Abstract
During the last half million years, pulses of gigantism in the anagenetic lineage of land snails of the subgenus Poecilozonites on Bermuda were correlated with glacial periods when lower sea level resulted in an island nearly an order of magnitude larger than at present. During those periods, the island was colonized by large vertebrate predators that created selection pressure for large size and rapid growth in the snails. Extreme reduction in land area from rising seas, along with changes in ecological conditions at the onset of interglacial episodes, marked extinction events for large predators, after which snails reverted to much smaller size. The giant snails were identical in morphology during the last two glacials when the predators included a large flightless rail Rallus recessus (marine isotope stages (MIS) 4-2) and a crane Grus latipes and a duck Anas pachysceles (MIS 6). In a preceding glacial period (MIS 10), when the fauna also included the tortoise Hesperotestudo bermudae, the snails were not only large, but the shells were much thicker, presumably to prevent crushing by tortoises. Evolution of Poecilozonites provides an outstanding example of dramatic morphological change in response to environmental pressures in the absence of cladogenesis.
Keywords: anagenesis, biogeography, extinction, Hesperotestudo, island area, sea-level cycles
1. Introduction
On the isolated North Atlantic island of Bermuda, 1000 km east–southeast of North Carolina, USA, land snails of the family Zonitidae, genus and subgenus Poecilozonites, evolved a remarkable diversity of shell sizes and shapes during the Pleistocene that Gould (1969, p. 407) considered to be an example of ‘an adaptive radiation comparable in scope with the classic insular speciation and ecologic differentiation of Darwin's finches’. With much more extensive collecting and refinement of stratigraphy and chronology (Hearty et al. 2004; Hearty & Olson in press), we established that this supposed radiation consists of a single anagenetic series with highly divergent shell morphologies that do not overlap in time (Hearty & Olson in press).
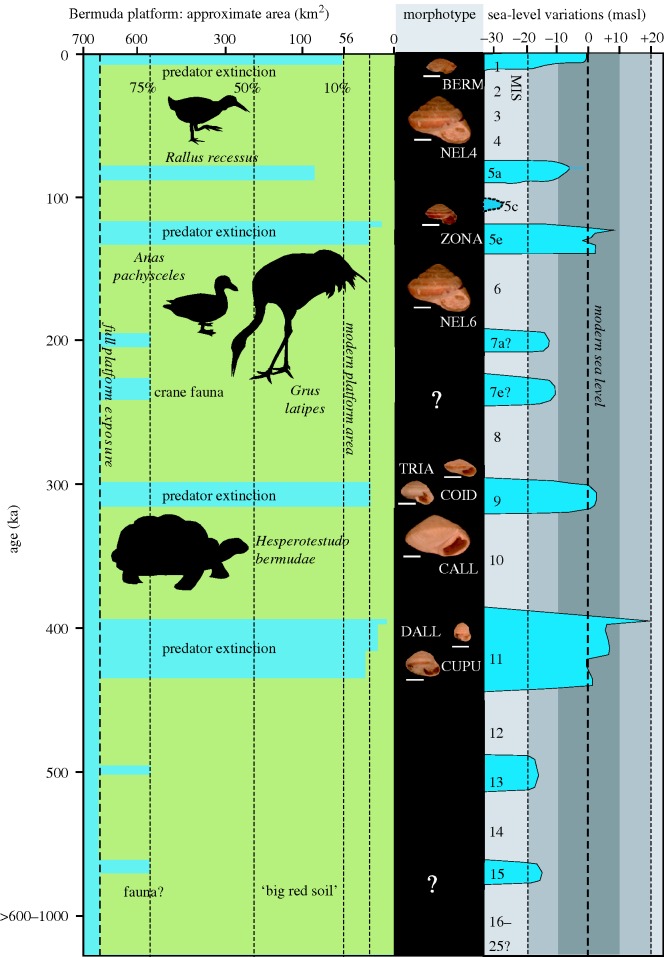
Changes in shell morphology in Poecilozonites are correlated with drastic changes in island area and environmental conditions caused by eustatic sea-level changes during the Pleistocene. During glacial periods, sea levels fall below the level of the Bermuda platform creating a large island with an area of >650 km2. During interglacial highstands, island area is reduced by an order of magnitude to a few tens of kilometres square—56 km2 at present with even greater reductions during interglacials marine isotope stages (MIS) 5e, 9 and 11 (figure 1).
Figure 1.
Anagenetic procession of snails (subgenus Poecilozonites) and their predators on Bermuda during the mid to late Quaternary stratigraphic order. Green on the left shows relative percentage of exposed land area of the Bermuda platform, with known vertebrate predators being associated with glacial lowstand periods. The central column shows the morphotypes of Poecilozonites associated with glacial and interglacial cycles (after Hearty & Olson in press). The right column identifies MIS events and various sea-level cycles during the Quaternary. Gigantism is expressed in glacial-age snails when vertebrate predators are present. The predators became extinct through reduction in habitat with interglacial flooding of the platform.
