Abstract
Traumatic fear memories can be inhibited by behavioral therapy for humans, or by extinction training in rodent models, but are prone to recur. Under some conditions, however, these treatments generate a permanent effect on behavior, which suggests that emotional memory erasure has occurred. The neural basis for such disparate outcomes is unknown. We found that a central component of extinction-induced erasure is the synaptic removal of calcium-permeable α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors (AMPARs) in the lateral amygdala. A transient up-regulation of this form of plasticity, which involves phosphorylation of the glutamate receptor 1 subunit of the AMPA receptor, defines a temporal window in which fear memory can be degraded by behavioral experience. These results reveal a molecular mechanism for fear erasure and the relative instability of recent memory.
Fear conditioning is the development of a fearful emotional response to a neutral cue [a conditioned stimulus (CS)] that occurs together with an unpleasant event [an unconditioned stimulus (US)]. Fear memories present as defensive reactions to the CS for a period of up to many months after learning (1). However, when conditioned subjects repeatedly encounter a CS without a reinforcing US, responses to a CS that no longer predicts harm are diminished in a process known as extinction. This paradigm is analogous to human exposure-based therapy for traumatic memories (2), in which maladaptive fear is suppressed by exposure to stimuli that were present at the time of trauma. However, because fear can recur after these treatments, extinction probably does not affect emotional memory, which can later be reactivated by specific cues (3, 4).
A number of studies indicate that a more lasting reduction of fear can be dependent on the conditions at the time of extinction, such as the age of the subjects (5), the intertrial spacing of stimuli (6–8), or the time elapsed since initial learning (9, 10). Because even strong reminder cues do not trigger the recurrence of conditioned fear in some cases (5–8), an intriguing possibility is that, under the right conditions, permanent erasure of fear memory occurs during the course of extinction. Such techniques could be useful in the treatment of emotional disorders. However, maximizing this approach requires an understanding of the cellular and molecular mechanisms underlying this fear erasure, which at present remain obscure.
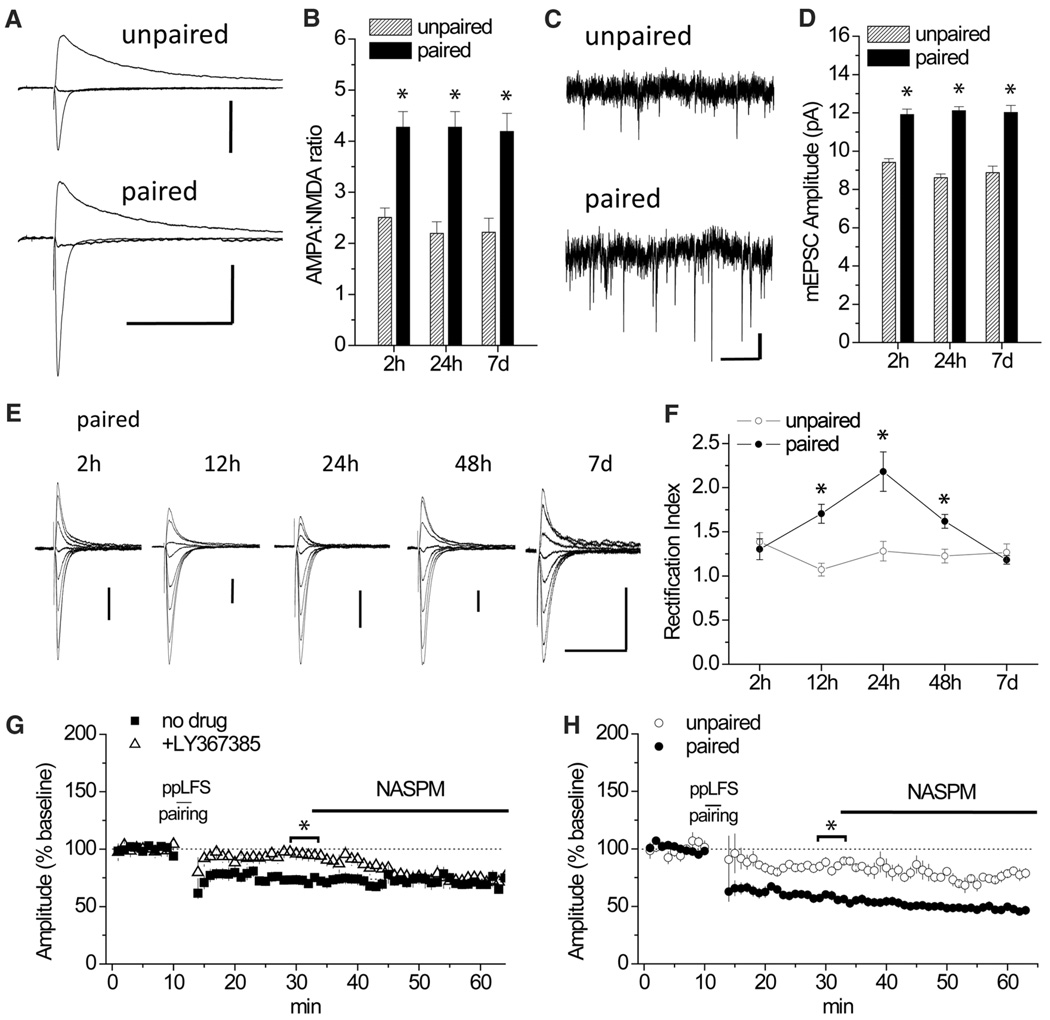
Overwhelming evidence indicates that plasticity within amygdala circuits underlies fear conditioning in animals and humans (11, 12). Cue-dependent fear conditioning is thought to be mediated by the potentiation of glutamatergic synaptic transmission in the lateral amygdala (LA) (13, 14). Correspondingly, we observed an enhancement of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptor (AMPAR) excitatory postsynaptic currents (AMPAR-EPSCs) at thalamic afferents to LA neurons after auditory fear conditioning in mice. This enhancement was evident as both an increase in the ratio of evoked AMPAR- to N-methyl-D-aspartate receptor (NMDAR)–mediated currents (Fig. 1, A and B, and fig. S1), as well as larger AMPAR miniature EPSC amplitudes (mEPSCs, Fig. 1, C and D, and fig. S2). These enhancements lasted for 7 days, suggesting that this potentiation may underlie the long-term maintenance of fear memory.
