Abstract
Extracellular ATP (eATP) and nitric oxide (NO) have emerged as crucial players in plant development, stress responses and cell viability. Glutathione (GSH) is an abundant reducing agent with proposed roles in plant growth, development and stress physiology. In a recent publication, we demonstrated that eATP and NO restore hypocotyl elongation of etiolated Arabidopsis seedlings treated with GSH. Here it is reported that exogenous ATP also restores root hair growth suggesting a role for ATP and NO in the regulation of redox balance associated to specific processes of plant morphogenesis. A tentative model integrating redox-, eATP- and NO-signaling pathways during root hair growth in Arabidopsis seedlings is presented.
Key words: Arabidopsis thaliana, extracellular ATP signaling, nitric oxide, redox system, root hair
Linking Signaling Pathways to Gain Understanding on the Regulation of Morphological Processes in Plants
The first living organisms evolved under reducing conditions a long time before the environment became oxidizing due to oxygen produced by photosynthesis. As a consequence, the most fundamental signaling pathways were initially adapted to reducing conditions. Plants, as sessile organisms have taken advantage of the regulatory mechanisms evolved on a changing redox-status and nowadays they are strongly dependent of environmental redox conditions. An efficient redox control together with other signaling mechanisms cooperates to maintain the steady-state redox homeostasis in plant cell. In this scenario, two small signaling molecules such as ATP and NO have a very ancient origin and are involved in cell redox physiology.1,2 ATP and NO play diverse biological functions in phylogenetically distant species. Intracellular ATP is the major source of energy for cellular reactions. However, ATP also acts as a signaling molecule at the extracellular face of the plasma membrane in animals as well as in plants.3 NO is an important regulatory molecule in eukaryotes. Several roles have been attributed to extracellular ATP (eATP) and NO in plants, ranging from cell viability to pathogen defence and cell death.2–4 NO production stimulated by eATP was shown in tomato suspension cells,5 hairy roots of Salvia miltiorrhiza6 as well as in algae.7 Reichler et al.8 showed that the intersection of eATP signaling and NO via the cellular messenger cGMP regulates pollen germination and pollen tube growth in Arabidopsis. Thus, the biochemical and molecular mechanisms underlying eATP and NO signaling pathways in plants have just begun to emerge. Lately, we have described an interconnection between eATP, NO and redox system during hypocotyl elongation in etiolated Arabidopsis seedlings.9 It was demonstrated that a fine-tuning of redox balance and endogenous NO level is associated to eATP action. Therefore, these findings open a new window in the poorly explored cross-talk between eATP, NO and redox balance in the plant kingdom.
Extracellular ATP Signaling Pathway in Plants
Despite the emerging role of eATP as a signal molecule, little is known about how eATP is transmitted to the apoplast. Apparently, ATP is released from plant cells by wounding10 or by plasma membrane ABC transporters.11 In addition, a G-protein complex participates in the fine-tuning of touch-induced ATP release in Arabidopsis roots.12 As summarized by Roux and Steinebrunner,3 cellular responses to eATP include perception, increases of cytoplasmic calcium concentration [Ca2+]cyt and superoxide production. Plants perceive eATP throughout receptors that are structurally distinct from those found in animals.13 Demidchik et al.14 proposed that in root cell protoplasts eATP is sensed at the plasma membrane by an unknown receptor and that reactive oxygen species (ROS) are produced by a co-localized NADPH oxidase. Subsequently, ROS production stimulates the activation of Ca2+ channels, which contributes to the increase of [Ca2+]cyt. NADPH oxidases are haem-containing flavoproteins with multiple transmembrane-spanning domains. These enzymes reduce oxygen to form superoxide anion. Previously, Song et al.15 showed that after eATP perception, [Ca2+]cyt level is transiently elevated and modulates eATP-induced superoxide accumulation via NADPH oxidase in Arabidopsis leaves. The spatial variation of Ca2+ channel activation by superoxide was also described in Arabidopsis root cells.16 More recently, the link between eATP, NO and phospholipid signaling has been revealed in tomato suspension cells.17
eATP, NO and Redox Balance Interplay during Root Hair Formation in Arabidopsis Seedlings
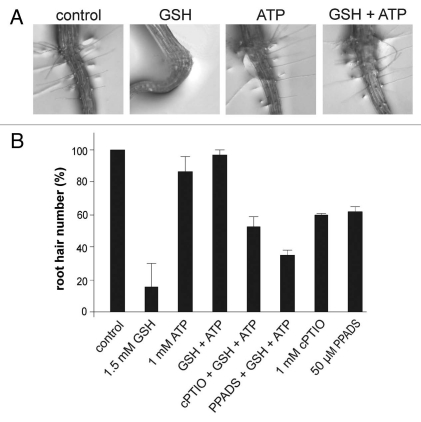
