Abstract
Benzene is an established leukemogen and hematotoxin in humans. However, the finding that benzene is a multiple-site carcinogen in rodents raises the possibility that other tissues could be susceptible to benzene-induced carcinogenicity, especially since a significant excess of squamous cell carcinomas and papillomas arise from epidermal and oral keratinocytes in benzene-exposed rats. Since inflammation and sustained hyperplasia are two integral components in carcinogenesis, the elaboration of proinflammatory cytokines and growth factors by keratinocytes might provide a mechanistic link between tumor initiation and promotion in benzene-induced cancers. We observed that the principal benzene metabolites, represented by hydroquinone, 1,4-benzoquinone, phenol, 1,2,4-benzenetriol, and catechol, significantly alters the production of transforming growth factor of (TGF)-α and interleukin (IL)-8 in human epidermal keratinocyte cultures. These cytokines represent the primary growth promoting factor and neutrophil chemotactant in the skin, respectively. Cytokine secretion correlated with the known redox potential of individual benzene metabolites and antioxidants, including dimethyl sulfoxide, 1,1,3,3-tetramethylthiourea, and N-acetylcysteine, attenuated the response. Binary combinations of selected benzene metabolites synergized in the induction of IL-8, while benzene, by itself, induced about a three-fold increase in IL-8 production. Taken together, our studies suggest that benzene and many of its phase I metabolites induce inflammatory cytokines and growth factors and this occurs through direct covalent binding or the generation of reactive oxygen species by autooxidation and reduction. The elaboration of proinflammatory cytokines and growth factors by keratinocytes in response to benzene and its principal metabolites may participate in benzene-induced skin carcinogenesis.
INTRODUCTION
Benzene is a known leukemogen and hematotoxin in humans (Yardley-Jones et al., 1991; Snyder and Kalf, 1994) and a multiple-site, complete carcinogen in rats and mice following long-term oral (Maltoni et al., 1989; Huff et al., 1989) or inhalation (Maltoni et al., 1989) exposure. In addition to lymphoid tissue, the lung and skin also represent targets for benzene-induced carcinomas. For example, mortality data from a large epidemiology study revealed a significant excess of melanomas and squamous cell carcinomas in benzene-exposed workers compared to an unexposed cohort (Bond et al., 1986). More recently, French et al. (1994) demonstrated the ability of benzene to induce papillomas when painted on the skin of TG.AC transgenic mice that harbor a v-Ha-ras oncogene, suggesting that benzene or its metabolites act as tumor promoters. Taken together these findings suggest that nonhematopoietic cells, particularly epidermal keratinocytes, in humans may be susceptible to benzene-induced carcinogenicity.
Benzene metabolites demonstrate significant dermatotoxicity in human and rodent skin. For example, while phenol is not a carcinogen (Boutwell and Bosch, 1959; Van Duuren et al., 1978), it is corrosive when applied to mouse skin and the subsequent influx of phagocytes, especially polymorphonuclear neutrophil leukocytes have been linked to phenol’s weak tumor promoting activity (Wilmer et al., 1994). At non-cytotoxic concentrations phenol also induces the expression of proinflammatory cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-8, and intracellular IL-1α in human keratinocyte cultures (Wilmer et al., 1994). While neither catechol nor hydroquinone have tumor promoting activity (Boutwell and Bosch, 1959; Van Duuren et al., 1978), the former is a potent cocarcinogen in mouse skin (Van Duuren et al., 1978) and the latter causes inflammation and allergic contact dermatitis from occupational exposure (Sax, 1988).
The metabolic activation of benzene is extensive and yields a number of reactive intermediates including the semiquinone and quinone forms of hydroquinone (HZ), 1,4-benzosemiquinone (BQ), 1,2,4-benzenetriol (BZT), catechol (CAT) (Yardley-Jones et al., 1991; Snyder and Kalf, 1994), and cis, cis-muconaldehyde (Witz et al., 1989). Besides binding covalently to critical macromolecules and low molecular weight compounds such as glutathione and cysteine, quinone metabolites can undergo redox cycling in the presence of O2 and cellular reductases and generate reactive oxygen species (ROS) capable of damaging macromolecules (Greenlee et al., 1981; Lewis et al. 1988). The increased production of ROS can also shift the intracellular redox balance to a prooxidant state, leading to the activation of the nuclear transcription factor (NF)-kB, which regulates proinflammatory cytokine gene expression (Schreck and Baeuerle, 1990). The secretion of proinflammatory cytokines and growth factors is important considering the integral roles that inflammation and sustained hyperplasia have in carcinogenesis (Slaga, 1984; DiGiovanni, 1994). In this regard, epidermal keratinocytes play an active role in skin inflammation via the synthesis and release of soluble proinflammatory cytokines, chemokines, and growth factors in response to diverse environmental agents (Kupper, 1990; Wilmer et al., 1994; Wilmer and Luster, 1995).