Fig. 1.
Fear conditioning alters AMPAR subunit composition and LTD at thalamo-LA synapses. Training entailed six CSs (auditory tones) and USs (footshocks) delivered in a paired or unpaired configuration. (A) EPSCs at membrane holding potential (Vh) = −70, 0, and +40 mV, scaled to peak amplitude at +40 mV, at 24 hours after conditioning. Scale bars, 100 pA × 200 ms. (B) AMPA:NMDA ratio as a function of time after conditioning. *P < 0.01 analysis of variance (ANOVA); Tukey’s post-hoc test for paired (n = 11 to 12 cells) versus unpaired (n = 7 to 10 cells) configuration. h, hours; d, days. (C) mEPSCs at 24 hours after conditioning. Scale bar, 5 pA × 1 s. (D) mEPSC amplitude as a function of time after conditioning. *P < 0.0001 ANOVA; Tukey’s posthoc paired versus unpaired cells. (E) AMPAR-EPSCs after paired conditioning at Vh = −70, −60, −40, −20, 0, +20, +40, and +50 mV, normalized to peak amplitude at −70mV. Scale bars, 50 pA × 40 ms. (F) Rectification index. *P < 0.05 ANOVA; Tukey’s post-hoc for paired (n = 6 to 9 cells) versus unpaired (n = 5 to 7 cells) configuration. (G) In slices from naïve mice, LTD induction by ppLFS pairing (with a 3-Hz paired-pulse stimulation at a 50-ms interpulse interval, for 3 min at −50 mV) without drug (n = 6 cells) or in the presence of LY367385 (100 µM, n = 6 cells), followed by normalization of EPSC amplitude by NASPM. (H) Enhanced LTD 24 hours after conditioning. Paired (n = 6 cells) and unpaired (n = 5 cells) configurations are shown. *P < 0.05, Student’s t test.
In principal neurons throughout the adult brain, AMPARs containing the glutamate receptor 2 subunit (GluA2) predominate (15). However, in vivo experience induces in other brain areas a switch from GluA2-containing to GluA2-lacking AMPARs (16–18), which are known as calcium-permeable (CP-AMPARs). We therefore assayed for CP-AMPARs at thalamic synapses before and after fear conditioning in mice. In naïve animals, current-voltage plots of AMPAR-EPSCs revealed a slight degree of inward rectification (fig. S3), which is a signature of CP-AMPARs, and rectification was eliminated by the CP-AMPAR antagonist 1-naphthylacetyl spermine (NASPM). Our examination of responses after fear conditioning revealed a slowly developing enhancement of rectification (Fig. 1, E and F, and figs. S4 and S5), mirroring an increase in AMPAR-EPSC inhibition by NASPM (fig. S6). Both increased rectification and NASPM sensitivity appeared within hours after conditioning, peaked at 24 hours, and disappeared by day 7. Thus, synapses were modified by the transient addition of CP-AMPARs even as transmission strength remained stable.
