Abstract
Plants respond to attack by herbivores or pathogens with the release of volatile organic compounds. Neighbouring plants can receive these volatiles and consecutively induce their own defence arsenal. This ‘plant communication’, however, appears counterintuitive when it benefits independent and genetically unrelated receivers, which may compete with the emitter. As a solution to this problem, a role for volatile compounds in within-plant signalling has been predicted. We used wild-type lima bean (Phaseolus lunatus) to quantify under field conditions the distances over which volatile signals move, and thereby determine whether these cues will mainly trigger resistance in other parts of the same plant or in independent plants. Independent receiver plants exhibited airborne resistance to herbivores or pathogens at maximum distances of 50 cm from a resistance-expressing emitter. In undisturbed clusters of lima bean, over 80 per cent of all leaves that were located around a single leaf at this distance were other leaves of the same plant, whereas this percentage dropped below 50 per cent at larger distances. Under natural conditions, resistance-inducing volatiles of lima bean move over distances at which most leaves that can receive the signal still belong to the same plant.
Keywords: extrafloral nectar, herbivore-induced volatiles, indirect defence, pathogen resistance, plant communication, Phaseolus lunatus
1. Introduction
After the initial report on ‘talking trees’ (Baldwin & Schultz 1983), studies on several species demonstrated resistance induction by airborne cues. Plants respond to attack by herbivores or pathogens with the release of volatile organic compounds (VOCs), which serve multiple defensive functions (Pichersky et al. 2006; Unsicker et al. 2009). Being released in response to attack, such VOCs indicate the presence of herbivores or pathogens. Neighbouring plants might, thus, gain a fitness benefit by monitoring these VOCs to pre-empt encounters witch their enemies and consecutively induce their own defence. This effect was first described for herbivore resistance (Baldwin & Schultz 1983; Rhoades 1983; Farmer & Ryan 1990; Karban et al. 2000; see Heil & Karban 2010 for a review) but can also affect resistance to pathogens (Shulaev et al. 1997; Yi et al. 2009).
Plant communication would, however, contradict our understanding of evolution when it benefits genetically independent receiver individuals at the cost of the emitter. What is the benefit for the emitter? As one explanation, a role of VOCs in within-plant signalling has been predicted (Farmer 2001) and was observed for sagebrush (Artemisia tridentata), lima bean (Phaseolus lunatus), poplar (Populus deltoides x nigra) and blueberry (Vaccinium corymbosum) (Karban et al. 2006; Frost et al. 2007; Heil & Silva Bueno 2007; Rodríguez-Saona et al. 2009). VOCs released from attacked organs prepare the as-yet unaffected parts of the same plant for resistance expression (Frost et al. 2008; Heil & Ton 2008). Because most herbivores and many pathogens move independently of the vascular system, VOCs appear particularly suitable for reaching parts of the plant that are spatially, but not anatomically, located close to the attacked organ (Heil & Karban 2010).
The question remained open, though, whether volatiles affect mainly other leaves of the same plant or those of independent neighbours. Because the ratio of ‘own’ versus ‘foreign’ leaves that can respond to volatiles depends on the distances over which these cues are exchanged at active concentrations, we used wild lima bean to determine signalling distances in nature. Emitters were induced and receiver shoots at different distances from the emitter (figure 1) were monitored for extrafloral nectar (EFN) secretion or resistance to bacteria. We then used undisturbed clusters of lima bean to estimate what percentage of leaves at the tested distances belongs to the same plant. Our results demonstrate that most leaves that receive lima bean VOCs at active concentrations usually belong to the same plant.
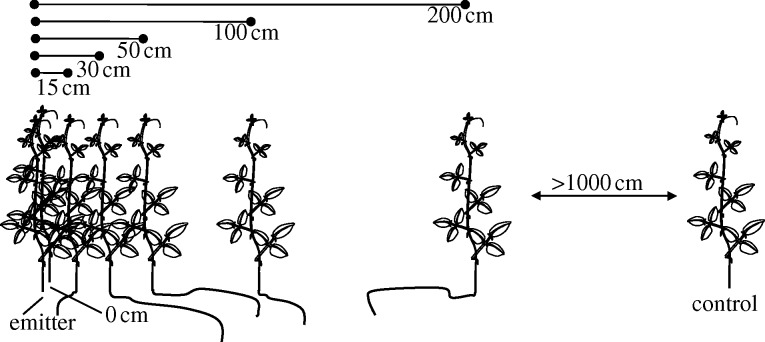
Figure 1.