The predominant interglacial snail morphotypes (WALS, CUPU, COID/TRIA, ZONA and BERM), known from the basal-most rocks in Bermuda, and from MIS 11, 9, 5 and 1 (figure 1), although varying somewhat in shell shape, are all about the same general medium-size averaging from 17.4 to 20.1 mm in maximum shell width (Hearty & Olson in press). During the three glacial periods for which there is a fossil record, these snails became gigantic, with shell widths averaging from 31.8 to 39.9 mm. Shell weights of NEL4, for example, were four times or more greater than in the interglacial ZONA that preceded it (Olson & Hearty 2007). Although known collectively as P. nelsoni, these giant morphotypes evolved independently (Hearty et al. 2004; Hearty & Olson in press). Here, we argue that this iterative gigantism was the result of selection pressure from vertebrate predators that colonized and evolved during glacial periods when the land area of Bermuda was at its maximum.
2. Material and methods
Chronostratigraphy, shell morphometrics and vertebrate paleontology are treated in our previous papers (Hearty et al. 2004; Olson et al. 2005; Olson & Hearty 2007; Hearty & Olson in press). Because the morphotypes of subgenus Poecilozonites are only temporal manifestations of a single anagentic lineage, binomial or trinomial designations are inappropriate so we designated them by four-letter abbreviations (Hearty & Olson in press), which we have followed here.
3. Results
During the last glacial period (MIS 2-4), the giant shells of NEL4 occur in association with the large, flightless rail Rallus recessus (Olson & Wingate 2001), which evolved from the large North American rails of the Rallus longirostris/elegans complex. These omnivores include snails in the diet (Eddleman & Conway 2002) and are capable of ingesting entire snails the size of ZONA (figure 2), the morph that prevailed through the preceding interglacial (MIS 5).
Figure 2.
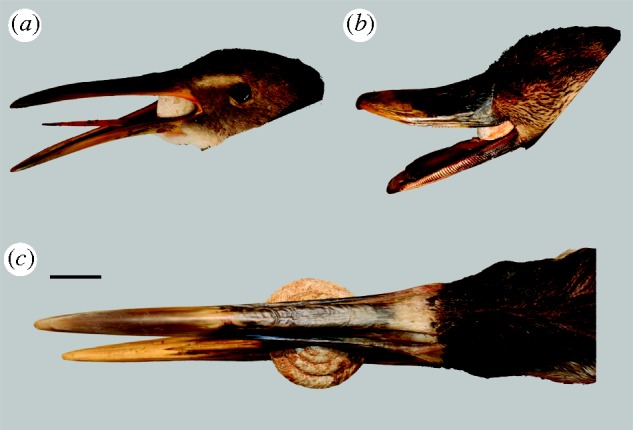
Manipulation of carcasses of modern birds with analogues in the fossil record of Bermuda to show potential swallowing ability of shells of Poecilozonites. (a) Clapper rail Rallus longirostris with shell of Poecilozonites zonatus (ZONA) nearly at the point of ingestion. (b) Black duck Anas rubripes ditto. (c) Whooping crane Grus americana, a species larger than the extinct endemic crane of Bermuda, with shell of Poecilozonites nelsoni (NEL4). The very large size of the snail would not have permitted intact ingestion by any bird known from the Quaternary of Bermuda. Scale bar, 2 cm.
During the penultimate glacial period (MIS 6), snails (NEL6) were also gigantic and identical in size and shape to NEL4 (Hearty et al. 2004; Hearty & Olson in press). MIS 6 in Bermuda was characterized by the ‘crane fauna’ (Olson et al. 2005) that included an endemic crane Grus latipes and an endemic duck Anas pachysceles (Wetmore 1960). The latter was a large dabbling duck the size of a mallard (Anas platyrhynchos) or the North American black duck (Anas rubripes), which include snails in the diet (Longcore et al. 2000; Drilling et al. 2002) and are large enough to ingest entire snails the size of interglacial morphs of Poecilozonites (figure 2). The Bermuda crane was derived from the North American sandhill crane (Grus canadensis), which also eats snails (Tacha et al. 1992) and was roughly similar in size (Wetmore 1960). Although fully capable of ingesting snails the size of interglacial morphs of Poecilozonites, even a larger species of crane would not have been capable of swallowing giant snails the size of NEL6 or NEL4 (figure 2).
