heritable changes in morphology, then our findings would suggest that water impoundment is an important, yet largely unrecognized, evolutionary driver acting on aquatic biological diversity.
Acknowledgements
All work carried out in this study conforms to institutional animal care protocols on file at Tulane University.
We thank Michael Guill for contributing to the study design and execution, David Corey for statistical consulting, Justin Mann for field assistance and Lee Attaway for laboratory assistance. This study was funded by the Louisiana Board of Regents.
References
- Baxter R. M.1977Environmental effects of dams and impoundments. Annu. Rev. Ecol. Syst. 8, 255–283 (doi:10.1146/annurev.es.08.110177.001351) [Google Scholar]
- Blake R. W.1983Fish locomotion. Cambridge, UK: Cambridge University Press [Google Scholar]
- Graf W. L.1999Dam nation: a geographic census of American dams and their large-scale hydrologic impacts. Water Resour. Res. 35, 1305–1311 (doi:10.1029/1999WR900016) [Google Scholar]
- Hendry A. P., Taylor E. B., McPhail J. D.2002Adaptive divergence and the balance between selection and gene flow: lake and stream stickleback in the misty system. Evolution 56, 1199–1216 (doi:10.1111/j.0014-3820.2002.tb01432.x) [DOI] [PubMed] [Google Scholar]
- Krabbenhoft T. J., Collyer M. L., Quattro J. M.2009Differing evolutionary patterns underlie convergence on elongate morphology in endemic fishes of Lake Waccamaw, North Carolina. Biol. J. Linn. Soc. 98, 636–645 (doi:10.1111/j.1095-8312.2009.01305.x) [Google Scholar]
- Langerhans R. B.2008Predictability of phenotypic differentiation across flow regimes in fishes. Integr. Comp. Biol. 48, 750–768 (doi:10.1093/icb/icn092) [DOI] [PubMed] [Google Scholar]
- Langerhans R. B., Reznick D. N.2010Ecology and evolution of swimming performance in fishes: predicting evolution with biomechanics. In Fish locomotion: an eco-ethological perspective (eds Domenici P., Kapoor B. G.), pp. 200–248 Enfield, NH: Science Publishers [Google Scholar]
- Langerhans R. B., Layman C. A., Langerhans A. K., DeWitt T. J.2003Habitat-associated morphological divergence in two Neotropical fish species. Biol. J. Linn. Soc. 80, 689–698 (doi:10.1111/j.1095-8312.2003.00266.x) [Google Scholar]
- Levins R.1968Evolution in changing environments. Princeton, NJ: Princeton University Press [Google Scholar]
- Lowe-McConnell R. H.1987Ecological studies in tropical fish communities. Cambridge, UK: Cambridge University Press [Google Scholar]
- Machado M. D., Heins D. C., Bart H. L., Jr2002Microgeographical variation in ovum size of the blacktail shiner, Cyprinella venusta Girard, in relation to stream flow. Ecol. Freshw. Fish 11, 11–19 (doi:10.1034/j.1600-0633.2002.1o103.x) [Google Scholar]
- McGuigan K., Franklin C. E., Moritz C., Blows M. W.2003Adaptation of rainbow fish to lake and stream habitats. Evolution 57, 104–118 (doi:10.1554/0014-3820(2003)057[0104:AORFTL]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Pringle C. M., Freeman M. C., Freeman B. J.2000Regional effects of hydrologic alterations on riverine macrobiota in the New World: tropical–temperate comparisons. BioScience 50, 807–823 (doi:10.1641/0006-3568(2000)050[0807:REOHAO]2.0.CO;2) [Google Scholar]
- Robinson B. W., Wilson D. S.1994Character release and displacement in fishes: a neglected literature. Am. Nat. 144, 596–627 (doi:10.1086/285696) [Google Scholar]
- Vogel S.1994Life in moving fluids, 2nd edn.Princeton, NJ: Princeton University Press [Google Scholar]
- West-Eberhard M. J.1989Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20, 249–278 (doi:10.1146/annurev.es.20.110189.001341) [Google Scholar]
- Zelditch M. L., Swiderski D. L., Sheets D. H., Fink W. L.2004Geometric morphometrics for biologists. San Diego, CA: Academic Press [Google Scholar]