Abstract
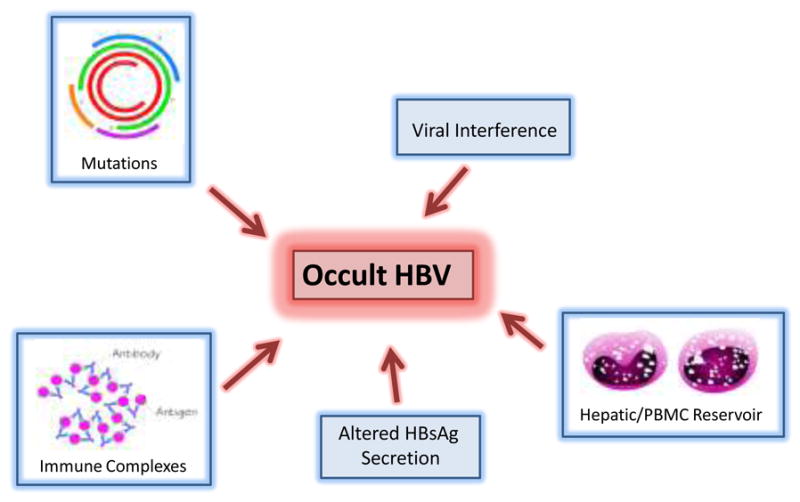
First reported in 1978, occult hepatitis B is a term used to describe the presence of hepatitis B virus (HBV) DNA without hepatitis B surface antigenemia. The prevalence of occult HBV is unclear and depends in part on the sensitivity of the hepatitis B surface antigen (HBsAg) and DNA assays used as well as the prevalence of HBV infection in the study population. The origin of occult HBV also remains in question. Several mechanisms have been hypothesized including mutations in the regulatory regions of the HBV genome, persistence of Ig-bound HBV immune complexes, viral interference, and blockage of free HBsAg secretion. Occult HBV has important clinical implications such as transmission through blood transfusion, reactivation in the setting of immunosuppression, and interference with hepatitis C treatment. To date, there is little date pertaining to the treatment of occult HBV outside of the setting of chemotherapy-induced HBV reactivation.
Keywords: occult HBV, chronic hepatitis B, HBV DNA, HBs antigenemia
Introduction
The hepatitis B virus accounts for significant morbidity and mortality worldwide. An estimated one third of the world population have been exposed to HBV and 400 million people are chronic carriers.[1] The spectrum of HBV-related disease is broad and includes acute hepatitis (which may progress to fulminant hepatic failure), an asymptomatic carrier state, an immunotolerant chronic carrier state, and patients with active, inflammatory disease characterized by the presence of a high viral load and high serum transaminases. The sine qua non of all of these states is the presence of HBsAg which is detectable via serum sampling. Another poorly recognized clinical entity, termed occult HBV, is defined as the presence of HBV viremia in the absence of detectable HBsAg. The viral load in occult HBV is usually low, less than 104 IU/mL.[2] Occult HBV is often observed in patients with hepatitis B core antibody (HBcAb) as the only HBV serological marker, though it has also been reported in patients with hepatitis B surface antibody (HBsAb) alone or even in those without any HBV serological markers. Where occult HBV fits in the clinical spectrum of HBV disease is not well known.
Our awareness of occult hepatitis B dates back to 1978 when it was shown that patients who received HBsAg negative, HBcAb positive blood were at risk for developing post-transfusion hepatitis B.[3] With the advent of sensitive PCR technology, the prevalence, virological aspects, and clinical implication of occult HBV have been further delineated. One study from 1988 showed that chimpanzees inoculated with HBsAg negative, HBV PCR positive serum from human donors developed acute hepatitis. HBV PCR amplification and cloning of HBV-DNA fragments demonstrated similar HBV-DNA sequences in the human donor and chimpanzee recipient. This work helped confirm the infectivity and clinical importance of occult HBV.[4]
Further studies have suggested that occult HBV may have a significant impact in several clinical contexts. First, the presence of occult HBV may speed the progression of liver fibrosis and increase the risk of developing hepatocellular carcinoma in patients with co-existing causes of liver damage. Second, in patients with chronic hepatitis C infection (HCV), occult HBV may decrease their response to HCV treatment. Third, occult HBV may acutely reactivate when an immunosuppressive state occurs. Finally, occult HBV may be transmitted during blood transfusion and organ transplantation, causing classic forms of hepatitis B. The question arises as to whether HBV treatment should be initiated in any of these scenarios. This review will address the epidemiology and natural history of occult HBV infection and review evidence regarding the role of treatment.
