Abstract
Background
Patients with systemic sclerosis (SSc) may develop exercise intolerance due to musculoskeletal involvement, restrictive lung disease, left ventricular dysfunction, or pulmonary vasculopathy (PV). The latter is particularly important since it may lead to lethal pulmonary arterial hypertension (PAH). We hypothesized that abnormalities during cardiopulmonary exercise testing (CPET) in patients with SSc can identify PV leading to overt PAH.
Methods
Thirty SSc patients from the Harbor-UCLA Rheumatology clinic, not clinically suspected of having significant pulmonary vascular disease, were referred for this prospective study. Resting pulmonary function and exercise gas exchange were assessed, including peakVO2, anaerobic threshold (AT), heart rate- VO2 relationship (O2-pulse), exercise breathing reserve and parameters of ventilation-perfusion mismatching, as evidenced by elevated ventilatory equivalent for CO2 (VE/VCO2) and reduced end-tidal pCO2 (PETCO2) at the AT.
Results
Gas exchange patterns were abnormal in 16 pts with specific cardiopulmonary disease physiology: Eleven patients had findings consistent with PV, while five had findings consistent with left-ventricular dysfunction (LVD). Although both groups had low peak VO2 and AT, a higher VE/VCO2 at AT and decreasing PETCO2 during early exercise distinguished PV from LVD.
Conclusions
Previously undiagnosed exercise impairments due to LVD or PV were common in our SSc patients. Cardiopulmonary exercise testing may help to differentiate and detect these disorders early in patients with SSc.
Introduction
Dyspnea on exertion, fatigue, and reduced exercise tolerance are common symptoms in patients with systemic sclerosis (SSc). These symptoms can often be explained by involvement of the musculoskeletal system, lungs, heart, chest wall, and/or pulmonary vasculature, in isolation or combination. Patients with SSc are at particular risk for developing pulmonary vasculopathy (PV) leading to pulmonary arterial hypertension (PAH). Untreated, PAH results in right ventricular failure, and early death [1].
PV impairs dilatation of affected pulmonary blood vessels, impeding pulmonary blood flow during exercise. This eventually leads to pulmonary hypertension and exercise intolerance. Initially, the degree of exercise limitation is determined by the ability of the right ventricle to hypertrophy and maintain adequate blood flow through the lungs. At this stage, pulmonary hypertension might only be visible during exercise [2], [3]. Over time, vasculopathy progresses and the right ventricular reserve fails to meet the pulmonary blood flow required for the increased O2 demand of exercise, leading to exertional dyspnea and fatigue and physical signs of pulmonary hypertension.
Early detection of PV may be desirable since timely therapeutic intervention improves outcomes in experimental models [4], [5]. Additionally, treatment of patients with early PAH can delay clinical worsening [6]. Pulmonary vasculopathy develops unevenly in the lungs. Thus, abnormal gas exchange findings characteristic of ventilation-perfusion mismatching, is an early abnormality during cardiopulmonary exercise testing (CPET) [7].
The gas exchange abnormalities during CPET in patients with PV reflect hypoperfusion of well-ventilated acini. Thus, ventilation (VE) is high compared to relatively low CO2 output (VCO2) and reduced end-tidal PCO2 (PETCO2), manifesting hypoperfusion of well-ventilated lung. In this study, we performed CPET in a group of referred SSc patients, without previously known or suspected PV. We expected that these patients would display heterogeneous gas exchange patterns during exercise, which cannot be explained by resting measurements alone. We hypothesized that we would find characteristic gas exchange patterns that would enable us to discriminate between the different causes of exercise intolerance, based on the exercise pathophysiology. We hypothesized that some of the patients would show gas exchange patterns during exercise that are characteristically found in patients with overt pulmonary vascular pathophysiology.
Methods
Ethics statement
This study was conducted in accordance with Good Clinical Practices and the current version of the revised Declaration of Helsinki [8]. The local Los Angeles Biomedical Research Institutional Review Board approved the protocol. A written informed consent was obtained from each patient prior to enrollment.
Study population
We prospectively screened 32 SSc patients referred from the Rheumatology Clinic at Harbor-UCLA Medical Center for CPET in order to determine if they had evidence of PV. Prior to referral, all patients had chest X-rays and/or high-resolution chest CT-scans. All patients had echocardiography with estimation of pulmonary artery pressure (PAP) prior to referral. Patients with estimated systolic PAP >35 mmHg, were excluded.
All patients had been diagnosed with SSc according to the criteria of the American College of Rheumatology (ACR) [9]. One patient refused to perform CPET and another could not perform CPET because of joint stiffness. Thus, thirty patients performed CPET.
Evaluations
6-minute walk test
All patients performed an unencouraged, standardized 6-minute walk test (6MWD), at least one hour before or after CPET [10].
Pulmonary function testing
Total lung capacity (TLC), forced vital capacity (FVC), forced expired volume in one second (FEV1), diffusing capacity for carbon monoxide (DLCO) and alveolar volume (VA) were all measured as part of CPET and are expressed as percent predicted.