Experimental setup. Single shoots of six receiver plants were placed at different distances from induced emitters and then investigated for their level of resistance to herbivores and pathogens.
2. Material and methods
We used a population of P. lunatus located in the coastal area of Oaxaca (México, Pacific coast, ∼15°55′ N and 97°09′ W). Plants were selected in 20 groups of each 7 individuals. We made use of the long (greater than 15 m) tendrils of lima bean, which allowed us to place six receiver shoots of naturally growing plants at different distances from an independent emitter (figure 1). One individual per group (the emitter) received one of two treatments. An aqueous 1 mM solution of jasmonic acid (JA) was applied to eight shoots. JA induces EFN and VOCs at concentrations similar to what is seen after natural herbivore damage (Kost & Heil 2006; Heil & Silva Bueno 2007); these VOCs prime receivers for EFN secretion (Heil & Kost 2006). An aqueous solution of 3 mg l−1 benzothiadiazole (BTH) was applied to 12 shoots. BTH induces resistance to pathogens and the release of methyl salicylate and nonanal, which prime resistance to bacterial pathogens in receivers (Yi et al. 2009). Emitters were treated at 09.00, and after drying, receivers were positioned at distances of 0, 15, 30, 50, 100 and 200 cm (figure 1). Distances between the closest groups were at least 10 m. In the groups with JA-treated emitters, the seven youngest leaves of all tendrils were mechanically damaged after 24 h to induce EFN secretion and then placed in mesh bags (Kost & Heil 2006). In the groups with BTH-treated emitters, the 10 youngest leaves of all shoots were challenged with Pseudomonas syringae pv syringae (strain 61 preselected for resistance to rifampicin) after 5 days of exposure (Yi et al. 2009). Controls received the same damage or challenging treatment but were growing more than 12 m from the closest emitter.
EFN secretion was quantified 6 h after damage as amounts of soluble solids per gram leaf dry mass (Kost & Heil 2006). Bacterial titres in the leaves were determined at day 0 (before applying bacteria) and at days 2 and 4 after challenge by counting colony-forming units (CFUs) as described previously (Yi et al. 2009). The results from all leaves of one shoot were averaged. Data were subjected to univariate analysis of variance (general linear model) with ‘distance’ and—in the case of BTH-treated groups—‘day’ as fixed factor(s) and ‘plant group’ as random factor. Post hoc analyses for distance were conducted as least significant distance (LSD) tests with SPSS 17.0.
To estimate the proportion of own leaves at different distances, we used concentric circles with a radius of 15, 30, 50, 100 and 200 cm around single lima bean leaves (n = 13 repetitions located along three spatially separated transects) and counted all leaves that belonged to the same plant and all leaves that belonged to other plants (because of the size of natural lima bean individuals these were mainly representing leaves of other species) in circles at the described distances around the leaf at the origin.
3. Results
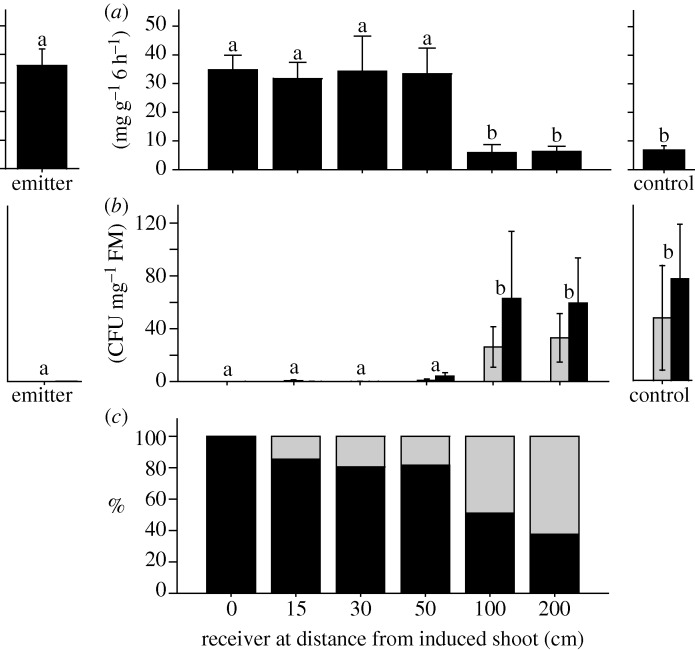
The distance from the induced emitter had a significant effect on the rates of EFN secretion of receivers (p < 0.001, figure 2) and in the numbers of CFUs that were obtained from their leaves (p < 0.05, figure 2). The effects in receivers at up to 50 cm did not differ significantly from those in the directly induced emitters, but they were significantly (post hoc LSD tests: p < 0.05) different from those at 100 and 200 cm and from the controls. In contrast, no significant differences could be detected among receivers at 100 and 200 cm and controls (figure 2). Thus, the resistance induction by volatile cues dropped from full levels to control levels at a distance between 50 and 100 cm, and no difference in the signalling distance was found between resistance to herbivores and to pathogens. More than 80 per cent of all leaves in circles with a radius of up to 50 cm around a single lima bean leaf were other leaves of the same plant, whereas this percentage dropped below 50 per cent in circles with a radius of 100 cm or more (figure 2).