Long-term depression (LTD), an in vitro model of experience-dependent synaptic weakening, has been associated with the trafficking of CP-AMPARs in other systems (16, 19–21). We therefore examined the effect of the presence of CP-AMPARs on LTD in mouse brain slices before and after fear conditioning. In naïve animals, LTD could be induced when LA neurons were mildly depolarized (−50 mV) in conjunction with paired-pulse low-frequency stimulation (ppLFS pairing) (Fig. 1G). The induction of this form of LTD was mediated by NMDARs and metabotropic glutamate receptor 1 (mGluR1) but not mGluR5 (fig. S7). When treated with NASPM after ppLFS pairing, neurons exhibited no further decrease in EPSC amplitude except when LTD had been blocked by inhibition of mGluR1 (Fig. 1G), indicating that LTD was expressed by selective removal of CP-AMPARs. At 24 hours after fear conditioning, when CP-AMPARs are elevated, LTD was of much greater magnitude (Fig. 1H). Synaptic removal of CP-AMPARs also mediated LTD at this time, because AMPAR rectification (fig. S8) and sensitivity to NASPM (Fig. 1H) were both eliminated by ppLFS pairing. These results suggest that CP-AMPAR incorporation after fear conditioning increases the capacity for synaptic weakening through mGluR1-dependent LTD.
Because synaptic CP-AMPARs reach their peak level at 1 day after fear conditioning, we hypothesized that the reversal of fear-dependent synaptic potentiation by removal of CP-AMPARs, and thus fear erasure, might be more easily achieved at this time by using specific extinction protocols. An extinction protocol that can fully eliminate fear, here referred to as reconsolidation update, has recently been described (6). This protocol targets a labile period after memory retrieval, when treatment with amnesic drugs leads to complete memory loss (22). Similarly, extinction training in this window permanently attenuates fear. The critical requirement for this effect is exposure to a single isolated CS (retrieval) between 10 min and 6 hours before extinction.
Thus, we subjected our mice to retrieval 30 min before extinction (Fig. 2A), and after extinction, fear relapse was examined in spontaneous recovery and renewal tests (Fig. 2, D to F). Spontaneous recovery develops passively with time and is measured in the extinction context, whereas renewal can be triggered at any time by exposure to the CS in the training context. As a negative control, we examined mice treated identically to retrieval subjects, except that retrieval was omitted and one additional CS was delivered during extinction to balance total CS exposure (Fig. 2, A to C, no retrieval). When extinction was performed on day 1 after conditioning (day 0), neither group exhibited spontaneous recovery on day 2 (Fig. 2D). However, no-retrieval controls exhibited significant renewal on day 2, and by day 7 they exhibited spontaneous recovery in addition to renewal after extinction on day 1 (Fig. 2E). In contrast, retrieval mice extinguished on day 1 displayed no fear relapse under any condition (Fig. 2, D and E), which is consistent with memory erasure. To determine whether reconsolidation update, like LTD-induced removal of CP-AMPARs (Fig. 1G), requires the activation of mGluR1, we examined fear relapse in mice that were treated systemically with 1-aminoindan-1,5-dicarboxylic acid [AIDA, 2 mg per kilogram of body weight (mg/kg)] 1 hour before retrieval extinction (Fig. 2G). AIDA-injected mice, but not saline controls, exhibited significant recurrence of fear in spontaneous recovery and renewal tests on day 7. Thus, reconsolidation update and CP-AMPAR–mediated LTD share a requirement for mGluR activation.
Fig. 2.