The purposes of the present study were to determine whether the principal benzene metabolites induce the production of proinflammatory cytokines and growth factors in normal human epidermal keratinocytes; cytokine production correlates with the potential of benzene metabolites to generate ROS; and binary combinations of benzene metabolites synergize in their induction of cytokines.
MATERIALS AND METHODS
Test Chemicals and Reagents
1,1,3,3-tetramethylthiourea (TMTU), p-nitro-phenylphosphate tablets, o-phenylenediamine • 2 HC1 tablets, diethanolamine, benzene, and bovine serum albumin (BSA, fraction V) were from Sigma Chemical Co. (St. Louis, MO); phenol and dimethylsulfoxide (DMSO) were from J.T. Baker Co. (Phillipsburg, NJ). Tween 20, CAT, HQ, BQ, and BZT were from Aldrich Chemical Co. (Milwaukee, WI). N-acetylesteine (NAC) and peroxidase-conjugated swine antigoat IgG were from Boehringer-Mannheim (Indianapolis, IN).
Cell Culture and Chemical Treatment
Cryopreserved normal human keratinocytes (NHK) from breast skin of adult females were provided by the Clonetics Corp. (San Diego, CA). Cell culturing, chemical treatment, and cytokine quantitation have been described previously (Wilmer et al., 1994; Wilmer and Luster, 1995). Briefly, keratinocytes were grown in 25 cm2 tissue cultures flasks in Keratinocyte Growth Medium® (KGM) composed of Keratinocyte Basal Medium® (KBM; modified MCDB 153 formulation; low calcium, 150 μM) supplemented with insulin, rh epidermal growth factor (EGF), bovine pituitary extract, hydrocortisone, and GA-1000 (Clonetics Corp., San Diego, CA). The cells were trypsinized and subcultured in 24-well plates (Costar Corp., Cambridge, MA) at a seeding density of 2.4 × 104 cells/cm2. After 24 hours, at which time the cells were approximately 70% confluent, the complete medium was changed to growth medium without hydrocortisone, bovine pituitary extract, and EGF. Benzene metabolites were dissolved in phosphate buffered saline (PBS) and sterilized through 0.2 μ filters (Corning Glass Works, Corning, NY). The solutions of benzene metabolites and metabolite-treated cell cultures were handled under gold safelights. Exposure of the cultures to benzene metabolites, delivered in 10 µl aliquots, was for 4 hours in a humidified 5% CO2/95% air atmosphere. After chemical exposure, the cultures were rinsed with KBM, and 1 ml of fresh complete medium without hydrocortisone was added into each well. Antioxidants were dissolved directly in growth-factor deficient medium without hydrocortisone, filter-sterilized, and added 1 ml volumes, 30 minutes before the benzene metabolites in order to allow for equilibration in the cultures. After exposure, the cultures were rinsed, and fresh growth factor-deficient medium in 4 ml aliquots was pipetted into each flask for a further 20-hour culture period. The culture supernatants were removed, sterile-filtered into cryotubes, and frozen at −70°C. Keratinocyte viability was assessed concurrently using a modified in situ trypan blue dye exclusion procedure (Wilmer and Luster, 1995).