Figure 2.
Distances in plant–plant communication. (a) EFN secretion was quantified as a measure of indirect resistance to herbivores in milligram soluble solids secreted per gram leaf fresh mass over 6 h. (b) Numbers of CFUs were determined in leaves that had been challenged with Pseudomonas syringae as a measure of resistance to pathogens and are expressed as CFU per mg leaf fresh mass. Bars represent means ±s.e., means marked with different letters were significantly different (p < 0.05 according to LSD post hoc test). Grey bar, day 2; black bar, day 4. (c) Proportions of own (black bar) versus foreign (grey bar) leaves in circles with a radius of the same distances at which receivers had been positioned. 0 cm, leaf in the centre of the circles.
4. Discussion
Sagebrush (A. tridentata) plants accumulated less natural damage when receiving volatile cues from genetically identical cuttings when compared with non-self cuttings (Karban & Shiojiri 2009). Although reliable self-recognition can reduce the danger of eavesdropping, communication among plants can cross species borders (Farmer & Ryan 1990; Karban et al. 2000; Glinwood et al. 2004). Genetic identity is no necessary prerequisite for functioning communication and plant VOCs do not necessarily represent ‘private messages’ (Gershenzon 2007). Sending the signal only over distances at which the receivers will usually be another part of the emitter (or, at least, a closely related plant of the same species) could, therefore, reduce the risk of providing competing neighbours with beneficial information. Our current study indicates that most leaves that were exposed to lima bean VOCs at active concentrations belonged to the same plant. This result is in line with the assumption that signalling by airborne cues mainly represents within-plant signalling, rather than communication among different individuals.
VOCs move freely through the air and their distribution depends on wind speed and air temperature, whereas their chemical nature and concentration are species-specific traits. It is difficult, therefore, to generalize our result without further studies. Interestingly, sagebrush emits high amounts of methyl jasmonate (Farmer & Ryan 1990) and can affect other plants at distances of up to 60 cm (Karban et al. 2006): the range that we found for lima bean. If this is to represent a general pattern, we would predict that large and anatomically complex plants emit volatiles at higher concentrations than small and anatomically simple plants. Intriguingly, the four species for which a role of VOCs in within-plant signalling has been demonstrated represent a tree, two shrubs and a liana (Karban et al. 2006; Frost et al. 2007; Heil & Silva Bueno 2007; Rodríguez-Saona et al. 2009). Besides the study on sagebrush (Karban et al. 2006), we are, however, not aware of another study that measured the distances (or concentrations) over which VOCs remain active and no study has correlated signalling distances with the relative proportion of own versus foreign leaves. Future studies will have to (i) control for signals exchanged among roots and (ii) determine dose–response relations between the concentration of volatile cues and the intensity of the response, in order to investigate how a correlation of signalling distance with the percentage of own leaves can be achieved at the genetic and the physiological level and whether similar mechanisms assure that also other cases of plant communication mainly remain a ‘soliloquy’.
Acknowledgements
We thank Ralf Krüger for help with the field experiments, an anonymous referee for valuable comments on an earlier version of this manuscript and CONACyT de México (Clave: 160379) for financial support.