Root hairs increase the root surface area allowing a most efficient anchorage in the soil and water uptake. Root hair formation is controlled by several endogenous and exogenous factors. In Arabidopsis, root hair formation occurs in light- and dark-grown seedlings. It has been demonstrated that cell elongation during post-embryonic hypocotyl and root hair growth are under the control of light-regulated developmental switch.18 However, it seems that light requirements for phytochrome action may be different for hypocotyl and root hair growth. Hypocotyl elongation and root hair growth are regulated by eATP.3,19,20 It was reported that root hair growth is under mechanistically different forms of redox control in Arabidopsis.21 Current knowledge of redox control in the apoplast and cytoplasm predicts that the plasma membrane is an important site for perception and transduction of environmental changes through redoxbased signaling.22 GSH-mediated inhibition of etiolated hypocotyl growth and rooting was described by Cano et al.23 in lupin. More recently, we demonstrated that in the presence of GSH, etiolated Arabidopsis seedlings showed inhibition of hypocotyl elongation and altered root system architecture including changes in primary root length, root hair number and agravitropic growth. All these morphological traits were restored by eATP and NO.9 In this addendum, we show that growth and differentiation of root hairs in Arabidopsis provide a simple and suitable model to study mechanisms underlying the interconnection between eATP, NO and redox status. Here, we examined root hair growth of 5-day-old Arabidopsis seedlings grown in the presence of GSH, ATP or combinatorial treatments in darkness. Harvested seedlings were recorded as digital images and root hair number was quantified. A similar amount of root hairs was detected in control and 1 mM ATP-treated seedlings (Fig. 1). However, in presence of 1.5 mM GSH, root hairs were almost undetected. Figure 1A and B shows that root hair development was almost completely recovered in GSH-treated seedlings upon the addition of exogenous 1 mM ATP. The purinoreceptor antagonist, PPADS and the specific NO scavenger, carboxy-PTIO (cPTIO) reduced the development of root hairs in ATP plus GSH-treated seedlings (Fig. 1B). In contrast, the addition of 100 µM H2O2 to GSH-treated seedlings did not restore the development of root hairs in GSH-treated seedlings (data not shown) suggesting that the recovery of root hair growth mediated by exogenous nucleotides is specific. Moreover, the NO donor s-nitroso-N-actyl-D-penicillamine (SNAP) added to GSH-treated seedlings did not restore the root hair number (data not shown). However, we can not discard that a combination of external factors such as GSH and darkness may affect NO release from SNAP.24 Our findings suggest that eATP is sensed by purinoreceptor-like proteins to exert its effect on root epidermal cells and that endogenous NO is implicated in this process (Fig. 1B). This is in agreement with the finding that NO is involved in auxin-induced root hair formation.25 Thus, eATP-NO action on GSH-treated seedlings grown in darkness is restricted not only to hypocotyl elongation but also to root hair development.
Figure 1.
eATP restores root hair growth of GSH-treated Arabidopsis seedlings in darkness. (A) Representative 5-d-old seedlings (Arabidopsis thaliana ecotype Col 0) grown vertically in the absence or presence of 1 mM AT P, 1.5 mM GSH, GSH + ATP in darkness. (B) Measurement of root hair number of etiolated seedlings grown in the absence or presence of 1 mM ATP, 1.5 mM GSH, GSH + ATP, 1 mM cPTIO, 50 µM PPAD S. Combinatorial treatments are indicated. Data are means (±SD) of at least three independent experiments.
Monshausen et al.26 demonstrated that ATRBOH C, a plasma membrane NADPH oxidase seems to be a key element in modulating root growth-related ROS production in Arabidopsis. In this way, our evidence is in agreement with the current model which assumes that root hair growth is dependent on eATP and ROS production.20,27 According to Foreman et al.27 ROS production by NADPH oxidase induces opening of Ca2+ channels and elongation of the root hair. Furthermore, ROS control the activity of Ca2+ channels required for polar growth.28 Wu and Wu6 demonstrated that eATP induces a rapid increase in the [Ca2+]cyt level, which is dependent on NO in Salvia miltiorrhiza hairy root culture. It was also demonstrated that a NADPH oxidase similar to the mammalian gp91phox is necessary to elongate root hairs in Arabidopsis.27 However, the balance between eATP and NO is tightly regulated3 possibly by impairing auxin transport.29 Other results also indicate that NO and cGMP operate downstream of auxin promoting root morphological changes.30 Furthermore, the involvement of NO in actin cytoskeleton and vesicle trafficking in root apices has been demonstrated.31 Thus, the currently available data allowed us to speculate that NO can directly influence the activity of target proteins through S-nitrosylation or acting as a Ca2+-mobilizing intracellular messenger. All these findings support an interesting convergence of eATP, NO and redox system operating in plants.