Cytokine Measurements
For IL-8, 96-well Immulon 4 plates (Dynatech Laboratories, Chantilly, VA) were coated with capture antibody (mouse antihuman IL-8 monoclonal antibody; R & D Systems, Minneapolis, MN) at a concentration of 2 μg/ml of coating buffer (0.1 ml/well) for 24 hours at 4°C. The plates were washed five times with PBS containing 0.5% Tween 20 and the free spaces were blocked by incubation for 2 hours with 1% BSA in PBS-Tween 20. Samples or standards (R & D Systems) were added in 0.1 ml aliquots and incubated for 2 hours at 37°C. The plates were rinsed and 0.1 ml of goat antihuman IL-8 polyclonal IgG antibody (R & D Systems), diluted to 0.5 μg/ml, was added to each well for an additional 2 hours. The plates were again washed and incubated with peroxidase-conjugated rabbit antigoat IgG antibody (Organon Teknika/Cappel, Durham, NC), diluted 1:7500, for 1 hour. After washing, the wells were incubated with peroxidase substrate (H2O2/tetramethylbenzidine; Sigma) for 20 minutes and the reaction was terminated by the addition of 50 μl of 2N sulfuric acid. TGF-α concentrations were determined using a commercial enzyme-linked immunosorbent assay (ELISA) system (Oncogene Science, Uniondale, NY) according to the manufacturer’s instructions. Absorbance was measured at 450 nM wavelength with a UV max® Kinetic Microplate Reader (Molecular Devices, Menlo Park, CA) using the ΔSoft Program (Macintosh v. 2.12, Biometallics, Inc., Princeton, NJ) for data collection and analysis.
Statistical Analyses
The concentration-response was analyzed initially by Bartlett’s test for homogeneity of variances, and in some cases the data were transformed by the square root to equalize variances prior to one-way analysis of variance (ANOVA). If the F-statistic was significant, post boc comparisons were made using Tukey’s honestly significant difference (HSD) procedure to determine whether the individual treatment groups were significantly different (p < 0.05) (Sokal and Rohlf, 1969). These tests were conducted using the Systat® statistical program (Macintosh v. 5, Systat, Inc., Evanston, IL).
RESULTS
Experiments were conducted to determine whether benzene metabolites stimulate IL-8 or TGF-α secretion in NHKs. Addition of BQ, HQ, or BZT induced a significant, concentration-related increase in the secretion of TGF-α in the absence of cell damage as assessed by trypan blue (Table 1). In contrast, the addition of phenol caused a significant increase in TGF-α only at a cytotoxic concentration. CAT did not induce TGF-α and had a slight suppressive effect on constitutive TGF-α secretion at noncytotoxic concentrations. Additionally, BQ, HQ, or BZT induced concentration-related increased in IL-8 secretion. At the highest, noncytotoxic concentration of metabolites examined, BQ, HQ, and BZT induced 27-fold, 23-fold and 60-fold increases, respectively, in IL-8 secretion. Phenol was strongly cytotoxic at 15 mM, but still induced a five-fold increase in IL-8 production. CAT did not affect IL-8 secretion. Neither BQ, HQ, nor BZT significantly increased IL-6, TNF-α, or MCP-1 secretion (data not shown). We had shown previously that noncytotoxic concentrations of phenol are capable of stimulating TNF-α secretion(Wilmer et al., 1994) and therefore, phenol was not tested further for its ability to stimulate TNF-α production.
Table 1.
TGF-α and IL-8 Secretion in NHK Cultures Treated with Benzene Metabolites
| Treatment | Concentration (μM) | TGF-α (pg/ml) | IL-8 (pg/ml) | Viability (% decrease from control) |
|---|---|---|---|---|
| Vehicle | 48 ± 5a | 4 ± 1 | — | |
| 1,4-Benzoquinone | 10 | 65 ± 6 | 1 ± 7 | — |
| 25 | 99 ± 17* | 40 ± 1* | — | |
| 50 | 70 ± 3* | 97 ± 66* | 39* | |
| Hydroquinone | 10 | 49 ± 4 | 7 ± 4 | — |
| 25 | 71 ± 11 | 9 ± 7 | — | |
| 50 | 83 ± 11* | 18 ± 15 | — | |
| 100 | 136 ± 16* | 49 ± 17* | — | |
| 125 | 128 ± 16* | 81 ± 39* | — | |
| 1,2,4-Benzenetriol | 50 | 46 ± 2 | 4 ± 3 | — |
| 75 | 77 ± 9 | 28 ± 10* | — | |
| 100 | 130 ± 43* | 216 ± 55* | 2 | |
| Catechol | 100 | NTb | 1 ± 1* | — |
| 200 | 42 ± 1 | 1 ± 1* | — | |
| 400 | 38 ± 5* | 0 ± 0* | — | |
| Phenol | 5 × 103 | 53 ± 7 | 4 ± 1 | — |
| 10 × 103 | 60 ± 15 | 1 ± 4 | — | |
| 15 × 103 | 66 ± 4* | 1 ± 15* | 80* | |
| PMA | 1 × 10−3 | 614 ± 22* | NT | — |
Represent mean ± SD of four to six cultures.