Assessment of restrictive lung disease
Restrictive lung disease was assessed by a combination of resting pulmonary function tests (PFTs), including diffusion capacity for carbon monoxide (DLCO), by Chest X-ray (CXR) and by high-resolution computed tomography (HRCT). An HRCT was performed if there were abnormalities in PFTs or CXR. An HRCT was not performed in patients with normal PFTs and a normal CXR, or a definite diagnosis of ILD based on these two measurements.
The available HRCT-scans in patients with suspected ILD (20 out of 30) were analyzed for signs of pulmonary venous occlusive disease (PVOD). Main characteristics were enlarged mediastinal lymph nodes, alveolar hemorrhage, centrilobular ground glass opacities and septal lines on HRCT.
Cardiopulmonary exercise testing
CPET was performed with upright cycling on a stationary cycle ergometer. The exercise protocol consisted of 3 minutes of rest and 3 minutes of unloaded cycling, followed by an incremental work rate between 5 and 15 watts per minute up to the patients' maximum tolerance, then 3 minutes of recovery. Gas exchange was measured breath-by-breath during the test, using a MedGraphics CPX-Ultima gas exchange system (Medical Graphics Corporation, St. Paul, Minnesota). Equipment was calibrated as previously described [11]. ECG and pulse oximetry were continuously monitored and blood pressure was measured every two minutes. Minute ventilation (VE), heart rate (HR), VO2/HR, VO2, VCO2, VCO2 vs VO2, VE/VO2, VE/VCO2, tidal volume (VT) vs VE, end-tidal PO2 (PETO2) and PCO2 (PETCO2) and the respiratory exchange ratio (RER) were averaged every 10 seconds. The anaerobic threshold (AT) was determined from gas exchange, by the V-slope method as previously described [12], in all patients. The AT was derived from a plot with VO2 (x-axis) and VCO2 (y-axis) on equal axis scaling, and was recognized as the point where VCO2 started to increase faster than VO2. AT prediction was performed as previously described [13], [14]. The other key variables were calculated and plotted as previously described [15], [16]. All studies were independently reviewed by two authors (DD and KW). Disagreements were adjudicated after review by a third author (JH), and consensus agreement among all three.
Categorizing Exercise Impairment
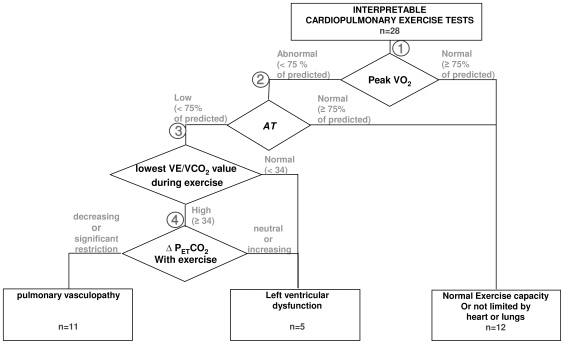
Patients with known severe heart or lung disease limiting exercise, or individuals with known PAH, were not referred by the Rheumatologists. An additional two patients with uninterpretable cardiopulmonary exercise test results were not included in the analysis (2 of 30 patients). The first patient stopped during the unloaded cycling phase due to joint pain. The second patient had a very noisy and chaotic breathing pattern. For both of these patients, peak VO2, the AT and VE/VCO2 at the AT could not be accurately determined. Thus, 28 patients were available for analysis. Figure 1 presents the algorithm utilized. Disagreement in the blinded interpretation of the CPET studies occurred in 2 of 28 interpretable cases. Agreement was reached in these two cases by review of a third author. The normal category included those with a normal peak VO2, normal anaerobic threshold (AT) normal ventilation-perfusion matching, and no exercise-induced hypoxemia (6 of 28 patients). The normal category also included six patients with a reduced peak VO2, but with normal AT, no abnormality in ventilation-perfusion matching or exercise-induced hypoxemia and an RER at peak exercise below 1.0, indicating submaximal effort. These patients were categorized as not being limited by heart or lung disease. Thus, 12 of the 28 patients were categorized as normal.
Figure 1. Categorizing referred SSc patients with normal and reduced exercise capacity, using cardiopulmonary exercise testing.