Epidemiology
The majority of published studies are focused on identification of occult HBV in clearly defined subpopulations, including those with liver disease and certain immunocompromised states such as HIV infection. Since HBV and HCV share common modes of transmission, the prevalence of occult HBV in patients with chronic HCV has been investigated. Cacciola, et al. analyzed serum and liver specimens from 200 HCV-infected patients and 50 patients with liver disease who were HBsAg and HCV negative. Intrahepatic HBV sequences were detected in 66 out of 200 (33%) HCV-infected patients. Forty-six of these 66 (69.6%) patients were positive for HBcAb. The authors also detected intrahepatic DNA sequences in a smaller percentage of non HCV-infected patients (7/50). Despite the high prevalence of intrahepatic DNA sequences in this study population, just 45 of the 73 subjects with detectable intrahepatic HBV DNA had detectable serum HBV DNA as measured by a nested PCR technique. This raises the question as to whether the presence of intrahepatic HBV DNA alone falls within the definition of occult HBV.[5] Our understanding of HBV virology provides evidence that HBV may exist in a minichromasomal form, or that specific sequences may incorporate into the host nucleus. Therefore, PCR amplification of hepatocytes may reveal presence of HBV sequences when active replication is not taking place. This suggests that simple detection of HBV sequence within hepatocytes may not be indicative of active HBV infection and should not be termed occult HBV. However, it is possible that incorporation rather than replication is associated with clinical events including increased risk of HCC.
As mentioned above, occult HBV seems to be more prevalent among immunocompromised individuals. Hofer et al. selected patients from the Swiss HIV Cohort Study whose HBV panel was positive only for HBcAb. They retrieved an average of 3.5 serum samples from the 57 patients in the study. HBV DNA was detected at least once in 51 (89.5%) patients. Furthermore, eight of 22 patients who were positive for HBV DNA and negative for HCV had persistent ALT elevation (> 6 months) that the authors felt was only attributable to HBV infection. Hofer concluded that the presence of HBcAb alone is indicative of chronic HBV infection and is often associated with chronic hepatitis and ALT elevation.[6] The authors, however, do not detail how they ruled out other causes of chronic liver disease (aside from HCV) in those with occult HBV. They also mention that liver biopsies were not available in these patients. Thus, it may be presumptive to state that occult HBV is the source of abnormal ALT values in these individuals.
Another study of 909 HIV-infected patients examined HBV prevalence using serologic markers and a real-time PCR assay (with a detection limit of 100 IU/mL). Forty-three patients (4.7%) were found to be HBV DNA positive. Twelve of those 43 patients (1.3% of the cohort) were HBsAg-negative, indicating occult HBV infection. Interestingly, five of the 12 patients with occult HBV were negative for all serologic markers.[7] It is not surprising therefore that the prevalence of HBV in this cohort was much lower than in the Hofer study since HBV DNA was tested for in all individuals, not just those who were HBcAb positive, thus increasing the denominator using a subgroup with lower prevalence of detectable HBV DNA.
A more recent study conducted at a tertiary care center in North India evaluated 53 HBsAg negative patients with HIV. Using a highly sensitive PCR kit (detection limit 10–30 copies/mL), the authors found 9 individuals (17%) with detectable HBV DNA. All of the occult HBV cases had one or more HBV serologic markers (HBcAb, HBsAb or both). Conversely, occult HBV was not found in the 29/53 patients without any HBV serologic markers.[8]
Compared to studies of unique patient groups, there is a paucity of data in “healthy” populations. General populations are best represented by broad surveys (e.g. NHANES III), but these data are not available. There are some data derived from blood donor screening that provide evidence of occult HBV prevalence in broader populations. A Canadian study published in 2007 evaluated the presence of occult HBV among HBcAb positive blood donors in the province of Quebec. A total of 1169 HBcAb reactive donations were tested for HBV DNA using an in-house nucleic acid testing assay. Twelve donors (1.03%) were HBV DNA positive and HBsAg negative. It was estimated that the viral load for these occult HBV donors was less than 38 copies/mL.[9] An Italian group investigated the prevalence of occult hepatitis B in the general population by testing for HBV DNA from liver tissue specimens. Ninety-eight patients without a history of liver disease underwent a variety of abdominal surgeries (i.e. gastric bypass, cholecystectomy, removal of benign liver tumors) during which liver biopsy was performed. PCR amplification of frozen liver specimen revealed 16 patients with detectable HBV DNA. Ten of the 16 were HBcAb positive.[10] Since the authors did not test for the presence of serum HBV DNA, it is unclear if these patients can be labeled as having occult HBV as noted above.
These studies show that the prevalence of occult hepatitis B is highly variable depending on the presence of comorbid diseases, the prevalence of HBV in the study population, the sensitivity of the assay used, and the definition of occult HBV (see table 1). Even though newer, more sensitive HBV DNA assays are available, it is difficult to determine whether this has led an increase in the prevalence of occult HBV due to the difference in study methodologies. The wide variability in detection of occult HBV seems more dependent upon cohort rather than test used, or the time period that the assays were run in.
Table 1.
