Abstract
The differential allocation theory predicts that females should invest more in offspring produced with attractive partners, and a number of studies support this prediction in birds. Females have been shown to increase reproductive investment when mated to males showing elaborated sexual traits. However, mate attractiveness might also depend on the interaction between male and female genotypes. Accordingly, females should invest more in offspring sired by individuals that are genetically dissimilar or carry superior alleles. Here, we show in zebra finches (Taeniopygia guttata) that pairs of unfamiliar genetic brothers and sisters are less likely to reproduce in comparison with randomly mated pairs. Among the brother–sister pairs, those that attempted to breed laid smaller clutches and of lower total clutch mass. Our results provide the first experimental evidence that females adjust their reproductive effort in response to the genetic similarity of their partners. Importantly, these results imply a female ability to assess relatedness of a social mate without prior association.
Keywords: kin recognition, inbreeding avoidance, breeding decision, clutch size
1. Introduction
Females may maximize their fitness by choosing attractive mates (Andersson 1994) and by investing more resources into offspring produced with an attractive partner (Sheldon 2000), as the potential reproductive value of such offspring might be higher (Burley 1988; Sheldon 2000). Attractive mates may provide genetic benefits in the form of additive ‘good genes’ and non-additive genetic benefits (Mays & Hill 2004; Neff & Pitcher 2005). Support for differential allocation theory has to date come mainly from avian studies in which females have been shown to invest more in offspring of more ornamented mates (e.g. Balzer & Williams 1998; Gil et al. 1999; Cunningham & Russell 2000). However, differential female investment in response to mate attractiveness has rarely been investigated in relation to mate genetic similarity. To our knowledge, in birds only one study reported adaptive adjustment of female reproductive effort in response to the genetic similarity of the partner. Specifically, female song sparrows (Melospiza melodia) paired to genetically dissimilar partners provisioned their brood at a higher rate compared with females paired to genetically similar males (Potvin & MacDougall-Shackleton 2009). However, the correlative nature of this study does not allow us to determine whether the genetic similarity was in fact causal component of the observed variation in the reproductive effort.
Here, we sought to explore whether females adjust their primary reproductive effort in relation to the genetic similarity of their partners, using an experimental approach. To create genetically similar breeding pairs, we used brothers and sisters of zebra finches that were raised in separation in foster nests from immediately after hatching and compared their breeding decisions with randomly mated birds. Since we paired males and females that were unfamiliar to each other, any potential decisions that are affected by relatedness must be attribute to the genetic similarity of the partners.
As a model species, we used zebra finches (Taeniopygia guttata). Mating of related individuals in this species has been shown to result in severe fitness costs (Fetherston & Burley 1990; Bolund et al. 2010) and it is suggested that female zebra finches are able to assess genetic relatedness of their potential partners (Schielzeth et al. 2008). However, in birds kin recognition without prior association with the kin is poorly documented. Avoidance of genetically related mates in zebra finches appeared significant only in one out of three experimental cohorts of birds (Schielzeth et al. 2008). Therefore, it is important to test whether birds are able to recognise kin without prior association in an independent study.
2. Material and methods
The study was conducted on zebra finches from a laboratory colony. All birds were kept in an air-conditioned room at 20 ± 2°C, under a 13 : 11 h incandescent light:dark photoperiod, lights on at 07.00 h. Birds were fed ad libitum with a standard mixture of seeds (Megan, Poland), along with a mixture of hard-boiled egg, and they also received cuttlebone and grit. Rearing conditions were kept constant during breeding experiments.
Unrelated birds randomly selected from the base population (inbreeding coefficient 0.010; Forstmeier et al. 2007) were paired in visually separated individual cages, equipped with external nest boxes and nesting material. Immediately after hatching two or three randomly chosen chicks from each nest were cross-fostered between nests of the same hatching day and similar brood size. The remaining chicks were left in their parental nests. Young birds were separated from their foster parents at the age of 40 days and remained in single-sex groups until they reached adulthood.
Mature offspring were paired in visually separated individual cages in the same room and at the same time. We created two groups, with different type of mating (inbred and control). In the inbred group, 22 females were paired with their genetic brothers raised in different nests. The control group comprised 26 females that were paired with unrelated, unfamiliar males. Nest boxes were inspected every morning between 09.00 and 10.00 h for a period of one month to record nest building and egg laying. Eggs were weighed to the nearest 0.01 g on the day of laying.
We used χ2-test to determine whether groups differed in probability of breeding. Differences in clutch size and total clutch mass between the groups were tested with a one-way ANOVA. Data that were not normally distributed (latency to laying) were analysed with a Kruskal–Wallis test.