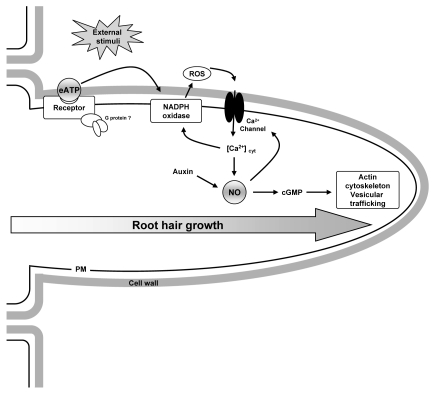
Furthermore, a complex signaling network including eATP, NO, auxin, ROS, [Ca2+]cyt and cGMP may take place during root hair growth in Arabidopsis seedlings. Finally, eATP and NO appear as multipurpose signaling messengers that regulate specific target molecules impacting in a cell-type specific manner in Arabidopsis seedlings. For such reasons, we should recognize that we are only beginning to understand the intricate puzzle that interplays in plant morphogenesis. To summarize previous evidence and the potential interactions between eATP, NO and their cross-talks with other signalings, a schematic model is shown in Figure 2. This model is based on those proposed by Wu and Wu,6 Demidchik et al.14 and Lombardo et al.25 It integrates both external and internal cues into the root hair cell. Nevertheless, crucial questions remain to be answered i.e., plant cell matrix, as well as plasma membrane contains a suite of proteins capable of being activated through exogenous ATP but which are their specific molecular targets? Overall, ROS, Ca2+ and NO seem to be critical messengers acting on multiple targets in the plant cell.
Figure 2.
Putative model integrating eATP and NO-regulated signaling pathways for root hair growth in Arabidopsis. eATP putative receptor, NADPH oxidase and Ca2+ channel are shown in the plasma membrane (PM). Depending on the cell type and the external stimulus, eAT P would induce Ca2+ channel activity and increase [Ca2+]cyt leading to NO accumulation and ROS production via NADPH oxidase stimulation. In addition, NO may regulate Ca2+ channels by feedback activation. NO may also promote the formation of cGMP messenger. In turn, actin cytoskeleton may act as a downstream effector of NO signal transduction pathway. In parallel, auxin through NO could modulate root hair growth.
Future studies will allow us to elucidate the expanding role of eATP, NO and the finely regulated cross-talk between auxin and redox system. Indeed, redox-dependent modulation by eATP and NO signaling may be critical to optimize root morphology as an adaptive strategy under environmental stress conditions.
Acknowledgements
This work was supported by grants from the University of Mar del Plata, ANPCyT, CONICET. M.C.T., L.L. and C.A.C. are members of CONICET. M.J.I. is a fellow of the same Institution. C.V.T. is member of CIC. The authors wish to thank Dr. Diego Fiol for his critical reading of the manuscript.
Abbreviations
- cPTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- eATP
extracellular adenosine triphosphate
- GSH
reduced glutathione
- NO
nitric oxide
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11579
References
- 1.Clark G, Roux SJ. Extracellular nucleotides: Ancient signaling molecules. Plant Sci. 2009;177:239–244. [Google Scholar]
- 2.Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol. 2003;54:109–136. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- 3.Roux SJ, Steinebrunner I. Extracellular ATP: an unexpected role as a signaler in plants. Trends Plant Sci. 2007;12:522–527. doi: 10.1016/j.tplants.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Chivasa S, Murphy AM, Hamilton JM, Lindsey K, Carr JP, Slabas AR. Extracellular ATP is a regulator of pathogen defence in plants. Plant J. 2009;60:436–448. doi: 10.1111/j.1365-313X.2009.03968.x. [DOI] [PubMed] [Google Scholar]
- 5.Foresi N, Laxalt AM, Tonón C, Casalongue C, Lamattina L. Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiol. 2007;145:589–592. doi: 10.1104/pp.107.106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu S, Wu J. Extracellular ATP-induced NO production and its dependence on membrane Ca2+ flux in Salvia miltiorrhiza hairy roots. J Exp Bot. 2008;59:4007–4016. doi: 10.1093/jxb/ern242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres J, Rivera A, Clark G, Roux SJ. Participation of extracellular nucleotides in the wound response of Dasycladus vermicularis and Acetabularia acetabulum (Dasycladales, Chlorophyta) J Phycol. 2008;44:1504–1511. doi: 10.1111/j.1529-8817.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 8.Reichler SA, Torres J, Rivera AL, Cintolesi VA, Clark G, Roux SJ. Intersection of two signalling pathways: extracellular nucleotides regulate pollen germination and pollen tube growth via nitric oxide. J Exp Bot. 2009;60:2129–2138. doi: 10.1093/jxb/erp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonón C, Terrile MC, Iglesias MJ, Lamattina L, Casalongue C. Extracellular ATP, nitric oxide and superoxide act coordinately to regulate hypocotyl growth in etiolated Arabidopsis seedlings. J Plant Physiol. 