Exercise intolerance was attributed to left ventricular dysfunction or pulmonary vascular disease. Normal is defined as either: a) normal in all cardiovascular and ventilatory aspects of exercise gas exchange, including normal ventilation-perfusion matching and normal peak VO2, or b) reduced peak VO2 with normal AT and no gas exchange abnormalities suggestive of heart, lung or pulmonary vascular disease. Diamonds (branch-points) address specific data: Branch-point 1: Right branch: If the peak VO2 is ≥75% of predicted with normal VE/VCO2 and PETCO2 @ AT and non-ventilatory limitation, the patient is considered to have normal heart and lung function. Left branch includes all with peak VO2 <75%. Branch-point 2: If the AT is normal and ventilation-perfusion matching and lung mechanics are normal (right branch), the patient is considered to be limited by poor effort and not limited by heart or lung disease. If the AT is reduced (left branch), the patient is likely to have left ventricular dysfunction or pulmonary vasculopathy. Branch-point 3: The VE/VCO2 @AT was used to assess matching of ventilation to perfusion. All patients with pulmonary vasculopathy would have ventilation/perfusion mismatching and an elevated VE/VCO2. A cut-off value of ≥34 was selected. If not elevated, they were considered to have left ventricular dysfunction. Branch point 4: PETCO2 usually increases from the beginning of exercise to the AT in patients with normal cardiopulmonary function and patients with left ventricular dysfunction (right branch). However, it usually decreases in patients with pulmonary arterial hypertension (left branch). Nine of the 11 patients classified as pulmonary vasculopathy had a decreasing PETCO2. Two had either no change or increasing PETCO2 from the start of exercise to the AT, possibly due to lung restriction. However, they hyperventilated above their AT. If the patient had moderate to severe restriction and marked decrease in DLCO, this signified interstitial lung disease with pulmonary vasculopathy.
Patients were categorized in the left ventricular dysfunction (LVD) group if they had a reduced peak VO2, AT, peak O2-pulse and Δ VO2/ΔWR - but without ventilation-perfusion mismatch or exercise-induced hypoxemia or RER at peak exercise <1.0. (5 of 28 patients were in this category).
Patients were categorized in the PV group if they had a reduced peak VO2 and AT, reduced peak O2-pulse and ΔVO2/ΔWR, and ventilation-perfusion mismatch (elevated VE/VCO2 at the AT or at the ventilatory compensation point (VCP) following AT). In addition, based on prior research [17], [18], suspected PV was separated from LVD by a decreasing PETCO2 from the start of exercise to AT (9 of 28 patients were in this category), in contrast to an increasing PETCO2 in LVD and normal subjects. Two other patients showed rising PETCO2 during exercise but were classified as suspected PV secondary to their restrictive lung disease with parallel loss of pulmonary capillary volume (low TLC and DLCO with normal FEV1/FVC), however breathing reserve was thought to be adequate without mechanical ventilatory limitation at peak exercise. This is based on a prior study [19] showing that lung restriction from pulmonary fibrosis, before functional lung restriction, is accompanied by exercise limiting PV.
Statistical analysis
A total of 28 of the SSc patients referred, with interpretable CPET studies, were analyzed; they were divided into 3 major categories: normal, LVD and PV, as described above. Continuous variables are expressed as mean ± SD. The three groups were individually compared to each other. Differences were analyzed using one way ANOVA, followed by Holm-Sidak testing for multiple comparisons. Nominal data were analyzed by Chi-square test for multiple groups. In all cases, a p value <0.05 was considered statistically significant.
Results
Table 1 shows the demographics according to diagnostic category. All patients tolerated CPET well, and there were no adverse events.
Table 1. Demographics for each exercise diagnosis in 30 scleroderma patients.
| Not interpretable exercise test results(n = 2) | Normal exercise capacity (NL)(n = 12) | Left Ventricular Dysfunction(LVD)(n = 5) | Pulmonary vasculopathy(PV)(n = 11) | NL vs. LVD | p-valueNL vs. PV | LVD vs. PV | |||
| M/F | 0/2 | 2/10 | 2/3 | 1/10 | |||||
| Limited/diffuse SSc | 2/0 | 9/3 | 3/2 | 9/2 | |||||
| NYHA Class I | 1/2 | 6/12 | 4/5 | 2/11 | |||||
| NYHA Class II | 1/2 | 6/12 | 1/5 | 8/11 | |||||
| NYHA Class III | 0/2 | 0/12 | 0/5 | 1/11 | |||||
| Age (years) | 51±1 | 52±7 | 41±11 | 49±14 | n/s (p = 0.31) | ||||
| BMI (kg/m2) | 28.2±7.2 | 28.5±7.7 | 27.0±3.9 | 26.4±5.7 | n/s (p = 0.73) | ||||
| ACA positive | 1/2 | 5/12 | 2/5 | 2/11 | n/s (p = 0.44) | ||||
| Scl-70 positive | 1/2 | 3/12 | 0/5 | 3/11 | n/s (p = 0.30) | ||||
ACA = anti-centromer antibodies.
Scl-70 = DNA-topoisomerase I antibodies.