Prevalence of Occult HBV
| Author | Study Population | Results | PCR Assay Detection Limit |
|---|---|---|---|
| Hofer, et al., Eur J. Clin Microbiol. Infect. Dis., 1998. | Swiss HIV Cohort. 57 anti-HBc +ve patients | 51 (89.5%) had detectable HBV DNA over 31 mos. median f/u time | Nested PCR1–10 copies |
| Shire, et al., J Acquir Immun Defic Syndr, 2007. | Univ. Cincinnati HIV database. 909 pts. studied | 43 pts. found to have +HBV DNA. 12/43 (28%) were sAg −ve. | Real timePCR100 IU |
| Gupta, et al. BMC Infectious Diseases, 2010 | Indian study of 53 HBsAg −ve, HIV pts. | 9 pts. (17%) were found to have occult HBV | 10–30 copies/mL |
| Chevrier, et al., Transfusion, 2007. | Canadian study of 1169 anti- HBc +ve blood donors. | 12 pts. (1%) were found to have occult HBV. | 38 copies/mL |
Mechanisms of occult HBV infection
While the exact mechanism behind occult HBV is unclear, several theories have been proposed. One possibility involves mutations in regulatory regions of the HBV genome that inhibit HBsAg production and viral replication. Studies have shown the presence of numerous mutations and deletions in the occult HBV genome. In one such study, Vivekanandan et al. sequenced HBV DNA from five cases of occult HBV infection as well as controls who were HBsAg positive. Although a variety of mutations were found, the overall locations of mutations were similar between the occult and nonoccult HBV samples. Furthermore, they did not identify any mutations that were shared by the occult strains but absent in the HBsAg positive strains. They did, however, find some differences in the methylation pattern between the occult and nonoccult samples and theorized that such epigenetic changes may play a role in occult HBV.[11] In contrast, an older study by Weinberger et al. did identify the major hydrophilic loop (MHL) of the S protein as an area of raised genetic variability. The frequency of mutation in the MHL of patients with occult HBV was 22.6/1000 amino acids, which was significantly higher than that in the non-MHL region (9.4/1000 aa) and HBsAg positive controls (7.5/1000 aa for MHL, 12/1000 aa for non-MHL).[12] Thus, the significance of these mutations remains unclear. The detection of wild-type HBV strains in occult HBV cases implies that other mechanisms may be involved.
Another possible explanation for the existence of occult HBV is the persistence of immune complexes consisting of HBsAg bound to HBsAb. Yotsuyanagi et al. studied the duration of viremia in the course of acute hepatitis B in eleven Japanese patients. They quantified the levels of free and Ig-bound HBV during the acute phase of infection, the window period when both HBsAg and HBsAb were absent from the serum, and after seroconversion to anti-HBs. Their results showed that the levels of free and Ig-bound HBV are equal in the acute phase, Ig-bound HBV predominates in the window period although free HBV is present, and free HBV is not detectable after seroconversion. The authors speculated that the immune complexes that persist after seroconversion are not infectious and therefore a HBV reservoir likely exists in the liver or peripheral blood mononuclear cells.[13] The presence of a HBV reservoir in hepatocytes is an interesting idea in light of the research by Raimondo et al. mentioned earlier.
The concept of viral interference may also help explain how HBV replication and gene expression is affected in those with occult HBV. As detailed above, occult HBV has been studied in individuals coinfected with HCV. Shih et al. conducted in vitro studies in which a human hepatoma cell line was cotransfected with HCV structural genes and cloned HBV DNA. They found that HBV-specific major transcripts and HBV antigens were reduced about two- to fourfold by the presence of HCV structural genes. Furthermore, secretion of HBV viral particles was suppressed about 20-fold. They speculated that these effects were mediated by the core protein of HCV, which may serve as a gene-regulatory protein in this situation.[14] Although this is an interesting finding, the mechanism for the suppressive effect of the HCV protein is unclear. This raises some doubt as to whether there is a direct interaction between the two viruses or whether other factors may be involved. It is possible that interferons or other cytokine mediators determine the relative suppression of one or both viruses when coinfection is present. Appearance of HBV following clearance of HCV was recently described in a clinical cohort of 161 dual-infected Taiwanese patients who received treatment with pegylated interferon and ribavirin. A sizable proportion (36.3%) of coinfected patients with undetectable pre-treatment HBV DNA developed HBV reactivation post-treatment.[15]
Another potential mechanism is the presence of a block to secretion of free HBsAg which would lead to only secretion of “Dane particles” without aggregate HBsAg in serum (see figure 1). Bruss et al. demonstrated that modification of the pre-S domain could limit virion excretion.[16]
FIGURE 1.
Occult HBV and cryptogenic liver disease
In roughly five percent of people with chronic liver disease, an etiology is never found. A few studies suggest that occult HBV may play a role in some of these cases. Chemin, et al. selected 50 patients with chronic hepatitis of unclear etiology. Results of commercially available PCR tests for HBV were negative in all these patients. However, using a newly developed PCR assay (with a detection limit of 350 copies/mL), the authors demonstrated the presence of occult HBV in 15/50 (30%) patients. This was confirmed by HBV DNA detection in liver biopsy samples. Histopathological analysis showed a wide range of disease, from mild reactive hepatitis (20–25% of cases) to more severe fibrosis and cirrhosis (26%). Interestingly, the patients with occult HBV had more advanced fibrosis (53% of HBV positive patients).[17] Although this study cannot provide a causal relationship between occult HBV infection and liver disease, the high proportion of occult HBV infection in non-A non-E chronic hepatitis cases is notable.
