Abstract
Tight regulation of the plasma membrane proton pump ATPase (H+-ATPase) is necessary for controlling the membrane potential that energizes secondary transporters. This regulation relies on the phosphorylation of the H+-ATPase penultimate residue, a theonine, and the subsequent binding of regulatory 14-3-3 proteins, which results in enzyme activation. Using phospho-specific antibodies directed against the phosphorylable Thr of either PMA2 (Plasma membrane H+-ATPase from N. plumbaginifolia) or PMA4, we showed that the kinetics and extent of phosphorylation differ between both isoforms according to the growth or environmental conditions like cold stress.1 Here, we used phospho-specific antibodies to follow PMA2 Thr phosphorylation upon acidification of the cytosol by incubating N. tabacum BY2 cells with four different weak organic acids. Increased PMA2 phosphorylation was observed for three of them, thus highlighting the role of the H+-ATPase in cell pH homeostasis.
Key words: H+-ATPase, regulation, phosphorylation, phospho-specific antibodies, pH homeostasis, cold stress
The H+-ATPase is a major enzyme of the plant plasma membrane. This P-type ATPase couples ATP hydrolysis with proton transport out of the cell and establishes pH and potential gradients across the plasma membrane, thereby activating secondary transporters. At the physiological level, this enzyme is implicated in diverse roles, such as cytosolic pH regulation, cell elongation or stomata aperture.2,3 H+-ATPase consists of ten membrane spanning regions and four cytosolic domains, among which the auto-inhibitory C-terminal region. The activation mechanism of the enzyme is well known and involves phosphorylation of its penultimate residue, a threonine, by an as yet unidentified protein kinase; phosphorylation in turn leads to the binding of regulatory 14-3-3 protein dimers and to the formation of an activated complex consisting of six H+-ATPases and six 14-3-3 proteins.4–7
Additional conserved phosphorylation sites in the enzyme C-terminal region have been shown to positively or negatively contribute to the enzyme regulation.8–10 More sites have been discovered by large-scale phospho-proteomics, but have not been studied to date.10,11 Most of these additional phosphorylated residues are located in the enzyme C-terminal autoinhibitory domain. This domain contains two to three inhibitory regions and a 14-3-3 binding region, partially super-imposed with an inhibitory region.12,13 All these recent data suggest that the activity of H+-ATPase is finely tuned. However, the complexity of this regulation makes it difficult at the present stage to propose a comprehensive view.
To follow and compare the activation status of two H+-ATPase isoforms belonging to different subfamilies, antibodies were designed for specifically recognizing the phosphorylated form of the penultimate Thr of either PMA2 (Plasma membrane H+-ATPase from N. plumbaginifolia) or PMA4, two broadly expressed isoforms belonging to subfamily I and II, respectively. This allowed us to find, for example, that PMA2, as opposed to PMA4, is strongly dephosphorylated upon cold stress. Both isoforms are strongly activated, upon subculturing N. tabacum BY2 suspension cells into a new media.1 However, they underwent dephosphorylation at different rates as the cell culture proceeded. These data showed the usefulness of these antibodies for determining the regulation of specific H+-ATPase isoforms and better understanding their physiological roles.
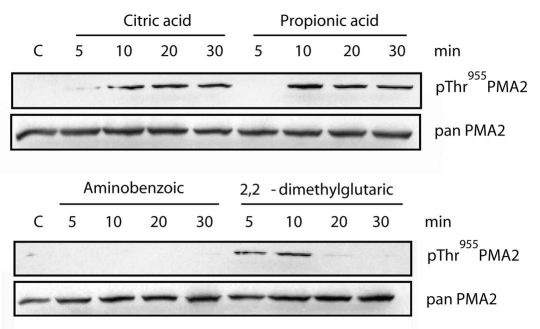
The primary function of the plasma membrane H+-ATPase is to transfer protons outside the cell. H+-ATPase is therefore considered as a possible regulator of the cytosolic pH homeostasis, for instance by preventing internal acidification. However, few data so far support this role for H+-ATPase. We addressed this point by adding to a N. tabacum BY2 cell culture weak organic acids, which are expected to permeate the membrane as a protonated form and dissociate once inside, resulting in cytosol acidification. This is expected to activate the plasma membrane H+-ATPase and so remove the proton excess out off the cell. A N. tabacum BY2 cell culture was treated with 5 mM of either citric acid, aminobenzoic acid, 2,2-dimethylglutaric acid, or propionic acid (Fig. 1). After several periods of time, a microsomal fraction was isolated and analyzed by western blotting. Among the four weak acids tested, propionic acid and citric acid induced strong and stable increase of PMA2 penultimate Thr phosphorylation. 2,2-dimethylglutaric acid induced a temporary increase of phosphorylation while aminobenzoic acid had no effect. The different responses might be explained either by different diffusion rates of the organic acids across the plasma membrane or by their possible toxicity. In addition, homeostasis of the intracellular pH results from the activity of several different enzymatic systems such as vacuolar H+-ATPase and H+-pyrophosphatase. Therefore it is also possible that, depending on the rate and/or extent of cytosol acidification, different responses are activated.
Figure 1.
Effect of various weak acids on the phosphorylation of the PMA 2 penultimate Thr residue of N. tabacum suspension cells. A 3-day old N. tabacum BY2 cell culture was treated with 1/10th volume of 50 mM of either aminobenzoic acid, 2,2-dimethylglutaric acid, citric acid or propionic acid, dissolved in the culture medium and brought beforehand to the same pH as the culture. After the indicated periods of time, cells were collected and a microsomal fraction was isolated and analyzed by western blotting using antibodies pThr955PMA 2 recognizing the PMA 2 penultimate activating Thr1 (upper) and pan PMA 2 recognizing a short sequence specific for PMA2,14 (lower). C, untreated cells.