2009 doi: 10.1016/j.jplph.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Jeter CR, Tang W, Henaff E, Butterfield T, Roux SJ. Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell. 2004;16:2652–2664. doi: 10.1105/tpc.104.023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas C, Rajagopal A, Windsor B, Dudler R, Lloyd A, Roux SJ. A role for ectophosphatase in xenobiotic resistance. Plant Cell. 2000;12:519–533. doi: 10.1105/tpc.12.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weerasinghe RR, Swanson SJ, Okada SF, Garrett MB, Kim SY, Stacey G, et al. Touch induces ATP release in Arabidopsis roots that is modulated by the heterotrimeric G-protein complex. FEBS Lett. 2009;583:2521–2526. doi: 10.1016/j.febslet.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:423–492. [PubMed] [Google Scholar]
- 14.Demidchik V, Shang Z, Shin R, Thompson E, Rubio L, Laohavisit A, et al. Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J. 2009;58:903–913. doi: 10.1111/j.1365-313X.2009.03830.x. [DOI] [PubMed] [Google Scholar]
- 15.Song CJ, Steinebrunner I, Wang X, Stout SC, Roux SJ. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol. 2006;140:1222–1232. doi: 10.1104/pp.105.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demidchik V, Shabala SN, Davies JM. Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J. 2007;49:377–386. doi: 10.1111/j.1365-313X.2006.02971.x. [DOI] [PubMed] [Google Scholar]
- 17.Sueldo DJ, Foresi N, Casalongue C, Lamattina L, Laxalt AM. Phosphatidic acid formation is required for extracellular ATP-mediated nitric oxide production in suspension-cultured tomato cells. New Phytol. 2010 doi: 10.1111/j.1469-8137.2009.03165.x. [DOI] [PubMed] [Google Scholar]
- 18.De Simone S, Oka Y, Inoue Y. Effect of light on root hair formation in Arabidopsis thaliana phytochrome-deficient mutants. J Plant Res. 2000;113:63–69. [Google Scholar]
- 19.Lew RR, Dearnaley JDW. Extracellular nucleotide effects on the electrical properties of growing Arabidopsis thaliana root hairs. Plant Sci. 2000;153:1–6. [Google Scholar]
- 20.Kim S, Sivaguru M, Stacey G. Extracellular ATP in plants. Visualization, localization and analysis of physiological significance in growth and signaling. Plant Physiol. 2006;142:984–992. doi: 10.1104/pp.106.085670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Fernandez R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, et al. Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc Natl Acad Sci USA. 1997;94:2745–2750. doi: 10.1073/pnas.94.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cano A, Artés F, Arnao MB, Sánchez-Bravo J, Acosta M. Inhibition of etiolated lupin hypocotyl growth and rooting by peroxides, ascorbate and glutathione. J Plant Physiol. 1996;147:721–728. [Google Scholar]
- 24.Floryszak-Wieczorek J, Milczarek G, Arasimowicz M, Ciszewski A. Do nitric oxide donors mimic endogenous NO-related response in plants? Planta. 2006;224:1363–1372. doi: 10.1007/s00425-006-0321-1. [DOI] [PubMed] [Google Scholar]
- 25.Lombardo MC, Graziano M, Polacco J, Lamattina L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav. 2006;1:28–33. doi: 10.4161/psb.1.1.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA. 2007;104:20996–21001. doi: 10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 28.Carol RJ, Dolan L. The role of reactive oxygen species in cell growth: lessons from root hairs. J Exp Bot. 2006;57:1829–1834. doi: 10.1093/jxb/erj201. [DOI] [PubMed] [Google Scholar]
- 29.Tang W, Brady SR, Sun Y, Muday GK, Roux SJ. Extracellular ATP inhibits root gravitropism at concentrations that inhibit polar auxin transport. Plant Physiol. 2003;131:147–154. doi: 10.1104/pp.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagnussat GC, Lanteri ML, Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003;132:1241–1248. doi: 10.1104/pp.103.022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasprowicz A, Szuba A, Volkmann D, Baluska F, Wojtaszek P. Nitric oxide modulates dynamic actin cytoskeleton and vesicle trafficking in a cell type-specific manner in root apices. J Exp Bot. 2009;60:1605–1617. doi: 10.1093/jxb/erp033. [DOI] [PMC free article] [PubMed] [Google Scholar]