The giant morph P. nelsoni callosus (CALL) occurs in the basal sediments of interglacial MIS 9 (Hearty & Olson in press) and we assume that it was probably present during glacial period MIS 10. In addition to being the size of NEL6 and NEL4, the shell was considerably thicker (figure 1) than in those morphs. No representative fauna from MIS 10 is yet known so we do not know if larger land birds colonized and evolved during that period, but a very different predator, the tortoise Hesperotestudo bermudae (Meylan & Sterrer 2000; Olson & Meylan 2010), is known from the same basal MIS 9 sediments as CALL. The only period when such a tortoise could have colonized and evolved on Bermuda was during MIS 10 (Olson et al. 2006). Although the large size of CALL may originally have been a response to large avian predators, the significant thickening of the shell was probably a response to the crushing ability of the tortoise. Living North American tortoises (Gopherus, Terrapene) are omnivorous and include snails in the diet (Ernst & Lovich 2009).
4. Discussion
Temperature, humidity and rainfall patterns would have fluctuated to some degree between glacial and interglacial periods, but the effects of most of those variables on shell evolution in land snails are often ambiguous (Goodfriend 1986). We rejected Gould's (1969) hypothesis that reduced availability of calcium carbonate during glacial periods was a limiting factor in snail evolution on Bermuda based on several different lines of evidence (Olson & Hearty 2007). Therefore, we consider that predation was the factor most likely to have been selecting for gigantism in Poecilozonites.
The simplest response of a snail to increased predation pressure would be an increase in size (Vermeij 1987, 1993), so that selection would favour snails too large to swallow whole. Rapid growth would be advantageous to achieve large size as quickly as possible and in some snails a more rapid growth rate also results in larger adult shell size (Goodfriend 1986). In a perhaps analogous situation, populations of lizards (Podarcis gaigeae) on certain small islands of the Aegean evolved giant forms that were thought to be the product of increased resource availability, leading to increased population densities and intraspecific competition (Pafilis et al. 2009), which involved increased cannibalism so that the proximate cause of rapid growth to a larger size was increased predation (Pafilis et al. 2009).
In the process of escalation (Vermeij 1987), predators may evolve new adaptations for processing shells to circumvent the responses of their prey, but on Bermuda the cycle of escalation was broken by habitat loss and extinction of predators during interglacials owing to loss of land area and prime habitat. The major interglacial inundations of MIS 11, 9, 5 and 1 are all associated with extinction events on Bermuda (Olson & Wingate 2000, 2001; Olson & Hearty, 2003; Hearty et al. 2004; Olson et al. 2005) so that the tortoise of MIS 10/9, the crane and duck of MIS 6 and the large rail of MIS 4-2 became extinct (figure 1) and do not occur in succeeding interglacial deposits. Differential extinction of predators during interglacial periods may have occurred because in the ‘final demise of species, reduction in suitable habitats should place high energy species (with larger body size and) relatively small population sizes, at greatest risk’ (Vermeij 1987, p. 393).
Selection for smaller size in interglacials may have involved a combination of factors. Lomolino (1984) argued that ecological release in the absence of predators is possibly more prevalent than previously realized. Another factor may involve ‘optimum body size for energy acquisition’ (Whittaker & Fernández-Palacios 2007, p. 186) such as has been suggested for insular mammals (Damuth 1993). Poecilozonites was small concurrently with reduced land area when the environment of Bermuda became much more maritime, introducing stresses from reduced diversity of vegetation, increased salt load, periodic drying or flooding, and perhaps increased population densities of snails with reduction of land area. In some snails, reduction in shell size is known to be population density dependent (Goodfriend 1986). Although a number of interacting variables may have facilitated the observed anagenetic pulses in shell size in Poecilozonites on Bermuda, predation, or the lack thereof, seems likely to be the most important process of evolutionary change in this lineage during the late Pleistocene and Holocene. The endemic subgenus Poecilozonites on Bermuda provides an extraordinary example of dramatic evolutionary change in response to environmental variables within a single lineage and a reminder that such evolutionary changes do not necessarily involve a process of speciation.
Acknowledgements
Bird carcasses used to determine swallowing capability were salvaged in compliance with federal and state laws and under the guidance of the Smithsonian Institutions' Animal Care and Use Committee.
We thank Wolfgang Sterrer and Lisa Green, Bermuda Aquarium, Museum and Zoo (BAMZ) for supporting our research and David B. Wingate and Frederick V. Grady for assistance in the field. Rüdiger Bieler, Joachim Gerber, Gustav Paulay, Gary Rosenberg and Geraat Vermeij commented on various drafts. Brian K. Schmidt assisted with graphics preparation. Louise Roth suggested useful references. This is contribution no. 141 of the Bermuda Biodiversity Project of the BAMZ.