A similar study by Berasain, et al. involved 101 patients who underwent liver biopsy because of persistently elevated ALT levels. The etiology of their liver disease was not determined from clinical, biochemical, and serological data obtained before biopsy. Histopathological findings included non-specific changes in 33 subjects (32.7%), NASH in 16 patients (15.8%), chronic hepatitis in 39 patients (38.6%), and cirrhosis in 13 subjects (12.9%). HBV DNA was detected in the serum of 19 (18.8%) patients. Once again, occult HBV infection was more frequently observed in those with advanced fibrosis than with minimal changes or NASH.[18]
Occult HBV and chronic HCV infection
Since HBV and HCV have similar modes of transmission, infection with both viruses is frequent. Many studies have been conducted to determine the clinical significance of occult HBV in patients with chronic HCV infection. While some of this work has focused on the relationship between co-infection and the severity of liver disease, other papers have explored how occult HBV affects HCV treatment outcomes. An Austrian study of 98 HBsAg negative patients with chronic HCV examined sera (n=82) or liver tissue (n=16) for HBV DNA using nested PCR. HBV DNA was found in 22% of sera and 19% of liver tissue specimens. Statistical analysis did not show a significant difference in biochemical (mean AST, ALT values) or histological (degree of fibrosis) markers of liver disease in patients with or without occult HBV.[19] In contrast, Cacciola et al., in a study of 200 patients with chronic HCV, did find a significant correlation between occult HBV infection and cirrhosis. Twenty-two of the 66 patients (33 percent) with HCV and occult HBV had cirrhosis as compared to 26 out of 134 patients (19 percent) only infected with HCV. The authors also studied a subgroup of 83 patients with HCV (65 with chronic hepatitis and 18 with cirrhosis) who underwent treatment with interferon alfa. Occult HBV was detected in 26 of 55 patients in whom interferon therapy was unsuccessful and in 7 of 28 patients in whom treatment was successful. These results, however, were not statistically significant.
Further studies of occult HBV’s influence on HCV treatment were conducted by De Maria, et al. who looked at the relationship between anti-HBc status and response to interferon alfa treatment in 285 patients with chronic HCV. They assessed response to interferon at three different endpoints: after 6 months, at the end of treatment, and 6 months after interferon discontinuation. They found that the anti-HBc positive subjects had a significantly lower response to interferon at 6 months and at the end of treatment compared to the anti-HBc negative individuals (respectively 42% vs. 66% and 32% vs. 57%). However, at 6 months after discontinuation of therapy, there was no difference between the groups.[20] To help elucidate a potential mechanism that explains the relationship between occult hepatitis B and HCV response to interferon, Japanese researchers evaluated intrahepatic mRNA levels of type-1 interferon receptor genes. This study involved 45 patients with chronic hepatitis C, 22 of which also had occult HBV as determined by a nested PCR assay. This group found that co-infection was significantly more frequent in IFN non-responders (18/24, 75%) than IFN-responders (4/21, 19%) regardless of HCV genotype. Furthermore, co-infection was associated with lower IFN-receptor mRNA levels.[21] Although the above data shows a high rate of occult HBV in those with chronic HCV infection, the clinical significance of occult HBV in this setting remains unclear.
A recently published study of HCV/HBV dual-infected patients treated with pegylated interferon and ribavirin has provided further insight into the complex relationship between HBV and HCV. Liu et al. treated 161 persons infected with both HBsAg and HCV RNA, and 160 matched, HCV-monoinfected persons using currently accepted doses and durations of therapy. In contrast to De Maria’s findings, Liu found that HBV/HCV co-infected patients clear HCV equally as well as HCV monoinfected patients. Furthermore, all 10 of the HCV monoinfected patients with occult HBV infection achieved HCV SVR and cleared occult HBV DNA with treatment. It is hard to reconcile these two findings with the observation that over three quarters of the dually infected patients who lost HCV RNA but still had elevated ALT post-treatment had detectable serum HBV DNA.[22]
Occult HBV and HCC
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide and the major risk factor for the development of HCC is chronic HBV infection. In a 2004 review article by Brechot, the pathogenesis of cancer in HBV infection is outlined. Studies have shown that HBV can become integrated into host cellular DNA. In some cases, this can then disrupt or promote expression of genes that govern cell growth and differentiation. The examination of viral DNA sequences present in HCC has implicated several HBV genes including truncated pre-S2/S, hepatitis B X gene, and hepatitis B spliced protein.[23] The possible role of occult HBV as a risk factor for the development of HCC has also been explored. Squadrito et al. followed a cohort of 380 HBsAg negative patients with chronic hepatitis for a median of 82.8 months. A large proportion of patients (35.5%) had occult HBV (which was detected by analysis of liver biopsy specimens). During the follow-up period, nine patients developed HCC; eight of those nine patients were positive for occult HBV.[24] A recent Japanese study evaluated occult HBV in patients with non-B, non-C (NBNC) hepatocellular carcinoma. Only 8 of 45 patients with NBNC-HCC had detectable serum HBV DNA. Furthermore, HBV sequencing of occult HBV patients in the NBNC-HCC group did not reveal any HCC-associated mutations. A large proportion of patients with HBV-HCC (25 of 30) did have these mutations, however. The authors postulated that many of the patients in the NBNC-HCC group may have had underlying NAFLD (a number of them were overweight and had diabetes mellitus) and that this disorder should be further investigated in terms of potential hepatocarcinogenesis.[25] In summary, while occult HBV is often found in patients with HCC, it is unclear what role it has in hepatocarcinogenesis.