NT, Not tested.
Significantly different from vehicle control, p < 0.05.
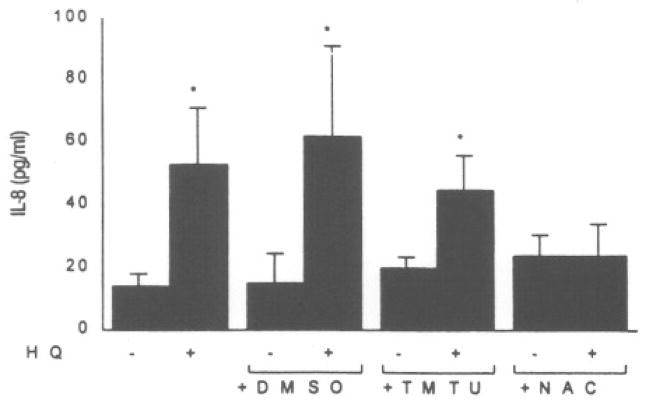
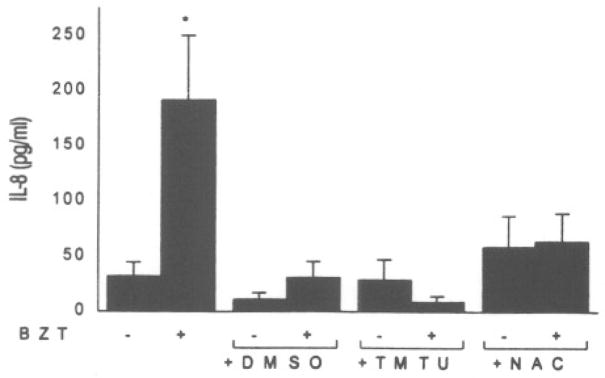
To assess the contribution of ROS generation in IL-8 induction, the antioxidants dimethylsulfoxide (DMSO), 1,1,3,3-tetramethylthiourea (TMTU), and N-acetylcysteine (NAC), were added to keratinocyte cell cultures in the presence of benzene metabolites. As demonstrated in Figure 1, while HQ induced a four-fold increase in IL-8 production, 0.5 mM NAC offered significant inhibition of HQ-induced IL-8 production. None of the treatment groups exhibited cytotoxicity. BZT also caused a significant increase in IL-8 production that was inhibited by all three antioxidants examined with NAC, on a molar basis, being the most effective nucleophile (Fig. 2).
FIG. 1.
Effect of antioxidants on hydroquinone (HQ)-induced IL-8 secretion in NHK cultures. Cells were pretreated for 30 minutes with 1% DMSO, 1 mM TMTU, or 0.5 mM NAC and exposed to HQ (125 μM). Asterisk (*) represents a significant (p < 0.05) increase from the vehicle control. Values represent the mean ± SD of four to six cultures.
FIG. 2.
Effect of antioxidants on 1,2,4-BZT-induced IL-8 secretion of NHK cultures. Cells were pretreated for 30 minutes with 1% DMSO, 1 mM TMTU, or 0.5 mM NAC and exposed to BZT (100 μM). Asterisk (*) represents a significant (p < 0.05) increase from the vehicle control. Values represent the mean ± SD of four to six cultures.
There have been a number of reports that combinations of benzene metabolites synergize in their ability to induce cytogenetic damage or toxicity (Robertson, et al., 1991). Various combinations of benzene metabolites were assessed for their ability to induce IL-8 secretion at non-cytotoxic concentrations. Of the combinations tested, CAT/HQ (0.1 mM each) resulted in synergistic increases in IL-8 secretion (Table 2). There was no evidence of cytotoxicity in any of the combinations depicted (data not shown).
Table 2.