Gas Exchange Patterns
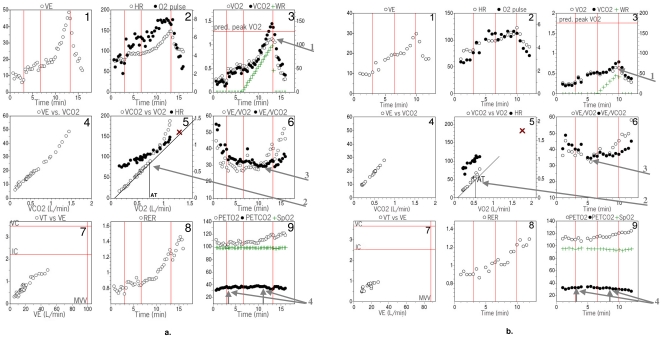
Figure 2 shows how the 15 variables taken from the CPET 9-panel plots of two representative SSc patients were analyzed. Figure 2a shows an SSc patient with a normal CPET response; Figure 2b shows another SSc patient with PV. The 4 arrows in Figures 2a and 2b correspond to the 4 branch-point parameters shown in Figure 1. The legend for figure 2 provides further detail.
Figure 2. Gas exchange response to exercise in two SSc patients.
Nine panel plots of a patient with normal exercise performance (Fig. 2a) and one with pulmonary vasculopathy (Fig. 2b). The protocol consisted of a 3-minute resting period, followed by 3 minutes of very-low-level cycle exercise, and then increasing cycle workload to the patient's maximum tolerance. Points are 20-second averages. Panel 1 is plot of ventilation against time. Panel 2 is plot of heart rate and O2-pulse against time. Panel 3 is plot of O2 uptake (VO2), CO2 output (VCO2) and work rate against time. Panel 4 is plot of minute ventilation (VE) against VCO2. Panel 5 is plot of VCO2 and HR against VO2. Panel 6 is plot of ventilatory equivalent for VO2 (VE/VO2) and VCO2 (VE/VCO2) against time. Panel 7 is plot of tidal volume against minute ventilation, with resting maximum voluntary ventilation on the X-axis and inspiratory capacity and vital capacity, measured at rest, on the Y-axis. Panel 8 is plot of gas exchange ratio (RER) against time. Panel 9 is plot of end tidal pO2 (PETO2), end tidal pCO2 (PETCO2) and pulse oximeter arterial oxyhemoglobin saturation against time. The normal subject (figure 2a) is a 59 year old female with scleroderma. Peak VO2 and AT are normal (panels 3 and 5) There are no signs of impaired oxygen flow, or ventilation/perfusion mismatching during exercise. Peripheral oxyhemoglobin saturation does not decrease during exercise. There is adequate breathing reserve. The subject with suspected pulmonary vasculopathy (figure 2b) is a 37 year old female with scleroderma. Peak VO2 and AT are reduced (panel 3, panel 5). Ventilatory equivalents are elevated and decrease only slightly during exercise (panel 6). End-tidal pCO2 is low and decreases during exercise (panel 9), consistent with reduced gas exchange efficiency rather than voluntary hyperventilation (RER is normal, panel 8). The patient stopped exercise because of leg pain. Four arrows are placed on each of Figures 2a and 2b that correspond to the branch-points described in Figure 1, Arrow 1 points to the peak VO2 in panel 3 (branch-point 1). Arrow 2 points to the AT in panel 5 (branch-point 2). Arrow 3 points to the VE/VCO2 at the AT in panel 6 (branch-point 3). Arrow 4 points to the changing PETCO2 from start of exercise to AT in panel 9.
Table 2 shows the 6 minute walk distance, key pulmonary function measurements, the presence of restrictive lung disease, pulse oximetry, and seven CPET parameters by diagnostic categories. Six patients achieved their predicted peak VO2, and another six stopped exercise prematurely without evidence of cardiovascular or pulmonary limitation. All 12 had linear increases in HR vs VO2 relationship towards their predicted value, normal AT, O2-pulse, and VE/VCO2 @ AT, as exemplified in figure 2a. We classified all 12 as normal. The other 16 patients achieved a symptom-limited test below their predicted peak VO2, and also had additional abnormalities. Of these 16, 5 were classified as LVD and 11 were classified as PV. Two of the latter also had significant restrictive lung disease with reduced FVC, TLC and DLCO. No patients were limited by obstructive lung disease, all had an adequate breathing reserve at peak exercise. In the patients who underwent HRCT due to clinical suspicion of interstitial lung disease (ILD), presence of ILD was found among all groups, with a trend to higher occurrence in the PV group. However, this difference did not reach statistical significance (p = 0.07).
Table 2. Physiologic measurements related to resting lung function and gas exchange during exercise in 28 scleroderma patients.