Occult HBV and blood transfusion/organ transplantation
As mentioned earlier, the risk of HBV transmission through blood transfusion of HBsAg-negative individuals was first recognized in the late 1970s. Although the risk of acquiring transfusion-transmitted viral hepatitis is low in developed countries, the risk of transfusion-transmitted HBV infection (1/63,000) remains higher than that of transfusion-transmitted HCV infection (1/103,000).[26] Occult HBV infection may partially account for this difference. It is generally accepted that blood donations containing anti-HBc as the only marker of HBV infection can transmit HBV. A recently published case report implicated a blood donor with anti-HBc, anti-HBs (12 IU/L) and HBV DNA (180 IU/mL) in the acquisition of acute HBV in two immunocompetent transfusion recipients.[27] This is an interesting finding because it suggests that low-level anti-HBs is poorly protective from infectivity when HBV DNA is present.
The clinical significance of occult HBV in patients undergoing liver transplantation has also been investigated. Livers from donors previously exposed to HBV can fail after transplantation due to severe HBV reactivation in the recipient. There is a reported 25–95% risk of transmitting HBV to recipients who receive a HBcAb-positive liver. Because of this high risk, prophylaxis with either a combination of hepatitis B immunoglobulin and lamivudine or at least HBIg alone is recommended.[28] Newer anti-viral agents (i.e. adefovir, telbivudine, entecavir, and tenofovir) have not been well studied in this setting. A survey study of 78 transplant physicians from the United States, Europe, Asia, and Australia was conducted in 2007 to gauge practice preferences regarding prophylactic therapy in recipients of an anti-HBc positive liver. The survey showed that almost two-thirds of transplant physicians consider lamivudine to be the preferred agent after anti-HBc liver donation. Many programs also use HBIg but there is a wide variation in how it is administered.[29]
Other studies have focused on occult HBV in the recipient and the risk of HBV reactivation following transplant. One small study by Ghisetti et al. collected liver samples from 23 HBsAg-negative patients (9 liver donors and 14 recipients) and 20 HBsAg-positive recipients (control group). They found intrahepatic DNA in 9/14 (64%) of the HBsAg-negative recipients (none of them had detectable serum HBV DNA). Despite the high frequency of occult HBV in the recipient group, none of them experienced de novo hepatitis B following transplantation (median follow up: 477 days).[30] Another study evaluating de novo HBV infection after liver transplantation did identify some recipients who had occult HBV prior to transplantation. Twenty of 570 HBsAg negative patients became HBsAg positive after transplantation. Eight of these 20 individuals were deemed to have reactivation of a latent HBV infection; HBV DNA was retrospectively detected in the pretransplant serum of seven patients and in native liver of one patient.[31] Occult HBV, therefore, appears to be clinically important in both liver transplant donors and recipients.
Occult HBV and hematological malignancy
Perhaps the most clinically relevant aspect of occult HBV involves its presence in individuals undergoing chemotherapy for hematological malignancies. Chemotherapy induced reactivation of HBV has been a known problem since the mid-1970s.[32] This phenomenon is especially important not only because it has been associated with severe liver dysfunction and fatal fulminant hepatitis, but also because reactivation often requires interruption of chemotherapy. It is speculated that chemotherapy-induced immunosuppression triggers rapid viral replication. At the time of immune system reconstitution, a T cell mediated immune response causes inflammation and concomitant hepatic necrosis. No uniform definition of HBV reactivation exists, but a commonly accepted one uses an ALT greater than three times the upper limit of normal in combination with a ten-fold rise in HBV DNA or an absolute value greater than 20,000 IU/mL.
Chemotherapy-induced HBV reactivation has been best characterized in patients with hematological malignancies. This may be due to the chemotherapeutic agents used in these disorders. In particular, corticosteroids and anthracyclines seem to be risk factors for HBV reactivation. A glucocorticoid responsive element on HBV DNA and in vitro stimulation of HBV DNA by anthracyclines help support this association.[33] Newer therapeutic agents such as rituximab have also been linked to HBV reactivation. Other immunomodulatory monoclonal agents (e.g. infliximab) are also implicated in HBV reactivation.