IL-8 Secretion in NHK Cultures in the Presence of Binary Combinations of Benzene
| Treatment (concentration) | IL-8 (pg/ml) |
|---|---|
| Vehicle | 4 ± 1a |
| CAT (0.1 mM) | 1 ± 1 |
| BZT (0.05 mM) | 4 ± 1 |
| CAT + BZT | 5 ± 2 |
| CAT (0.1 mM) | 1 ± 1 |
| HQ (0.1 mM) | 49 ± 17 |
| CAT + HQ | 119 ± 23* |
Represent mean ± SD of four to six cultures.
Significantly different from vehicle control, p < 0.05.
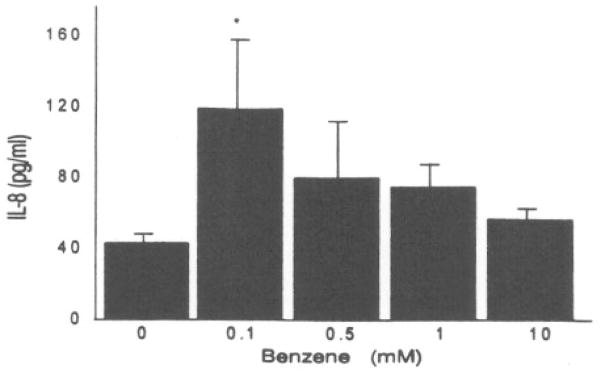
We determined whether benzene, alone, was capable of inducing IL-8 production in human keratinocyte cultures. The lowest concentration of benzene (0.1 mM) induced a modest but significant increase in IL-8 production (Fig. 3). There was no evidence of cytotoxicity at any benzene concentration tested.
FIG. 3.
Induction of IL-8 secretion in NHK cultures by benzene. Asterisk (*) represents a significant (p < 0.05) increase from the vehicle control. Values represent the mean ± SD of three cultures.
DISCUSSION
Benzene metabolites have four attributes associated with carcinogens: inflammatory properties (King et al., 1988; MacEachern and Laskin, 1992; Irons et al., 1992), cytotoxicity (Dean, 1985; Snyder and Kalf, 1994), genotoxicity (Dean, 1985; Yardley-Jones et al., 1991; Snyder and Kalf, 1994), and aneuploidy induction by mitotic spindle damage (Zhang et al., 1994). Regarding mediators of inflammation, several studies have implicated proinflammatory cytokines such as IL-1α (King et al., 1988; Renz and Kalf, 1991), TNF-α (MacEachern and Laskin, 1992), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Irons et al., 1992) in the pathophysiologic effects of benzene metabolites on bone marrow hematopoietic and stromal cells. In an earlier study, we demonstrated that the secretion of TNF-α and IL-8, as well as the synthesis and intracellular accumulation of IL-1α, in cultured human keratinocytes are associated with the weak tumor-promoting and irritant properties of phenol (Wilmer et al., 1994). In the present study, several benzene metabolites were shown to induce concentration-related increases in keratinocyte-derived TNF-α and IL-8. This response was relatively specific as the same metabolites did not stimulate cytokines such as TNF-α, IL-6, and macrophage chemotactic protein-1 (MCP-1) (data not shown).
TGF-α (Imamoto et al., 1991; Germolec et al., 1996) and, to a lesser extent, IL-8 (Tuschil et al., 1992) act as keratinocyte growth factors that when overexpressed can induce mitotic activity in the epidermis beyond that associated with normal cell proliferation. IL-8 is also a potent chemotactic and activating factor for neutrophils (Kunkel et al., 1991). We observed that not only benzene metabolites but also low concentrations of the parent compound can stimulate IL-8 secretion. Cytochrome P-450 activity in the skin and in the epidermis is well established (Mukhtar et al., 1992), and benzene and phenol are metabolized principally by ethanol-inducible cytochrome P-450 IIE1 (Koop et al., 1989).