3. Results and disscusion
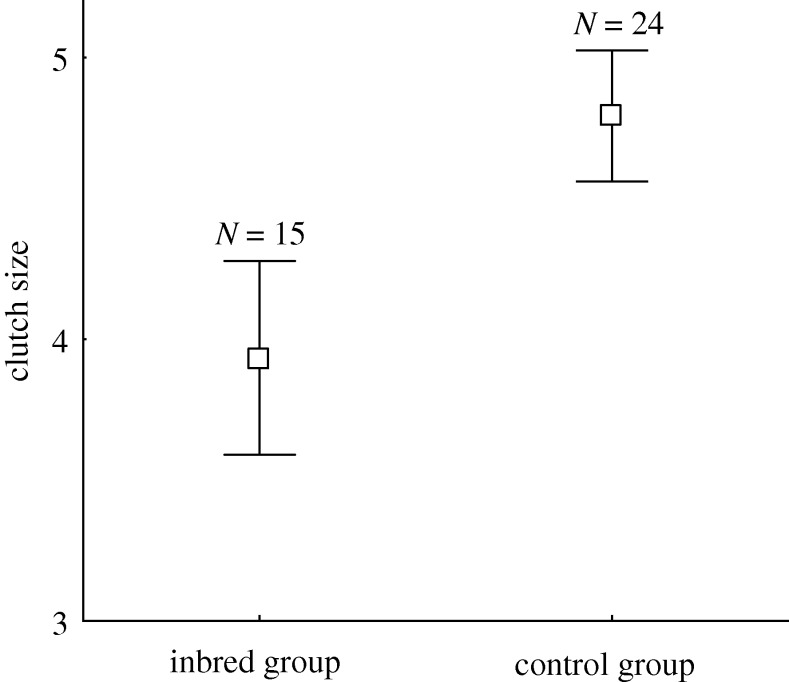
Our data show that zebra finch females adjusted their primary reproductive effort according to the genetic similarity of their partner. We found the pairs of mutually unfamiliar genetic brothers and sisters to be less likely to breed (15 out of 22 females started egg laying) than the pairs of unrelated randomly mated individuals (24 out of 26; χ12 = 4.55; p = 0.033). There was no significant difference in latency to onset of egg laying between the groups (median = 6 days in both groups, H = 0.55; p = 0.814). More importantly, females paired to their unfamiliar brothers laid smaller clutches (F1,38 = 6.80; p = 0.040; figure 1), of lower total clutch mass (mean ± s.e.; inbred group: 4.03 ± 0.37 g; control group: 5.28 ± 0.31 g; F1,36 = 13.78; p = 0.014). Average egg mass tended also to be smaller among clutches laid by inbred pairs (mean ± s.e.; inbred group: 1.01 ± 0.02 g; control group: 1.07 ± 0.02 g; F1,36 = 3.51; p = 0.07).
Figure 1.
Mean ± s.e. clutch size produced by females mated to their unfamiliar brothers (inbred group) and mated to unfamiliar, unrelated male (control group).
Because inbreeding typically leads to a significant reduction in fitness (e.g. Fetherston & Burley 1990; Keller & Waller 2002; Bolund et al. 2010), avoiding breeding with a genetically similar partner should be beneficial. However, such behaviour may also incur costs of missing a breeding opportunity if a non-related mate is not available (e.g. Kokko & Ots 2006). Zebra finches are a relatively short-lived bird species (Zann 1996), so a decision to refuse breeding may have important fitness consequences. This may explain why most of sibling pairs decided to breed. However, females mated to their unfamiliar genetic brothers invested less in current brood in comparison with females paired with unrelated partners, possibly to account for lower prospects of inbred offspring and own future prospects of survival and reproduction (Stearns 1992). Thus, females seem to follow ‘the best of a bad job’ strategy. A number of studies have shown that females adjust their reproductive effort according to the phenotypic attractiveness of their mates (Burley 1988; Gil et al. 1999; Reyer et al. 1999; Cunningham & Russell 2000; Sheldon 2000; Rutstein et al. 2004; Pryke & Griffith 2009), however, our study seems to provide the first experimental evidence demonstrating adjustment of primary reproductive effort according to the genetic similarity of the social mate.
Our study provides strong support that zebra finches can assess the relatedness of their mates. Because the sibling pairs were reared in separate broods, kin recognition in this case occurred without prior association. This corroborates previous results of research on the Japanese quail (Coturnix japonica), showing female preference for unfamiliar mates of intermediate relatedness (i.e. cousins; Bateson 1982) and of a recent study on zebra finches in which females were shown to prefer spending time with unfamiliar, unrelated males to unfamiliar genetic brothers (Schielzeth et al. 2008). Schielzeth et al. (2008) suggested that this case of kin recognition might be accounted for by self-referent phenotype matching. However, our experimental design does not allow us to elucidate the precise mechanism of kin recognition because the birds were exposed to their kin during early development (parents or siblings). Thus, the birds could have used family phenotype matching to discriminate between kin and non-kin.