Hepatic decompensation and death attributable to HBV reactivation is estimated to occur in 5–40% of HBV carriers who undergo chemotherapy. For this reason, prophylaxis with lamivudine 100mg qday has been recommended for HBsAg positive patients with HBV DNA levels less than 2000 IU/mL (those with higher viral loads should be assessed for long term treatment). While studies have focused on lamivudine in this population, AASLD guidelines suggest that newer agents (adefovir, entecavir, and tenofovir) may be more appropriate if long-term immunosuppression (greater than 12 months) is anticipated. Studies have shown that prophylaxis is superior to delaying treatment until there is serological evidence of reactivation. Hsu et al. randomized HBV carriers with newly diagnosed non-Hodgkin’s lymphoma to either prophylactic or therapeutic lamivudine treatment groups. The prophylactic group started lamivudine on day 1 of the first course of chemotherapy while the therapeutic group was only started on lamivudine when HBV reactivation was detected. The primary endpoint was incidence of HBV reactivation during the 12 months after starting chemotherapy. The authors found that fewer prophylactic group patients had HBV reactivation (11.5% vs. 56%), HBV-related hepatitis (7.7% vs. 48%), or severe hepatitis (0 vs. 36%).[34]
Although the HBV reactivation rate is lower (around 5%) in patients with occult HBV undergoing chemotherapy, there is a significant risk of morbidity and mortality. A 2006 study by Hui et al. examined 244 HBsAg negative lymphoma patients treated with chemotherapy. Eight of the 244 patients (3.3%) experienced HBV reactivation, three of which had fulminant hepatic failure. In all eight cases, a 100-fold increase in HBV DNA occurred (by a median of 18.5 weeks) prior to serological evidence of hepatitis. The authors concluded that close surveillance for a 100-fold increase in HBV DNA is warranted in HBsAg negative patients so that antiviral therapy can be initiated early.[35]
There is some controversy as to how to prevent HBV reactivation in HBsAg negative patients who are about to undergo chemotherapy for hematological malignancies. A recent meeting report by the Scottish Viral Hepatitis Group and the Scottish Diagnostic Virology Group recommends screening all potential chemotherapy patients with serum HBsAg and anti-HBc. They suggest administering lamivudine 100mg qday (starting 1 week prior to chemotherapy and ending 6 months after) to those patients who are HBsAg negative and HBcAb positive.[36] In response to Hui’s study, they state that following serial HBV DNA levels has not been tested in a prospective trial and may be cost prohibitive. As Hui showed, however, a large proportion of HBsAg negative patients may be HBcAb positive (62.3% in his study which was conducted in Hong Kong) depending on the prevalence of HBV in the study population. Treating for HBcAb positivity alone would therefore result in a high rate of overtreatment. This emphasizes the need for widespread availability of sensitive PCR assays to detect occult HBV and thereby further risk stratify HBsAg negative patients.
Conclusion
A recently published treatment algorithm for the management of chronic hepatitis B makes reference to occult HBV but does not discuss whether treatment is warranted. The authors’ recommendations on when to begin treatment of HBeAg-negative chronic HBV is predicated on the HBV DNA level (>2000 IU/mL) and an elevated ALT (1–2 times the upper limit of normal).[37] They do acknowledge, however, retrospective studies that have shown that up to one third of patients with normal ALT levels may have significant fibrosis or inflammation on liver biopsy.[38] Furthermore, the article points out that low HBV DNA levels do not necessarily indicate the absence of progressive liver disease. Fung et al. reported that 15% of patients with HCC have HBV DNA levels < 103 copies/mL.[39] Given the limitations of HBV DNA and ALT testing, the question arises whether occult HBV should be treated. Certainly it would be interesting conduct further studies examining the frequency of histologic changes in occult HBV. Since the available oral regimens for chronic HBV are well tolerated, it would seem reasonable to treat occult HBV assuming that more data can be obtained to substantiate its role in the progression of liver disease.
In summary, although we have been aware of the presence of occult HBV for over three decades, there remain many unanswered questions regarding its prevalence, pathogenesis, and clinical significance. In part, this is due to years of debate surrounding its existence and relevance. Both animal models (woodchuck hepatitis virus) and human studies have provided more insight into occult HBV. The potential role of occult HBV in certain areas such as HCV infection, HIV infection, HCC, solid organ transplantation, and reactivation of HBV in immunosuppressed patients certainly warrants further investigation. Perhaps these will show that the term “occult” may be a misnomer for this type of HBV infection.