Increasing evidence demonstrates that ROS participate in the signaling mechanisms of inflammatory cytokine production (DeForge et al., 1992; Simeonova and Luster, 1995). In this regard, the present study suggests that the ability of benzene metabolites to induce cytokines correlates with their ability to autooxidize and generate ROS. Benzene metabolites have been characterized to display redox potential in the following order: BZT>HQ>CAT (Lewis et al., 1988). Consistent with these observations, the inhibition of IL-8 production by antioxidants indicates that BZT mediates IL-8 secretion via the generation of ROS. The induction of IL-8 by BZT was attenuated by the three antioxidants tested, while HQ-mediated IL-8 secretion was blocked only by NAC, a precursor of glutathione. The effect of HQ could be the result of direct arylation of critical macromolecules or low molecular weight sulfhydryls or oxidation of cell components by ROS. However, our data suggest that HQ induces IL-8 predominantly through direct covalent binding, as only NAC afforded significant protection.
Combinations, rather than single benzene metabolites, are likely responsible for cytotoxicity and cytokine induction in vivo. The synergism between HQ and CAT in stimulating IL-8 production is particularly noteworthy as it parallels the findings of Robertson et al. (1991) who observed at a 16-fold increase above the additive frequency of micronuclei expected for the individual metabolites in human lymphocyte cultures treated with combinations of HQ and CAT. The synergism may result from an interaction between CAT and HQ within cells that enhances the formation of a reactive metabolite, or increases the flux of ROS responsible for stimulating IL-8.
In conclusion, evidence is presented that exposure of human epidermal keratinocytes to benzene and its metabolites at relatively low concentrations can induce selective cytokines and growth factors. Membrane damage and cytotoxicity is not a prerequisite for these responses. In the mortality study by Bond et al. (1986), the findings of a small but significant excess of skin tumors in benzene workers illustrates that future epidemiologic studies on benzene carcinogenesis may need to focus in a prospective manner on additional tissues such as the skin. Since most epidemiologic studies rely on mortality records for evidence of correlations with occupational exposure to benzene, it is likely that skin cancers are underreported as a cause of death owing to their early detection and treatment Huff et al., 1989). This is important considering that 900,000 new cases of skin cancer are diagnosed annually (Brandt, 1996). Clearly, the occurrence of tumors in rats exposed to benzene through different routes (Maltoni et al., 1989; Huff et al., 1989), as well as in TG.AC transgenic mice exposed dermally (French et al., 1994), have already suggested that epidermal keratinocytes are a target for malignant transformation. The elaboration of proinflammatory cytokines and growth factors by keratinocytes in response to benzene and its principal metabolites might provide a mechanistic link between tumor initiation and promotion that will be important in understanding benzene-induced skin carcinogenesis.
References
- BOND GG, MCLAREN EA, BALDWIN CL, COOK RR. An update for mortality among chemical workers exposed to benzene. Br J Ind Med. 1986;43:685–691. doi: 10.1136/oem.43.10.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUTWELL RK, BOSCH DK. The tumor-promoting action of phenol and related compounds for mouse skin. Cancer Res. 1959;19:413–424. [PubMed] [Google Scholar]
- BRANDT TP. Skin cancer screening. In: Herold AH, Woodard LJ, editors. The Medical Clinics of North America. Cancer Screening and Diagnosis. 1. Vol. 80. W.B. Saunders Company; Philadelphia: 1996. pp. 99–112. [DOI] [PubMed] [Google Scholar]