Reconsolidation update performed soon after fear conditioning (FC) erases fear. (A) Experimental groups. Ret, retrieval; Ext, extinction. (B and C) CS-evoked freezing during conditioning and extinction. (D to F) Freezing was averaged for the first and last four trials of extinction. Spontaneous recovery (Spont Rec) and renewal were assessed by test comparison with the last four trials of extinction. Repeated-measures ANOVA revealed a significant group × test interaction in (D) [F3,39 = 10.1, P < 0.0001 (n = 7 to 8 mice)] and (E) [F3,36 = 9.6, P < 0.0001 (n = 7 to 8 mice)] but not in (F) [F3,36 = 0.34, P = 0.80 (n = 6 to 8 mice)]. *P < 0.001, Tukey’s post-hoc comparison with the last four trials. #P < 0.0001. (G) Effect of systemic AIDA 1 hour before retrieval extinction (Ret-Ext). Repeated-measures ANOVA, treatment × test: F3,48 = 4.95, P < 0.01 (n = 8 to 10 mice). *P < 0.01, Tukey’s post-hoc comparison with the last four trials. #P < 0.01.
We next examined whether synaptic depression underlies fear memory erasure in mouse brain slices prepared after reconsolidation update on day 1. Here we included two additional controls to determine how retrieval and no-retrieval extinction might differentially affect synaptic properties (Fig. 3A). Unpaired controls experienced CS/US cues that were explicitly unpaired to control for stimulus effects unrelated to memory formation. Context-only controls underwent paired conditioning and were exposed to the extinction arena without any tones. As expected, context-only mice displayed high tone-evoked freezing 2 hours after context exposure (Fig. 3B). Although no-retrieval controls showed significantly less freezing than context-only mice in the test of spontaneous recovery, fear renewal in the conditioning context eliminated this difference. Conversely, under both test conditions, retrieval mice displayed lower freezing than context-only controls (Fig. 3B), and when tested on day 7, this difference was still present (fig. S9).
Fig. 3.
Reconsolidation update dampens amygdala transmission via removal of synaptic CP-AMPARs. (A) Treatment groups for synaptic physiology. (B) Freezing during spontaneous recovery and renewal tests 2 hours after extinction. *P < 0.01 ANOVA, Tukey’s post-hoc test (n = 6 mice each). (C to F) AMPAR properties 2 hours after extinction, including AMPA:NMDA ratio [(C), n = 7 to 9 cells], AMPAR-mEPSC amplitude [(D), n = 8 to 18 cells], AMPAR-EPSC rectification index [(E), n = 7 to 13 cells], and AMPAR-EPSC sensitivity to NASPM [(F), n = 5 to 6 cells]. *P < 0.01 ANOVA, Tukey’s post-hoc test. Scale bars for (C) to (E) are as follows: (C) 100 pA × 40 ms, (D) 5 pA × 20 ms, (E) 50 pA × 40 ms. (F) Representative traces from time points a and b; scale bars, 100 pA × 20 ms. (G) ppLFS pairing LTD followed by test for CP-AMPARs by NASPM (50 µM). *P < 0.01 ANOVA followed by Tukey’s post-hoc comparison of retrieval with context-only and no-retrieval. Representative traces from time points a, b, and c are shown; scale bars, 100 pA × 20 ms.
Electrophysiological recordings 2 hours after reconsolidation update revealed a significant decrease in AMPAR-mediated transmission in the retrieval group as compared to context-only and no-retrieval controls (Fig. 3, C and D, and fig. S9), demonstrating that reconsolidation update reverses fear-related synaptic strengthening. This reversal was accompanied by the selective removal of synaptic CP-AMPARs, because rectification of AMPAR-EPSCs and sensitivity to NASPM was greatly reduced after reconsolidation update (Fig. 3, E and F). Because no effect of extinction on AMPAR-EPSCs was seen in no-retrieval animals, as compared to context-only controls, we conclude that reconsolidation update reduces transmission by removing CP-AMPARs.
Furthermore, ppLFS pairing revealed that LTD was significantly enhanced in context-only and no-retrieval animals (Fig. 3G), indicating that CP-AMPAR incorporation had increased the capacity for synaptic weakening. Conversely, because of prior synaptic removal of CP-AMPARs in retrieval animals, further synaptic depression was occluded. NASPM had no effect after LTD, which indicates that changes in LTD could not be explained by reduced coupling of synaptic activity to CP-AMPAR trafficking.
In the above experiments, reconsolidation update was performed on day 1 after fear conditioning, when CP-AMPARs are transiently elevated and susceptible to removal by reconsolidation update. To examine whether erasure is effective after CP-AMPARs have subsided, we performed reconsolidation update on day 7. In that case, we observed significant recurrence of fear in both no-retrieval and retrieval groups on day 14 (Fig. 2F). Furthermore, on day 7, reconsolidation update conferred no benefit over no-retrieval extinction in the reduction of fear, suggesting that an abundance of CP-AMPARs at the time of extinction enables erasure.