Acknowledgments
Bristol-Myers Squibb virology fellows research training grant to PS NIH NIDDK 5K24 DK070528 to KES
References
- 1.Mulrooney-Cousins PM, Michalak TI. Persistent occult hepatitis B virus infection: Experimental findings and clinical implications. World J Gastroenterol. 2007;13(43):5682–5686. doi: 10.3748/wjg.v13.i43.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allain JP. Occult hepatitis B virus infection. Transfusion Clinique et Biologique. 2004;11:18–25. doi: 10.1016/j.tracli.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Hoofnagle JH, Seef LB, Bales ZB, Zimmerman HJ. The type B hepatitis after transfusion with blood containing antibody to hepatitis B core antigen. N Engl J Med. 1978;298:1379–83. doi: 10.1056/NEJM197806222982502. [DOI] [PubMed] [Google Scholar]
- 4.Thiers V, Nakajima E, Kremsdorf D, et al. Transmission of hepatitis B from hepatitis-B-seronegative subjects. Lancet. 1988;2:1273–76. doi: 10.1016/s0140-6736(88)92891-7. [DOI] [PubMed] [Google Scholar]
- 5.Cacciola I, Pollicino T, Squadrito G, et al. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. New England Journal of Medicine. 1999;341:22–26. doi: 10.1056/NEJM199907013410104. [DOI] [PubMed] [Google Scholar]
- 6.Hofer M, Joller-Jemelka H, Grob P, et al. Frequent Chronic Hepatitis B Virus Infection in HIV-Infected Patients Positive for Antibody to Hepatitis B Core Antigen Only. Eur J Clin Microbiol Infect Dis. 1998;17:6–13. doi: 10.1007/BF01584356. [DOI] [PubMed] [Google Scholar]
- 7.Shire N, Rouster S, Stanford SD, et al. The Prevalence and Significance of Occult Hepatitis B Virus in a Prospective Cohort of HIV-Infected Patients. J Acquir Immune Defic Syndr. 2007;44:309–314. doi: 10.1097/QAI.0b013e31802e29a9. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Singh S. Occult Hepatitis B Virus infection in ART-Naïve HIV-infected patients seen at a Tertiary Care Centre in North India. BMC Infectious Diseases. 2010;10:1–7. doi: 10.1186/1471-2334-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevrier MC, St-Louis M, Perreault J, et al. Detection and characterization of hepatitis B virus of anti-hepatitis core antigen-reactive blood donors in Quebec with an in-house nucleic acid testing assay. Transfusion. 2007;47:1794–1802. doi: 10.1111/j.1537-2995.2007.01394.x. [DOI] [PubMed] [Google Scholar]
- 10.Raimondo G, Navarra G, Mondello S, et al. Occult hepatitis B in liver tissue of individuals without hepatic disease. Journal of Hepatology. 2008;48:743–746. doi: 10.1016/j.jhep.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Vivekanandan P, Kannangai R, Ray SC, et al. Comprehensive Genetic and Epigenetic Analysis of Occult Hepatitis B from Liver Tissue Samples. Clinical Infectious Diseases. 2008;46:1227–1236. doi: 10.1086/529437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberger K, Bauer T, Bohm S, et al. High genetic variability of the group specific a-determinant of hepatitis B virus surface antigen (HBsAg) and the corresponding fragment of the viral polymerase in chronic virus carriers lacking detectable HBsAg in serum. J General Virol. 2000;81:1165–1174. doi: 10.1099/0022-1317-81-5-1165. [DOI] [PubMed] [Google Scholar]
- 13.Yotsuyanagi H, Yasuda K, Iino S, et al. Persistent Viremia After Recovery From Self-Limited Acute Hepatitis B. Hepatology. 1998;27:1377–1382. doi: 10.1002/hep.510270526. [DOI] [PubMed] [Google Scholar]
- 14.Shih CM, Lo SJ, Miyamura T, et al. Suppression of Hepatitis B Virus Expression and Replication by Hepatitis C Virus Core Protein in HuH-7 Cells. Journal of Virology. 1993;67:5823–5832. doi: 10.1128/jvi.67.10.5823-5832.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu CJ, Chuang WL, Lee CM, et al. Peginterferon Alfa-2a Plus Ribavirin for the Treatment of Dual Chronic Infection With Hepatitis B and C Viruses. Gastroenterology. 2009;136:496–504. doi: 10.1053/j.gastro.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 16.Bruss V, Vieluf Functions of the Internal Pre-S Domain of the Large Surface Protein in Hepatitis B Virus Particle Morphogenesis. Journal of Virology. 1995;69:6652–6657. doi: 10.1128/jvi.69.11.6652-6657.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chemin I, Zoulim F, Merle P, et al. High incidence of hepatitis B infections among chronic hepatitis cases of unknown aetiology. Journal of Hepatology. 2001;34:447–454. doi: 10.1016/s0168-8278(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 18.Berasain C, Betes M, Panizo A, et al. Pathological and virological findings in patients with persistent hypertransaminasaemia of unknown aetiology. Gut. 2000;47:429–435. doi: 10.1136/gut.47.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazemi-Shirazi L, Petermann D, Muller C. Hepatitis B virus DNA in sera and liver tissue of HbsAg negative patients with chronic hepatitis C. Journal of Hepatology. 2000;33:785–790. doi: 10.1016/s0168-8278(00)80311-6. [DOI] [PubMed] [Google Scholar]