| Normal exercise capacity (NL)(n = 12) | Left Ventricular Dysfunction(LVD)(n = 5) | Pulmonary vasculopathy(PV)(n = 11) | NL vs. LVD | p-valueNL vs. PV | LVD vs. PV | |||
| Aerobic capacity | 6-MWD (m) | 444±78 | 394±66 | 351±76 | 0.22 | 0.01 | 0.31 | |
| Peak V̇O2(% predicted) | 73.5±13.1 | 46.9±5.8 | 48.8±12.0 | <0.001 | <0.001 | 0.76 | ||
| AT(% predicted) | 102.0±17.8 | 66.0±11.5 | 71.5±19.4 | <0.001 | <0.001 | 0.58 | ||
| Cardiac Function | Peak O2 pulse(% predicted) | 87.1±13.1 | 65.5±6.5 | 72.6±17.6 | 0.009 | 0.03 | 0.37 | |
| Δ V̇O2/ΔWR ((ml/min)/W) | 9.1±0.9 | 7.2±0.9 | 6.5±2.0 | 0.001 | <0.03 | 0.45 | ||
| Ventilatory inefficiency | V̇E/V̇CO2 AT * | 29.8±2.9 | 30.2±2.4 | 39.2±8.3 | 0.87 | <0.001 | 0.002 | |
| PETCO2 AT * (mmHg) | 37.9±4.5 | 37.4±4.0 * | 31.0±2.5 * | 0.82 | <0.001 | 0.004 | ||
| Difference PETCO2 AT – PETCO2Start (mmHg) | 3.2±2.3 | +3.9±2.0 * | −1.3±2.6 * | 0.93 | <0.001 | 0.002 | ||
| Lung function/ imaging | FVC(% predicted) | 94.9±13.8 | 92.1±23.1 | 75.7±18.5 | 0.75 | 0.01 | 0.08 | |
| FEV1/FVC(% predicted) | 95.5±6.6 | 91.0±8.6 | 95.4±8.2 | n/s (p = 0.60) | ||||
| DLCO(% predicted) | 89.8±22.6 | 71.8±12.9 | 54.7±17.6 | 0.18 | <0.001 | 0.05 | ||
| FVC/DLCO(no unit) | 1.14±0.17 | 1.30±0.28 | 1.45±0.29 | 0.17 | 0.01 | 0.37 | ||
| Presence of ILD | 3/12 | 2/5 | 8/11 | n/s (p = 0.07) | ||||
| Pulse oximetry | Resting SpO2(%) | 96.8±1.81 | 95.8(2.17 | 96.5(2.07 | n/s (p = 0.82) | |||
| Nadir SpO2(%) | 91.9(5.52 | 94.4(3.78 | 90.8(6.14 | n/s (p = 0.48) | ||||
* = p<0.05, left ventricular dysfunction group vs. pulmonary vasculopathy group.
None of these patients showed signs of PVOD.
Exercise Capacity
The cause of exercise limitation discerned from all 28 interpretable cardiopulmonary exercise tests was determined using the algorithm shown in Figure 1. Its branch-points systematically examined each of the key parameters from the 9-panel plots (Figure 2). Figure 2 shows where the branch-point data were obtained in each patient's 9-panel plot. Exercise capacity was significantly reduced due to identifiable defects in 16 of the 28 patients. In these patients, peak VO2 and VO2 at AT were <75% of the absolute predicted value and/or oxygen pulse reached a plateau at a significantly reduced value above the AT (Figure 2b, panel 2).
Several measurements in Table 2 are of special interest. FVC values were mildly reduced, 6MWD was moderately reduced, and DLCO values were markedly reduced from normal in the PV group (p<0.001). However, reductions were qualitatively similar in the two cardiovascular disorders, the difference did not reach statistical significance. The FVC/DLCO ratio showed the same results: Only the normal and the PV group showed a difference which reached statistical significance (p = 0.01). The difference between the normal and the LVD group, as well as the difference between the PV and the LVD group were not statistically significant (p = 0.17 and p = 0.37, respectively).
Peak VO2, AT, peak O2 pulse and Δ VO2/ΔWR were all reduced in patients with PV and LVD, but the magnitudes and patterns of these reductions did not distinguish the two disorders. As single parameters, only PETCO2@AT (p = 0.004), VE/VCO2@AT (p = 0.002) and the changes in PETCO2 from early exercise to the AT (p = 0.002) distinguished LVD from PV.
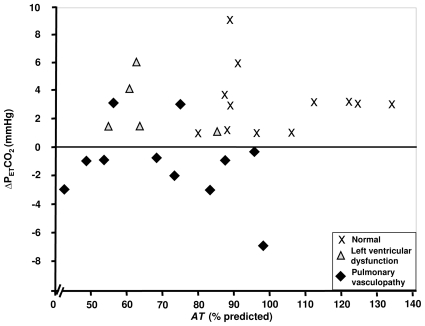
The directional change in PETCO2 at the start of exercise to the AT (ΔPETCO2) tends to be negative (decreases to the AT), as has been previously shown in patients with idiopathic PAH [17], [18]. In contrast, PETCO2 increases from the start of exercise to the AT in the normal subjects and the patients with LVD (Fig. 3). There was no significant difference between the normal (3.2±2.3 mm Hg) and LVD (3.9±2.0) groups (p = 0.93) in the PETCO2 change. However, the PV group (−1.3±2.6) differed significantly from both (p<0.001 and p = 0.002, respectively).
Figure 3. Difference between PETCO2 at AT and PETCO2 at start of exercise, plotted against AT, percent predicted, for SSc patients with normal exercise tolerance, left ventricular dysfunction, and pulmonary vasculopathy.