References
- Damuth J.1993Copés rule, the island rule and the scaling of mammalian population density. Nature 365, 748–750 (doi:10.1038/365748a0) [DOI] [PubMed] [Google Scholar]
- Drilling N., Titman R., McKinney F.2002Mallard Anas platyrhynchos. Birds North Am. 658, 1–44 [Google Scholar]
- Eddleman W. R., Conway C. J.2002Clapper Rail Rallus longirostris. Birds North Am. 340, 1–32 [Google Scholar]
- Ernst C., Lovich J. E.2009Turtles of the United States and Canada. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- Goodfriend G. A.1986Variation in land-snail shell form and size and its causes: a review. Syst. Zool. 35, 204–223 (doi:10.2307/2413431) [Google Scholar]
- Gould S. J.1969An evolutionary microcosm: Pleistocene and recent history of the land snail P. (Poecilozonites) in Bermuda. Bull. Mus. Comp. Zool. 138, 407–531 [Google Scholar]
- Hearty P. J., Olson S. L.In press Geochronology, biostratigraphy, and changing shell morphology in the land snail subgenus Poecilozonites during the Quaternary of Bermuda. Palaeogeogr. Palaeoclimatol. Palaeoecol. [Google Scholar]
- Hearty P. J., Olson S. L., Kaufman D. S., Edwards R. L., Cheng H.2004Stratigraphy and geochronology of pitfall accumulations in caves and fissures, Bermuda. Quatern. Sci. Rev. 23, 1151–1171 (doi:10.1016/j.quascirev.2003.09.008) [Google Scholar]
- Lomolino M. V.1984Immigrant selection, predation, and the distributions of Microtus pennsylvanicus and Blarina brevicauda on islands. Am. Nat. 61, 376–382 [Google Scholar]
- Longcore J. R., Mcauley D. G., Hepp G. R., Rhymer J. M.2000American black duck Anas rubripes. Birds North Am. 481, 1–36 [Google Scholar]
- Meylan P. A., Sterrer W.2000Hesperotestudo (Testudines: Testudinidae) from the Pleistocene of Bermuda, with comments on the phylogenetic position of the genus. Zool. J. Linn. Soc. 128, 51–76 (doi:10.1111/j.1096-3642.2000.tb00649.x) [Google Scholar]
- Olson S. L., Hearty P. J.2003Extirpation of a breeding colony of short-tailed albatross (Phoebastria albatrus) on Bermuda by Pleistocene sea-level rise. Proc. Natl Acad. Sci. USA 100, 12 825–12 829 (doi:10.1073/pnas.1934576100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson S. L., Hearty P. J.2007Fallacies underlying the assumption of calcium limitation on the evolution of land snails in Bermuda. Veliger 49, 132–139 [Google Scholar]
- Olson S. L., Meylan P. A.2010A second specimen of the Pleistocene Bermuda tortoise, Hesperotestudo bermudae Meylan and Sterrer. Chelonian Conserv. Biol. 8, 211–212(for 2009) (doi:10.2744/CCB-0766.1) [Google Scholar]
- Olson S. L., Wingate D. B.2000Two new species of flightless rails (Aves: Rallidae) from the middle Pleistocene crane fauna of Bermuda. Proc. Biol. Soc. Wash. 113, 356–368 [Google Scholar]
- Olson S. L., Wingate D. B.2001A new species of large flightless rail of the Rallus longirostris/elegans complex (Aves: Rallidae) from the late Pleistocene of Bermuda. Proc. Biol. Soc. Wash. 114, 509–516 [Google Scholar]
- Olson S. L., Wingate D. B., Hearty P. J., Grady F. V.2005Prodromus of vertebrate paleontology and geochronology of Bermuda. Monografies de la Societat d'Història Natural de les Balears 12, 219–232 [Google Scholar]
- Olson S. L., Hearty P. J., Pregill G. K.2006Geological constraints on evolution and survival in endemic reptiles on Bermuda. J. Herpetol. 40, 394–398 (doi:10.1670/0022-1511(2006)40[394:GCOEAS]2.0.CO;2) [Google Scholar]
- Pafilis P., Meiri S., Foufopoulos J., Valakos E.2009Intraspecific competition and high food availability are associated with insular gigantism in a lizard. Naturwissenschaften 96, 1107–1113 (doi:10.1007/s00114-009-0564-3) [DOI] [PubMed] [Google Scholar]
- Tacha T. C., Nesbitt S. A., Vohs P. A.1992Sandhill crane Grus canadensis. Birds North Am. 31, 1–24 [Google Scholar]
- Vermeij G. J.1987Evolution and escalation: an ecological history of life. Princeton, NJ: Princeton University Press [Google Scholar]
- Vermeij G. J.1993A natural history of shells. Princeton, NJ: Princeton University Press [Google Scholar]
- Wetmore A.1960Pleistocene birds in Bermuda. Smithsonian Misc. Coll. 140, 1–11 [Google Scholar]
- Whittaker R. J., Fernández-Palacios J. M.2007Island biogeography, 2nd ednOxford, UK: Oxford University Press [Google Scholar]