- 20.De Maria N, Colantoni A, Friedlander L, et al. The Impact of Previous HBV Infection on the Course of Chronic Hepatitis C. The American Journal of Gastroenterology. 2000;95:3529–3536. doi: 10.1111/j.1572-0241.2000.03371.x. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda R, Ishimura N, Hamamoto S, et al. Co-Infection by Serologically-Silent Hepatitis B Virus May Contribute to Poor Interferon Response in Patients With Chronic Hepatitis C by Down-Regulation of Type-I Interferon Receptor Gene Expression in the Liver. J Med Virol. 2001;63:220–227. doi: 10.1002/1096-9071(200103)63:3<220::aid-jmv1004>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Liu CJ, Chuang WL, Lee CM, et al. Peginterferon Alfa-2a Plus Ribavirin for the Treatment of Dual Chronic Infection With Hepatitis B and C Viruses. Gastroenterology. 2009;136:496–504. doi: 10.1053/j.gastro.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 23.Brechot C. Pathogenesis of Hepatitis B Virus-Related Hepatocellular Carcinoma: Old and New Paradigms. Gastroenterology. 2004;127:S56–S61. doi: 10.1053/j.gastro.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Squadrito G, Pollicino T, Cacciola I, et al. Occult Hepatitis B Virus Infection Is Associated with the Development of Hepatocellular Carcinoma in Chronic Hepatitis C Patients. Cancer. 2006;106:1326–1330. doi: 10.1002/cncr.21702. [DOI] [PubMed] [Google Scholar]
- 25.Kusakabe A, Tanaka Y, Orito E, et al. A weak association between occult HBV infection and non-B non-C hepatocellular carcinoma in Japan. J Gasteroenterol. 2007;42:298–305. doi: 10.1007/s00535-006-1999-3. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber GB, Busch MP, Kleinman SH, et al. The risk of transfusion-transmitted viral infections. N Engl J Med. 1996;334:1685–1690. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 27.Levicnik-Stezinar S, Rahne-Potokar U, Candotti D, et al. Anti-HBs positive occult hepatitis B virus carrier blood infectious in two transfusion recipients. Journal of Hepatology. 2008;48:1022–1025. doi: 10.1016/j.jhep.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Mas A. Liver transplantation for hepatitis B virus. Pre-emptive and peri-operative prophylaxis. Digestive and Liver Disease. 2009;41S:S191–S194. doi: 10.1016/S1590-8658(09)60443-6. [DOI] [PubMed] [Google Scholar]
- 29.Perrillo R. Hepatitis B Virus Prevention Strategies for Antibody to Hepatitis B Core Antigen-Positive Liver Donation: A Survey of North American, European, and Asian-Pacific Transplant Programs. Liver Transplantation. 2009;15:223–232. doi: 10.1002/lt.21675. [DOI] [PubMed] [Google Scholar]
- 30.Ghisetti V, Marzano A, Zamboni F, et al. Occult Hepatitis B Virus Infection in HBsAg Negative Patients Undergoing Liver Transplantation: Clinical Significance. Liver Transplantation. 2004;10:356–362. doi: 10.1002/lt.20093. [DOI] [PubMed] [Google Scholar]
- 31.Roche B, Samuel D, Gigou M, et al. De novo and apparent de novo hepatitis B virus infection after liver transplantation. Journal of Hepatology. 1997;26:517–526. doi: 10.1016/s0168-8278(97)80416-3. [DOI] [PubMed] [Google Scholar]
- 32.Galbraith RM, Eddleston AL, Williams R, et al. Fulminant hepatic failure in leukaemia and choriocarcinoma related to withdrawal of cytotoxic drug therapy. Lancet. 1975;2:528–530. doi: 10.1016/s0140-6736(75)90897-1. [DOI] [PubMed] [Google Scholar]
- 33.Lalazar G, Rund D, Shouval D, et al. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. British Journal of Hematology. 2007;136:699–712. doi: 10.1111/j.1365-2141.2006.06465.x. [DOI] [PubMed] [Google Scholar]
- 34.Hsu C, Hsiung CA, Su I, et al. A Revisit of Prophylactic Lamivudine for Chemotherapy-Associated Hepatitis B Reactivation in Non-Hodgkin s Lymphoma: A Randomized Trial. Hepatology. 2008;47:844–853. doi: 10.1002/hep.22106. [DOI] [PubMed] [Google Scholar]
- 35.Hui C, Cheung W, Zhang H, et al. Kinetics and Risk of De Novo Hepatitis B Infection in HBsAg-Negative Patients Undergoing Cytotoxic Chemotherapy. Gastroenterology. 2006;131:59–68. doi: 10.1053/j.gastro.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Meeting report. The management of chronic hepatitis B in the immunocompromised patient: Recommendations from a single topic meeting. Journal of Clinical Virology. 2008;41:243–254. doi: 10.1016/j.jcv.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 37.Keefe E, Dieterich D, Han S, et al. A Treatment Algorithm for the Management of Chronic Hepatitis B Virus Infection in the United States: 2008 Update. Clinical Gastroenterology and Hepatology. 2008;6:1315–1341. doi: 10.1016/j.cgh.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Lai M, Hyatt BJ, Nasser I, et al. The clinical significance of persistently normal ALT in chronic hepatitis B infection. Journal of Hepatology. 2007;47:760–767. doi: 10.1016/j.jhep.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 39.Fung J, Lai C-L, But D, et al. Prevalence of fibrosis and cirrhosis in chronic hepatitis B: implications for treatment and management. Am J Gastroenterol. 2008;103:1421–6. doi: 10.1111/j.1572-0241.2007.01751.x. [DOI] [PubMed] [Google Scholar]