- DEAN BJ. Recent findings on the genetic toxicology of benzene, toluene, xylenes, and phenols. Mutat Res. 1985;154:153–181. doi: 10.1016/0165-1110(85)90016-8. [DOI] [PubMed] [Google Scholar]
- DEFORGE LE, FANTONE JC, KENNEY JS, REMICK DG. Oxygen radical scavengers selectively inhibit interleukin-8 production in human whole blood. J Clin Invest. 1992;90:2123–2129. doi: 10.1172/JCI116097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIGIOVANNI J. Multistage skin carcinogenesis in mice. In: Waalkes MP, Ward JM, editors. Carcinogenesis. Raven Press; NY: 1994. pp. 265–299. [Google Scholar]
- FRENCH JE, LIBBUS BL, HANSEN L, SPALDING J, TICE RR, MAHLER J, TENNANT RW. Cytogenetic analysis of malignant skin tumors induced in chemically treated TG.AC transgenic mice. Mol Carcinog. 1994;11:215–226. doi: 10.1002/mc.2940110407. [DOI] [PubMed] [Google Scholar]
- GERMOLEC DR, YOSHIDA T, GAIDO K, WILMER JL, SIMEONOVA PP, KAYAMA F, BURLESON F, DONG W, LANGE RW, LUSTER MI. Arsenic induces overexpression of growth factors in human keratinocytes. Toxicol Appl Pharmacol. 1996;41:308–318. doi: 10.1006/taap.1996.0288. [DOI] [PubMed] [Google Scholar]
- GREENLEE WF, SUN JD, BUS JS. A proposed mechanism of benzene toxicity: formation of reactive intermediates from polyphenol metabolites. Toxicol Appl Pharmacol. 1981;59:187–195. doi: 10.1016/0041-008x(81)90189-7. [DOI] [PubMed] [Google Scholar]
- HUFF JE, HASEMAN JK, DEMARINI DM, EUSTIS S, MARONPOT RR, PETERS AC, PERSING RL, CHRISP CE, JACOBS AC. Multiple-site carcinogenicity in Fischer 344 rats and B6C3F1 mice. Environ Health Perspect. 1989;82:125–163. doi: 10.1289/ehp.8982125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMAMOTO A, BELTRAN LM, DIGIOVANNI J. Evidence for autocrine/paracrine growth stimulation by transforming growth factor-α during the process of skin tumor promotion. J Mol Carcinog. 1991;4:52–60. doi: 10.1002/mc.2940040109. [DOI] [PubMed] [Google Scholar]
- IRONS RD, STILLMAN WS, COLAGIOVANNI DB, HENRY VA. Synergistic action of the benzene metabolite hydroquinone on myelopoietic stimulating activity of granulocyte/macrophage colony-stimulating factor in vitro. Proc Natl Acad Sci USA. 1992;89:3691–3695. doi: 10.1073/pnas.89.9.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING AG, WARTY D, LANDRETH KS. Bone marrow stromal cell regulation of B-lymphopoiesis. I. The role of macrophages, IL-1 and IL-4 in pre-B cell maturation. J Immunol. 1988;141:2016–2026. [PubMed] [Google Scholar]
- KOOP DR, LAETHEM CL, SCHNIER GG. Identification of ethanol-inducible P450 isozyme 3a (P450 IIE1) as a benzene and phenol hydroxylase. Toxicol Appl Pharmacol. 1989;98:278–288. doi: 10.1016/0041-008x(89)90233-0. [DOI] [PubMed] [Google Scholar]
- KUNKEL TJ, STANDIFORD T, KASHARA K, STREITER RM. Interleukin −8: the major neutrophil chemotactic factor in the lung. Exp Lung Res. 1991;17:17–23. doi: 10.3109/01902149109063278. [DOI] [PubMed] [Google Scholar]
- KUPPER TS. Role of epidermal cytokines. In: Oppenheim JJ, Shevach EM, editors. lmmunophysiology: The Role of Cells and Cytokines in Immunity and Inflammation. Oxford University Press; NY: 1990. pp. 285–305. [Google Scholar]
- LEWIS JG, STEWART W, ADAMS DO. Role of oxygen radicals in induction of DNA damage by metabolites of benzene. Cancer Res. 1988;48:4762–4765. [PubMed] [Google Scholar]
- MACEACHERN L, LASKIN DL. Increased production of tumor necrosis factor-α by bone marrow leukocytes following benzene treatment of mice. Toxicol Appl Pharmacol. 1992;113:260–266. doi: 10.1016/0041-008x(92)90123-a. [DOI] [PubMed] [Google Scholar]
- MALTONI C, CILIBERTI A, COTTI C, BELPOGGI F. Benzene, an experimental carcinogen: Results of the long-term bioassays performed at the Bologna Institute of Oncology. Environ Health Perspect. 1989;82:109–124. doi: 10.1289/ehp.8982109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUKHTAR H, AGARWAL R, BICKERS DR. Cutaneous metabolism of xenobiotics and steroid hormones. In: Mukhtar H, editor. Pharmacology of the Skin. CRC Press, Inc; Boca Raton, FL: 1992. pp. 89–109. [Google Scholar]