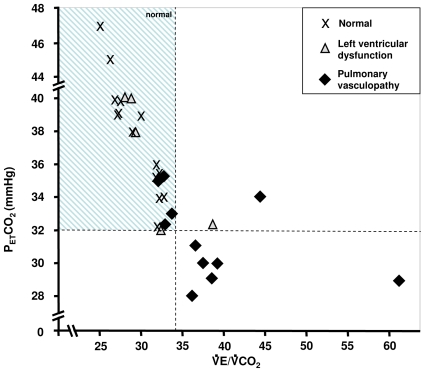
Figure 4 shows the relationships of PETCO2 to VE/VCO2 at the AT of all patients. Although there is some overlap, most patients with pulmonary vasculopathy had a lower PETCO2 and higher VE/VCO2 than the normal and LVD groups.
Figure 4. PETCO2 as a function of VE/VCO2 at the anaerobic threshold in 28 SSc patients.
The FEV1/FVC ratio was normal in all subjects. However, on average, our patients with PV tended to have lower FVC than the normal and LVD groups, (Table 2). To distinguish those patients with PV and restriction from those without or less restriction, we plotted the FVC against VE/VCO2 and PETCO2 at AT (Figures 5a and 5b). Approximately half of the patients with ventilation-perfusion mismatch (high VE/VCO2 and low PETCO2 at the AT) had significant reductions in FVC, while the others had PV with no or minimal restriction (normal FVC).
Figure 5. FVC as a function of PETCO2at AT (Fig. 5a) and PETCO2 at the AT(Fig. 5b) in 28 SSc patients with normal exercise tolerance, left ventricular dysfunction, and pulmonary vasculopathy.
Discussion
Previous studies have described lung gas exchange abnormalities at rest and during exercise in SSc patients [20]. However, this is the first study to show that abnormal gas exchange patterns during exercise, characteristic of PV, can be seen in patients with SSc without elevated pulmonary artery pressure on echocardiography or of having pulmonary vascular disease based on clinical suspicion. These abnormal CPET patterns may represent early PV, which with time, may lead to clinical and symptomatic pulmonary hypertension at rest.
Using CPET, we found evidence of possible PV in an SSc patient population, which was asymptomatic for the disease. A decreased peak VO2 along with a reduced AT has been the primary marker of reduced exercise capacity in patients with cardiovascular limitations to exercise [21]. However, patients with multi-organ diseases like scleroderma, are frequently exercise-limited with unclear cause. The 6MWD cannot be expected to define pathophysiology or differentiate causes of reduced exercise capacity. Therefore assessment of measures beyond 6MWD and peak VO2 measurements is needed to identify the specific pathophysiology underlying exercise intolerance.
In this study, we hypothesized that measures of ventilatory efficiency, specifically PETCO2 and VE/VCO2 and their patterns of change during exercise, added to other gas exchange measures evaluating peak and sustainable cardiac output, or VO2, could be used to differentiate patterns which indicate possible PV from other causes of exercise gas exchange abnormalities. Elevated VE/VCO2 values at the AT or VCP are important non-invasive measurements of ventilation-perfusion mismatching due to loss of pulmonary vasculature, and can be identified without maximal exercise. The additional finding of a low PETCO2@AT was even more discriminatory when used to differentiate early LVD from early PV (Table 2).
Because of loss of perfusion to ventilated lung, there is less CO2 laden blood to release CO2 to the airspaces for a given ventilation in patients with suspected PV. Thus, to eliminate the metabolic CO2, ventilation must increase resulting in an elevated ratio of VE to VCO2. In left ventricular failure, it is also common for portions of the lung to be well-ventilated, but be poorly perfused. Thus, VE/VCO2 is commonly used as an index of the severity of LVD [22], [23]. Due to its pathogenesis, VE/VCO2 should invariably be increased in patients with PV [18], [24], [25]. Because of the loss of vascularity to lung acini, PETCO2 is diluted in proportion to the fraction of underperfused acini. Thus, PETCO2 is decreased as VE/VCO2 is increased, the degree depending on disease severity. In less severe stages of pulmonary vascular disease, small increases in VE/VCO2 are accompanied by large decreases in PETCO2 [18] (Figure 4). Thus, a reduced PETCO2 at the AT or VCP is a valuable marker of blood vessel loss, and may be sensitive in detecting early pulmonary vascular disease.
It has also been shown, in the transition from the start of exercise to the AT, that PETCO2 tends to decrease in PAH, whereas the PETCO2 tends to increase in LVD [17]. This observation appears to occur in SSc patients with suspected PV as well.
Figure 5 relates the degree of lung restriction (reduced FVC) to the elevation of VE/VCO2 (Fig. 5a) and dilution of PCO2 (Fig. 5b) at the AT or VCP. All three groups had reductions in FVC, but it is mainly the PV group that had the abnormally high VE/VCO2 and low PETCO2 values. This might become therapeutically relevant in patients with SSc and borderline pulmonary hypertension. Presumably, the best candidates for specific therapy would be those patients with the highest VE/VCO2 and lowest PETCO2 values and least lung restriction. However, the validation of this hypothesis is subject to further studies.