References
- Baldwin I. T., Schultz J. C.1983Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science 221, 277–279 (doi:10.1126/science.221.4607.277) [DOI] [PubMed] [Google Scholar]
- Farmer E. E.2001Surface-to-air signals. Nature 411, 854–856 (doi:10.1038/35081189) [DOI] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A.1990Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl Acad. Sci. USA 87, 7713–7716 (doi:10.1073/pnas.87.19.7713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost C., Appel H., Carlson J., De Moraes C., Mescher M., Schultz J.2007Within-plant signalling by volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol. Lett. 10, 490–498 (doi:10.1111/j.1461-0248.2007.01043.x) [DOI] [PubMed] [Google Scholar]
- Frost C., Mescher M. C., Carlson J., De Moraes C. M.2008Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol. 146, 818–824 (doi:10.1104/pp.107.113027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenzon J.2007Plant volatiles carry both public and private messages. Proc. Natl Acad. Sci. USA 104, 5257–5258 (doi:10.1073/pnas.0700906104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinwood R., Ninkovic V., Pettersson J., Ahmed E.2004Barley exposed to aerial allelopathy from thistles (Cirsium spp.) becomes less acceptable to aphids. Ecol. Entomol. 29, 188–195 (doi:10.1111/j.0307-6946.2004.00582.x) [Google Scholar]
- Heil M., Karban R.2010Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol. 25, 137–144 (doi:10.1016/j.tree.2009.09.010) [DOI] [PubMed] [Google Scholar]
- Heil M., Kost C.2006Priming of indirect defences. Ecol. Lett. 9, 813–817 (doi:10.1111/j.1461-0248.2006.00932.x) [DOI] [PubMed] [Google Scholar]
- Heil M., Silva Bueno J. C.2007Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl Acad. Sci. USA 104, 5467–5472 (doi:10.1073/pnas.0610266104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M., Ton J.2008Long-distance signalling in plant defence. Trends Plant Sci. 13, 264–272 (doi:10.1016/j.tplants.2008.03.005) [DOI] [PubMed] [Google Scholar]
- Karban R., Shiojiri K.2009Self-recognition affects plant communication and defense. Ecol. Lett. 12, 502–506 (doi:10.1111/j.1461-0248.2009.01313.x) [DOI] [PubMed] [Google Scholar]
- Karban R., Baldwin I., Baxter K., Laue G., Felton G.2000Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125, 66–71 (doi:10.1007/PL00008892) [DOI] [PubMed] [Google Scholar]
- Karban R., Shiojiri K., Huntzinger M., McCall A. C.2006Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication. Ecology 87, 922–930 (doi:10.1890/0012-9658(2006)87[922:DRISVA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Kost C., Heil M.2006Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J. Ecol. 94, 619–628 (doi:10.1111/j.1365-2745.2006.01120.x) [Google Scholar]
- Pichersky E., Noel J. P., Dudareva N.2006Biosynthesis of plant volatiles: nature's diversity and ingenuity. Science 311, 808–811 (doi:10.1126/science.1118510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades D. F.1983Responses of alder and willow to attack by tent caterpillars and webworms: evidence for pheromonal sensitivity of willows. In Plant resistance to insects (ed. Hedin P. A.), pp. 55–68 Washington, DC, USA: American Chemical Society [Google Scholar]
- Rodríguez-Saona C. R., Rodríguez-Saona L. E., Frost C. J.2009Herbivore-induced volatiles in the perennial shrub, Vaccinium corymbosum, and their role in inter-branch signaling. J. Chem. Ecol. 35, 163–175 (doi:10.1007/s10886-008-9579-z) [DOI] [PubMed] [Google Scholar]
- Shulaev V., Silverman P., Raskin I.1997Airborne signalling by methyl salicylate in plant pathogen resistance. Nature 385, 718–721 (doi:10.1038/385718a0) [Google Scholar]
- Unsicker S. B., Kunert G., Gershenzon J.2009Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 12, 479–485 (doi:10.1016/j.pbi.2009.04.001) [DOI] [PubMed] [Google Scholar]
- Yi H.-S., Heil M., Adame-Álvarez R.-M., Ballhorn D., Ryu M.2009Airborne induction and priming of plant resistance to a bacterial pathogen. Plant Physiol. 151, 2152–2161 (doi:10.1104/pp.109.144782) [DOI] [PMC free article] [PubMed] [Google Scholar]