- RENZ JF, KALF GF. Role for interleukin-1 (IL-1) in benzene-induced hematotoxicity: Inhibition of conversion of pre-IL-1α to mature cytokine in murine macrophages by hydroquinone and prevention of benzene-induced hematotoxicity in mice by IL-1α. Blood. 1991;78:938–944. [PubMed] [Google Scholar]
- ROBERTSON ML, EASTMOND DA, SMITH MT. Two benzene metabolites, catechol and hydroquinone, produce a synergistic induction of micronuclei and toxicity in cultured human lymphocytes. Mutat Res. 1991;249:201–209. doi: 10.1016/0027-5107(91)90147-g. [DOI] [PubMed] [Google Scholar]
- SAX NI. Hydroquinone. Dangerous Properties of Industrial Materials Report. Van Nostrand Reinhold; NY: 1988. pp. 51–60. [Google Scholar]
- SCHRECK R, BAEUERLE PA. NF-κB as inducible transcriptional activator of the granulocyte-macrophage colony-stimulating factor gene. Mol Cell Biol. 1990;10:1281–1286. doi: 10.1128/mcb.10.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMEONOVA PP, LUSTER MI. Iron and reactive oxygen species in the asbestos-induced tumor necrosis factor-α response from alveolar macrophages. Am J Resp Cell Mol Biol. 1995;12:676–683. doi: 10.1165/ajrcmb.12.6.7539275. [DOI] [PubMed] [Google Scholar]
- SLAGA TJ. Mechanisms involved in two-stage carcinogenesis in mouse skin. In: Slaga TJ, editor. Mechanisms of Tumor Promotion. Tumor Promotion and Skin Carcinogenesis. Vol. 2. CRC Press, Inc; Boca Raton, FL: 1984. pp. 1–16. [Google Scholar]
- SNYDER R, KALF GF. A perspective on benzene leukemogenesis. CRC Crit Rev Toxicol. 1994;24:177–209. doi: 10.3109/10408449409021605. [DOI] [PubMed] [Google Scholar]
- SOKAL RR, ROHLF FJ. Biometry: The Principles and Practice of Statistics in Biological Research. WH Freeman; San Francisco: 1969. [Google Scholar]
- TUSCHIL A, LAM C, HASLBERGER A, LINDLEY I. Interleukin-8 stimulates calcium transients and promotes epidermal cell proliferation. J Invest Dermatol. 1992;99:294–298. doi: 10.1111/1523-1747.ep12616634. [DOI] [PubMed] [Google Scholar]
- VAN DUUREN BL, WITZ G, GOLDSCHMIDT BM. Structure-activity relationships of tumor promoters and co-carcinogens and interaction of phorbol myristate acetate and related esters with plasma membranes. In: Slaga TJ, Sivak A, Boutwell RK, editors. Carcinogenesis: Mechanisms of Tumor Promotion and Cocarcinogenesis. Vol. 2. Raven Press; NY: 1978. pp. 491–507. [Google Scholar]
- WILMER JL, BURLESON FG, KAYAMA F, KANNO J, LUSTER MI. Cytokine induction in human epidermal keratinocytes exposed to contact irritants and its relation to chemical-induced inflammation in mouse skin. J Invest Dermatol. 1994;102:915–922. doi: 10.1111/1523-1747.ep12383512. [DOI] [PubMed] [Google Scholar]
- WILMER JL, LUSTER MI. Chemical induction of interleukin-8, a proinflammatory chemokine, in human epidermal keratinocyte cultures and its relation to cytogenetic toxicity. Cell Biol Toxicol. 1995;11:37–50. doi: 10.1007/BF00769991. [DOI] [PubMed] [Google Scholar]
- WITZ G, LATRIANO L, GOLDSTEIN BD. Metabolism and toxicity of trans, trans-muconaldehyde, an open-ring microsomal metabolite of benzene. Environ Health Perspect. 1989;82:19–22. doi: 10.1289/ehp.898219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YARDLEY-JONES A, ANDERSON D, PARKE DV. The toxicity of benzene and its metabolism and molecular pathology in human risk assessment. Br J Ind Med. 1991;48:437–444. doi: 10.1136/oem.48.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG L, VENKATESH P, ROBERTSON-CREEK ML, SMITH MT. Detection of 1,2,4-benzenetriol induced aneuploidy and microtubule disruption by fluorescence in situ hybridization and immunocytochemistry. Mut Res. 1994;320:315–327. doi: 10.1016/0165-1218(94)90084-1. [DOI] [PubMed] [Google Scholar]