Study limitations
Our diagnostic algorithm categorizing exercise pathophysiology, based on patterns of exercise gas exchange, was designed to identify scleroderma patients with characteristic patterns of PV and normal pulmonary artery pressure on echocardiography. We did not perform right-heart catheterization in our patients, as the study aim was to detect patterns of early PV in patients who might not have yet progressed to clinical resting pulmonary hypertension, so that an elevated PAP during a resting right heart catheterization might not have been evident, given a normal systolic PAP on echocardiography. Only long-term longitudinal evaluation of these patients will enable us to discern the rates of progression of these abnormalities, and may provide insight into the natural course of PV in patients with SSc.
True dead space/tidal volume ratio can only be calculated using arterial blood gas measurements. We did not do arterial blood sampling during exercise in order to avoid discomfort, and the potential for sudden peripheral vasospasm in SSc patients. However, increased VE/VCO2 beyond that found in normal subjects [26], and simultaneously decreased PETCO2 at the AT, as well as specific changes in the patterns of these two variables as work rate is increased, strongly suggest that dead space ventilation is increased.
Although more patients with systemic sclerosis suffer from the limited type than from the diffuse type, the distribution between diffuse and limited SSc may have been shifted towards patients with the limited form of the disease in our cohort, as only a few patients were found to have the diffuse form. Thus, our findings might be influenced by an overrepresentation of patients with the limited form of SSc.
The differential diagnosis of pulmonary veno-occlusive disease (PVOD) in SSc patients, an important clinical question, is challenging. We could not definitely exclude PVOD in our subjects, as this would require histological confirmation. However, this procedure is not recommended as it carries a significant risk [27]. HRCT, which was performed in all patients with suspicion of ILD (20 out of 30 patients) did not show any findings consistent with PVOD such as enlarged mediastinal lymph nodes, alveolar hemorrhage, or septal lines in any of the patients. In the remaining 10 patients, HRCT was not indicated as clinical status, PFT, chest x-ray and (except for one asymptomatic patient) VE/VCO2 were normal, and hence the probability of PVOD is considered very low. Furthermore, in these patients the nadir SpO2 during exercise were significantly higher than the values found in PVOD patients reported by Montani et al [28].
We conclude that routine CPET may be a sensitive method to detect developing exercise intolerance and provide additional information on the mechanism of exercise limitation in SSc. More detailed analysis of the specific pathophysiological mechanism underlying the developing exercise intolerance, such as PV and LVD, might clarify the treatment direction and therefore might help in preventing progression. However, there are no data to prove this, and further investigations are warranted.
Acknowledgments
The work was performed at Harbor-UCLA Medical Center, Torrance, CA.
Footnotes
Competing Interests: DD received an unrestricted research grant from Actelion Pharmaceuticals, and received occasional speaker fees from Actelion. RO received speaker and consulting fees, as well as research grant support from Actelion. SR received speaker and consulting fees as well as research grant support from Actelion. This does not alter the adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This work was partly supported by an unrestricted grant from Actelion Pharmaceuticals (http://www.actelion.com). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mukerjee D, St George D, Coleiro B, Knight C, Denton CP, et al. Prevalence and outcome in systemic sclerosis associated pulmonary hypertension: application of a registry approach. Ann Rheum Dis. 2003;62:1088–1093. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tolle JJ, Waxman AB, Van Horn TL, Pappagianopoulos PP, Systrom DM. Exercise-induced pulmonary arterial hypertension. Circulation. 2008;118:2183–2189. doi: 10.1161/CIRCULATIONAHA.108.787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovacs G, Maier R, Aberer E, Brodmann M, Scheidl S, et al. Borderline pulmonary arterial pressure is associated with decreased exercise capacity in scleroderma. Am J Respir Crit Care Med. 2009;180(9):881–886. doi: 10.1164/rccm.200904-0563OC. [DOI] [PubMed] [Google Scholar]
- 4.Miyauchi T, Yorikane R, Sakai S, et al. Contribution of endogenous endothelin-1 to the progression of cardiopulmonary alterations in rats with monocrotaline-induced pulmonary hypertension. Circ Res. 1993;73:887–897. doi: 10.1161/01.res.73.5.887. [DOI] [PubMed] [Google Scholar]