Having established a role for CP-AMPARs in reconsolidation update, we next investigated the molecular requirements for their synaptic delivery. Conditioned fear requires glutamate receptor 1 (GluA1)–containing AMPARs (23, 24), whose phosphorylation-dependent trafficking underlies synaptic plasticity (25–27). Phosphorylation of the protein kinase A (PKA) target serine-845 (S845A) in GluA1 has also been shown to regulate the stability of CP-AMPARs (19). We therefore examined CP-AMPAR dynamics in GluA1 phosphorylation site knockin (KI) mutant mice. Alanine substitution of the protein kinase C (PKC)/calcium-calmodulin–dependent kinase II target residue serine-831 (S831A) did not prevent an increase in rectification 24 hours after conditioning (Fig. 4, A and B). However, mutation of the PKA site S845A abolished fear-induced enhancement of CP-AMPAR currents (Fig. 4, A to C). Despite their failure to accumulate CP-AMPARs, however, S845A synapses were nevertheless potentiated by fear conditioning (Fig. 4, D to F). However, because of the absence of CP-AMPAR dynamics, LTD was unaffected by fear conditioning or extinction in S845A mutants (Fig. 4G).
Fig. 4.
GluA1 phosphorylation regulates LTD and fear erasure by controlling synaptic CP-AMPAR levels. (A) AMPAR-EPSCs at Vh = −70, −60, −40, −20, 0, +20, +40, and +50 mV, scaled to peak amplitude at −70 mV, in homozygous GluA1 S831A mice (n = 6 each) and S845A knockin mutant mice (n = 6 to 8) 24 hours after conditioning. Scale bars, 100 pA × 40 ms. (B) Rectification index 24 hours after conditioning. *P < 0.02, Student’s t test. (C to F) For S845A mutants 24 hours after conditioning, the NASPM sensitivity of AMPAR-EPSCs [(C), n = 5 to 6 cells], the AMPA:NMDA ratio [(D) and (E), n = 9 to 14 cells], and the AMPAR-mEPSC amplitude [(F), n = 7 to 8 cells] are shown. *P < 0.001, Student’s t test. Scale bars in (D), 100 pA × 200 ms. (G) ppLFS pairing in S845A knockin mice 2 hours after reconsolidation update on day 1 (as in Fig. 2). (H and I) Spontaneous recovery and renewal in S845A knockin mice and wild-type littermates after reconsolidation update. (H) Repeated measures ANOVA, group × test interaction: for the wild type, F3,33 = 3.29, P < 0.05 (n = 5 to 8 mice); for S845A mice, F3,33 = 1.69, P = 0.19 (n = 5 to 8 mice). (I) Repeated measures ANOVA, group × test interaction: for the wild type, F3,36 = 4.72, P < 0.01 (n = 6 to 8 mice); for S845A mice, F3,36 = 0.54, P = 0.66 (n = 6 to 8 mice). *P < 0.01 Tukey’s post-hoc comparison with the last four trials. #P < 0.01.
Because CP-AMPAR synaptic removal underlies reconsolidation update, we next examined fear memory erasure in GluA1 mutants. Freezing in S845A animals was equivalent to that in wild-type littermates during conditioning on day 0 and reconsolidation update on day 1 (Fig. 4, H and I, and fig. S10). However, retrieval extinction was accompanied by significant renewal of fear on day 2 in S845A mutants but not in wild-type littermates (Fig. 4H). In addition, on day 7 after reconsolidation update on day 1, S845A retrieval mice displayed fear recurrence in both spontaneous recovery and renewal tests (Fig. 4I), whereas wild-type littermates did not. Conversely, S831A mutants did not relapse under these conditions (fig. S11), indicating that serine-845 phosphorylation is a specific prerequisite for memory erasure during reconsolidation update. These results do not rule out effects of S845A mutation in brain regions beyond the amygdala. However, a wealth of data illustrates the requirement for LA synaptic plasticity in conditioned fear (11, 12), supporting our conclusion that the reversal of synaptic strengthening by CP-AMPAR trafficking (Fig. 3) mediates fear erasure.
Our data reveal that even as fear memories are maintained by persistent increases in excitatory transmission, underlying changes in the molecular composition of AMPARs make these imprints vulnerable to erasure. These effects demonstrate the behavioral significance of GluA1 serine-845 phosphorylation and CP-AMPAR dynamics, which occur in several brain regions after in vivo experience (15), and suggest that this form of metaplasticity may loosen constraints on behavioral reversals in other models. In particular, a switch from GluA2-containing to GluA2-lacking AMPARs occurs in the ventral tegmental area (16) and nucleus accumbens (18) after cocaine experience. Thus, CP-AMPAR trafficking may also constitute a strategy for the erasure of drug memories. Indeed, it is also possible that CP-AMPARs regulate memory erasure and editing through their accumulation at additional sites throughout the brain.