This data supports the role H+-ATPase in pH homeostasis and highlights the strong potential of using phospho-specific antibodies to follow enzyme activation in the plant according to different environmental conditions. In addition, one should also take advantage of them as a tool for in vitro phosphorylation tests using different subcellular fractions of N. tabacum BY2 cells. This approach might lead to the isolation of the kinase and phosphatase involved in the modification of the penultimate Thr residue. Indeed, these enzymes are still undiscovered in spite of the fact that phosphorylation of this residue has been demonstrated more than a decade ago.
Acknowledgements
The work performed in this laboratory was supported by grants from the Interuniversity Poles of Attraction Program (Belgian State, Scientific, Technical, and Cultural Services) and the Belgian National Fund for Scientific Research. We thank Joseph Nader for his excellent technical assistance. GD was a post-doctoral researcher of the Belgian National Fund for Scientific Research.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11572
References
- 1.Bobik K, Duby G, Nizet Y, Vandermeeren C, Stiernet P, Kanczewska J, et al. Two widely expressed plasma membrane H+-ATPase isoforms of Nicotiana tabacum, are differentially regulated by phosphorylation of their penultimate threonine. Plant J. 2010;62:291–301. doi: 10.1111/j.1365-313X.2010.04147.x. [DOI] [PubMed] [Google Scholar]
- 2.Palmgren MG. Plant plasme membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:817–845. doi: 10.1146/annurev.arplant.52.1.817. [DOI] [PubMed] [Google Scholar]
- 3.Duby G, Boutry M. The plant plasma membrane proton pump ATPase: a highly regulated P-type ATPase with multiple physiological roles. Pflugers Arch. 2009;457:645–655. doi: 10.1007/s00424-008-0457-x. [DOI] [PubMed] [Google Scholar]
- 4.Fuglsang AT, Visconti S, Drumm K, Jahn T, Stensballe A, Mattei B, et al. Binding of 14-3-3 protein to the plasma membrane H+-ATPase AHA2 involves the three C-terminal residues Tyr(946)-Thr-Val and requires phosphorylation of Thr(947) J Biol Chem. 1999;274:36774–36780. doi: 10.1074/jbc.274.51.36774. [DOI] [PubMed] [Google Scholar]
- 5.Maudoux O, Batoko H, Oecking C, Gevaert K, Vandekerckhove J, Boutry M, et al. A plant plasma membrane H+-ATPase expressed in yeast is activated by phosphorylation at its penultimate residue and binding of 14-3-3 regulatory proteins in the absence of fusicoccin. J Biol Chem. 2000;275:17762–17770. doi: 10.1074/jbc.M909690199. [DOI] [PubMed] [Google Scholar]
- 6.Kanczewska J, Marco S, Vandermeeren C, Maudoux O, Rigaud JL, Boutry M. Activation of the plant plasma membrane H+-ATPase by phosphorylation and binding of 14-3-3 proteins converts a dimer into a hexamer. Proc Natl Acad Sci USA. 2005;102:11675–11680. doi: 10.1073/pnas.0504498102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ottmann C, Marco S, Jaspert N, Marcon C, Schauer N, Weyand M, et al. Structure of a 14-3-3 coordinated hexamer of the plant plasma membrane H+-ATPase by combining X-ray crystallography and electron cryomicroscopy. Mol Cell. 2007;25:427–440. doi: 10.1016/j.molcel.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Duby G, Poreba W, Piotrowiak D, Bobik K, Derua R, Waelkens E, et al. Activation of plant plasma membrane H+-ATPase by 14-3-3 proteins is negatively controlled by two phosphorylation sites within the H+-ATPase C-terminal region. J Biol Chem. 2009;284:4213–4221. doi: 10.1074/jbc.M807311200. [DOI] [PubMed] [Google Scholar]
- 9.Fuglsang AT, Guo Y, Cuin TA, Qiu Q, Song C, Kristiansen KA, et al. Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant Cell. 2007;19:1617–1634. doi: 10.1105/tpc.105.035626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niittyla T, Fuglsang AT, Palmgren MG, Frommer WB, Schulze WX. Temporal analysis of sucrose-induced phosphorylation changes in plasma membrane proteins of Arabidopsis. Mol Cell Proteomics. 2007;6:1711–1726. doi: 10.1074/mcp.M700164-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Nuhse TS, Stensballe A, Jensen ON, Peck SC. Phosphoproteomics of the Arabidopsis plasma membrane and a new phosphorylation site database. Plant Cell. 2004;16:2394–2405. doi: 10.1105/tpc.104.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Axelsen KB, Venema K, Jahn T, Baunsgaard L, Palmgren MG. Molecular dissection of the C-terminal regulatory domain of the plant plasma membrane H+-ATPase AHA2: mapping of residues that when altered give rise to an activated enzyme. Biochemistry. 1999;38:7227–7234. doi: 10.1021/bi982482l. [DOI] [PubMed] [Google Scholar]
- 13.Speth C, Jaspert N, Marcon C, Oecking C. Regulation of the plant plasma membrane H+-ATPase by its C-terminal domain: what do we know for sure? Eur J Cell Biol. 2010;89:145–151. doi: 10.1016/j.ejcb.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Moriau L, Michelet B, Bogaerts P, Lambert L, Michel A, Oufattole M, et al. Expression analysis of two gene subfamilies encoding the plasma membrane H+-ATPase in Nicotiana plumbaginifolia reveals the major transport functions of this enzyme. Plant J. 1999;19:31–41. doi: 10.1046/j.1365-313x.1999.00495.x. [DOI] [PubMed] [Google Scholar]