In conclusion, our data suggest that zebra finches are able to recognize unfamiliar kin without prior association and thus avoid producing inbred offspring. Moreover, the low potential value of inbred offspring makes females invest less in their brood. Further investigation of the underlying kin discrimination mechanisms and phenotypic traits used as cues in kin discrimination is required to understand the evolutionary processes shaping breeding decisions in birds.
Acknowledgements
The study was carried out under a licence from the Local Ethical Committee at the Jagiellonian University.
The authors thank M. Herdegen for assistance in the experiment. J. Kubacka and W. Forstmeier provided helpful comments on the manuscript. The study was supported by the Polish Ministry of Science and Higher Education in years 2006–2010 and partly by DS/WBiNoZ/INoŚ/757/09.
References
- Andersson M.1994Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- Balzer A. L., Williams T. D.1998Do female zebra finches vary primary reproductive effort in relation to mate attractiveness? Behaviour 35, 297–309 [Google Scholar]
- Bateson P. P. G.1982Preferences for cousins in Japanese quail. Nature 295, 236–237 (doi:10.1038/295236a0) [Google Scholar]
- Bolund E., Martin K., Kempenaers B., Forstmeier W.2010Inbreeding depression of sexually selected traits and attractiveness in the zebra finch. Anim. Behav. 1, 1–9 (doi:10.1016/j.anbehav.2010.01.014) [Google Scholar]
- Burley N.1988The differential-allocation hypothesis: an experimental test. Am. Nat. 132, 611–628 (doi:10.1086/284877) [Google Scholar]
- Cunningham E. J. A., Russell A. F.2000Egg investment influenced by male attractiveness in the mallard. Nature 404, 74–77 (doi:10.1038/35003565) [DOI] [PubMed] [Google Scholar]
- Fetherston I. A., Burley N. T.1990Do zebra finches prefer to mate with close relatives? Behav. Ecol. Sociobiol. 27, 411–414 (doi:10.1007/BF00164067) [Google Scholar]
- Forstmeier W., Segelbacher G., Mueller J. C., Kempenaers B.2007Genetic variation and differentiation in captive and wild zebra finches (Taeniopygia guttata). Mol. Ecol. 16, 4039–4050 (doi:10.1111/j.1365-294X.2007.03444.x) [DOI] [PubMed] [Google Scholar]
- Gil D., Graves J., Hazon N., Wells A.1999Male attractiveness and differential testosterone investment in zebra finch eggs. Science 286, 126–128 (doi:10.1126/science.286.5437.126) [DOI] [PubMed] [Google Scholar]
- Keller L. F., Waller D. M.2002Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241 (doi:10.1016/S0169-5347(02)02489-8) [Google Scholar]
- Kokko H., Ots I.2006When not to avoid inbreeding. Evolution 60, 467–475 (doi:10.1111/j.0014-3820.2006.tb01128) [PubMed] [Google Scholar]
- Mays H. L., Hill G. E.2004Choosing mates: good genes versus genes that are a good fit. Trends Ecol. Evol. 19, 554–559 (doi:10.1016/j.tree.2004.07.018) [DOI] [PubMed] [Google Scholar]
- Neff B. D., Pitcher T. E.2005Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 14, 19–38 (doi:10.1111/j.1365-294X.2004.02395.x) [DOI] [PubMed] [Google Scholar]
- Potvin D. A., MacDougall-Shackleton E. A.2009Parental investment amplifies effects of genetic complementarity on growth rates in song sparrows, Melospiza melodia. Anim. Behav. 78, 943–948 (doi:10.1016/j.anbehav.2009.07.023) [Google Scholar]
- Pryke S. R., Griffith S. C.2009Genetic incompatibility drives sex allocation and maternal investment in a polymorphic finch. Science 323, 1605–1607 (doi:10.1126/science.1168928) [DOI] [PubMed] [Google Scholar]
- Reyer H. U., Frei G., Som C.1999Cryptic female choice: frogs reduce their clutches when amplexed by undesired male. Proc. R. Soc. Lond. B 266, 2101–2107 (doi:10.1098/rspb.1999.0894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutstein A. N., Gilbert L., Slater P. J. B., Grave J. A.2004Mate attractiveness and primary resource allocation in the zebra finch. Anim. Behav. 68, 1087–1094 (doi:10.1016/j.anbehav.2004.02.011) [Google Scholar]
- Schielzeth H., Burger C., Bolund E., Forstmeier W.2008Assortative versus disassortative mate preferences in the zebra finch based on self-referent phenotype matching. Anim. Behav. 76, 1927–1934 (doi:10.1016/j.anbehav.2008.08.014) [Google Scholar]
- Sheldon B. C.2000Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 15, 397–402 (doi:10.1016/S0169-5347(00)01953-4) [DOI] [PubMed] [Google Scholar]
- Stearns S. C.1992The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- Zann R. A.1996The zebra finch: a synthesis of field and laboratory studies. Oxford, UK: Oxford University Press [Google Scholar]