Coactivation of NMDARs and mGluR1, which removes synaptic CP-AMPARs during in vitro LTD, may distinguish reconsolidation update from conventional extinction. Interestingly, pharmacological enhancement of NMDAR currents alleviates the recurrence of fear after extinction (28, 29). Our results suggest that this effect may be attributable to CP-AMPAR synaptic removal. Similarly, mGluR1 may also be an effective drug target for improving the long-term success of extinction, or exposure-based therapy, in preventing the return of emotional responses.
Extinction-induced erasure has been interpreted as interference with the reconsolidation of memory by CS exposure (6). The progressive consolidation of memories onto new substrates, or even their translocation to different brain areas (30), may dictate that recent and remote memories undergo different forms of editing. Our data indicate that soon after initial consolidation, changes in synaptic properties render the permanence of recent memory highly reversible. This period may be important for the fine-tuning of information stored by the fear system, as well as a useful point for intervention in alleviating traumatic memories.
Supplementary Material
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/science.1195298/DC1
Materials and Methods
Figs. S1 to S11
References
References and Notes
- 1.Gale GD, et al. J. Neurosci. 2004;24:3810. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNally RJ. Clin. Psychol. Rev. 2007;27:750. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Herry C, et al. Eur. J. Neurosci. 2010;31:599. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- 4.Pape HC, Pare D. Physiol. Rev. 2010;90:419. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gogolla N, Caroni P, Lüthi A, Herry C. Science. 2009;325:1258. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 6.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Science. 2009;324:951. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiller D, et al. Nature. 2010;463:49. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urcelay GP, Wheeler DS, Miller RR. Learn. Behav. 2009;37:60. doi: 10.3758/LB.37.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huff NC, Hernandez JA, Blanding NQ, LaBar KS. Behav. Neurosci. 2009;123:834. doi: 10.1037/a0016511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maren S, Chang CH. Proc. Natl. Acad. Sci. U.S.A. 2006;103:18020. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues SM, Schafe GE, LeDoux JE. Neuron. 2004;44:75. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Phelps EA, LeDoux JE. Neuron. 2005;48:175. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 13.McKernan MG, Shinnick-Gallagher P. Nature. 1997;390:607. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 14.Rogan MT, Stäubli UV, LeDoux JE. Nature. 1997;390:604. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 15.Liu SJ, Zukin RS. Trends Neurosci. 2007;30:126. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Bellone C, Lüscher C. Nat. Neurosci. 2006;9:636. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 17.Clem RL, Barth A. Neuron. 2006;49:663. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Conrad KL, et al. Nature. 2008;454:118. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He K, et al. Proc. Natl. Acad. Sci. U.S.A. 2009;106:20033. [Google Scholar]
- 20.Ho MT, et al. J. Neurosci. 2007;27:11651. doi: 10.1523/JNEUROSCI.2671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu SQ, Cull-Candy SG. Nature. 2000;405:454. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- 22.Nader K, Hardt O. Nat. Rev. Neurosci. 2009;10:224. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 23.Humeau Y, et al. J. Neurosci. 2007;27:10947. doi: 10.1523/JNEUROSCI.2603-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rumpel S, LeDoux J, Zador A, Malinow R. Science. 2005;308:83. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 25.Boehm J, et al. Neuron. 2006;51:213. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Nature. 2000;405:955. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 27.Lee HK, et al. Cell. 2003;112:631. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 28.Mao SC, Hsiao YH, Gean PW. J. Neurosci. 2006;26:8892. doi: 10.1523/JNEUROSCI.0365-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker DL, Ressler KJ, Lu KT, Davis M. J. Neurosci. 2002;22:2343. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacco T, Sacchetti B. Science. 2010;329:649. doi: 10.1126/science.1183165. [DOI] [PubMed] [Google Scholar]
- 31.Supported by grants from NIH (R01NS036715) and the Howard Hughes Medical Institute. R.L.C. was supported by a postdoctoral fellowship from NIH (F32 MH087037-01). We thank G. Thomas, D. Linden, L. Volk, and A. Lade for helpful comments.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.