- 5.Nishida M, Eshiro K, Okada Y, Takaoka M, Matsumura Y. Roles of endothelin ETA and ETB receptors in the pathogenesis of monocrotaline-induced pulmonary hypertension. J Cardiovasc Pharmacol. 2004;44:187–191. doi: 10.1097/00005344-200408000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Galiè N, Rubin LJ, Hoeper M, Jansa P, Al-Hiti H. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371:2093–2100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 7.Sun XG, Oudiz RJ, Hansen JE, Wasserman K. Exercise Pathophysiology in Primary Pulmonary Vascular Hypertension. Circulation. 2001;104:429–435. doi: 10.1161/hc2901.093198. [DOI] [PubMed] [Google Scholar]
- 8.WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. 2008;19 Available: http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed 2010 Nov. [PubMed] [Google Scholar]
- 9.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis. Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 10.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 11.Huszczuk A, Whipp BJ, Wasserman K. A respiratory gas exchange simulator for routine calibration in metabolic studies. Eur Respir J. 1990;3:465–468. [PubMed] [Google Scholar]
- 12.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 13.Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. . Am Rev Respir Dis. 1985;131:700–708. doi: 10.1164/arrd.1985.131.5.700. [DOI] [PubMed] [Google Scholar]
- 14.Davis JA, Storer TW, Caiozzo VJ. Prediction of normal values for lactate threshold estimated by gas exchange in men and women. J Appl Cardiol. 1997;76:157–164. doi: 10.1007/s004210050228. [DOI] [PubMed] [Google Scholar]
- 15.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Philadelphia: Lippincott Williams & Wilkins; 2005. Measurements During Integrative Cardiopulmonary Exercise Testing. In: Principles of Exercise Testing and Interpretation. 4th ed. pp. 104–106. [Google Scholar]
- 16.Hansen JE, Sun XG, Yasunobu Y, Garafano RP, Gates G, et al. Reproducibility of cardiopulmonary exercise measurements in patients with pulmonary arterial hypertension. Chest 126; 2004:816–824. doi: 10.1378/chest.126.3.816. [DOI] [PubMed] [Google Scholar]
- 17.Hansen JE, Ulubay G, Chow BF, Sun XG, Wasserman K. Mixed-expired and end-tidal CO2 distinguish between ventilation and perfusion defects during exercise testing in patients with lung and heart diseases. Chest. 2007;132:977–983. doi: 10.1378/chest.07-0619. [DOI] [PubMed] [Google Scholar]
- 18.Yasunobu Y, Oudiz RJ, Sun XG, Hansen JE, Wasserman K. End-tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest. 2005;127:1637–1646. doi: 10.1378/chest.127.5.1637. [DOI] [PubMed] [Google Scholar]
- 19.Hansen JE, Wasserman K. Pathophysiology of activity limitation in patients with interstitial lung disease. Chest. 1996;109:1566–1576. doi: 10.1378/chest.109.6.1566. [DOI] [PubMed] [Google Scholar]
- 20.Schwaiblmair M, Behr J, Fruhmann G. Cardiorespiratory Responses to incremental exercise in patients with systemic sclerosis. Chest. 1996;110:1520–1525. doi: 10.1378/chest.110.6.1520. [DOI] [PubMed] [Google Scholar]
- 21.Wasserman K, Sun XG, Hansen JE. Effect of biventricular pacing on the exercise pathophysiology of heart failure. Chest. 2007;132:250–261. doi: 10.1378/chest.06-2872. [DOI] [PubMed] [Google Scholar]
- 22.Kleber FX, Vietzke G, Wernecke KD, Bauer U, Opitz C, et al. Impairment of ventilatory efficiency in heart failure: prognostic impact. Circulation. 2000;101:2803–2809. doi: 10.1161/01.cir.101.24.2803. [DOI] [PubMed] [Google Scholar]
- 23.Gitt AK, Wasserman K, Kilkowski C, Kleemann T, Kilkowski A, et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002;106:3079–3084. doi: 10.1161/01.cir.0000041428.99427.06. [DOI] [PubMed] [Google Scholar]
- 24.Deboeck G, Niset G, Lamotte M, Vachiery JL, Naeije R. Exercise testing in pulmonary arterial hypertension and in chronic heart failure. Eur Respir J. 2004;23:747–751. doi: 10.1183/09031936.04.00111904. [DOI] [PubMed] [Google Scholar]
- 25.Markowitz DH, Systrom DM. Diagnosis of Pulmonary Vascular Limit to Exercise by Cardiopulmonary Exercise Testing. J Heart Lung Trans. 2004;23:88–95. doi: 10.1016/s1053-2498(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 26.Sun XG, Hansen JE, Garatchea N, Storer TW, Wasserman K. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med. 2002;166:1443–1448. doi: 10.1164/rccm.2202033. [DOI] [PubMed] [Google Scholar]
- 27.Montani D, O'Callaghan DS, Savale L, Jaïs X, Yaïci A, et al. Pulmonary veno-occlusive disease: recent progress and current challenges. Respir Med. 2010;104(Suppl 1):S23–32. doi: 10.1016/j.rmed.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Montani D, Achouh L, Dorfmüller P, Le Pavec J, Sztrymf B, et al. Pulmonary veno-occlusive disease: clinical, functional, radiologic, and hemodynamic characteristics and outcome of 24 cases confirmed by histology. Medicine (Baltimore) 2008;87(4):220–233. doi: 10.1097/MD.0b013e31818193bb. [DOI] [PubMed] [Google Scholar]