Abstract
In plants, polar transport of the hormone auxin between cells connects cell polarity and pattern formation and is thus required for plant development. The direction of auxin transport is determined by polar localization of PIN auxin efflux transporters. The dynamic polar localization of PIN proteins depends on constitutive endocytic recycling to and from the plasma membrane. However, it was not well understood how PIN polarization is connected to regulators of cell polarity. In a paper that was published in the January issue of PLoS Biology, 2010 we described the involvement of the ROP GTPase associated scaffold Interactor of Constitutive active ROP 1 (ICR1) in recruitment of PIN proteins to polar domains in the plasma membrane. icr1 mutant plants and embryos have severe developmental aberrations that are caused by abnormal auxin distribution. ICR1 functions at or close to the plasma membrane where it is required for exocytosis. ICR1 transcription is quickly induced by auxin but is post-transcriptionally repressed at the site of stable auxin maximum in embryos and roots. Thus, ICR1 integrates auxin-regulated transcription with ROP modulated cell polarity.
Key words: Rho, auxin, ROP, cell polarity, Arabidopsis, development, pattern formation
The plant hormone auxin is transported between cells by a unique polar mechanism resulting in formation of local auxin maxima and gradients.1 Auxin functions through regulation of gene expression2 and its asymmetric distribution is required for almost all developmental processes in plants.1,3–6
Directionality of auxin transport depends on polar subcellular distribution of PINFORMED (PIN) family of efflux transporters.7–9 PIN polarity is a result of constitutive endocytic recycling that requires the function of brefeldin A (BFA)-sensitive ADP ribosylation factor GDP/GTP Exchange Factors (ARF GEFs)10,11 and of clathrin and endosomal Rab5/Ara7-regulated endocytosis.1,12,13 Presumably, slow lateral diffusion of PIN proteins in the membrane enables their polarization by a constitutive endocytic recycling machinery.14 However, how PIN recycling is directed to result in their polar distribution was largely unknown.
In eukaryotes, Rho superfamily small GTPases function as master regulators of cell polarity.15 Plants have a single family of Rho associated proteins called ROPs (Rho of Plants) or RACs.16,17 Previously, we have identified a ROP interacting coiled coil scaffold protein ICR1 (Interactor of Constitutively active ROP 1) and showed that it affects cell polarity.18 ICR1 interacts with number of proteins, including the octameric vesicle tethering exocyst complex subunit SEC3A. It was shown that ROPs could recruit ICR1 as well as ICR1-SEC3A complexes to the plasma membrane.18 The primary root meristem of icr1 mutant and RNAi silenced plants collapses soon after germination,18 resembling mutants affected in basal localization of PIN proteins and multiple pin loss-of-function mutants.1 These data suggested that ICR1 might form a link between Rho-regulated cell polarity and polar auxin transport.
In a recent publication,19 we demonstrated that ICR1 functions in recruitment of PINs to polar domains in the plasma membrane through its involvement in polarized secretion (Fig. 1). We further demonstrated that ICR1 expression is quickly induce by auxin but suppressed at the site of the stable auxin maximum at the root tip (Fig. 2). Our results imply that ICR1 is part of an auxin-regulated feedback loop, integrating auxin mediated gene expression with ROP-modulated cell polarity.
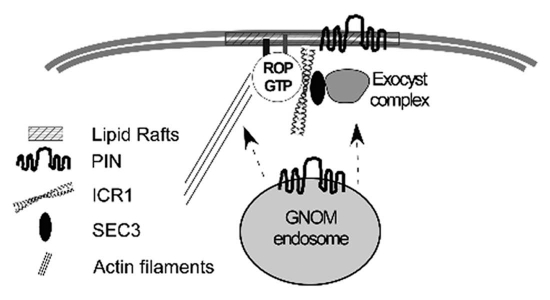
Figure 1.
A model describing the possible involvement of ICR1 in recruitment of PIN proteins to polar domains in the plasma membrane. ICR1 is composed of coiled coil domains and functions as a scaffold that interacts with activated GTP-bound ROPs as well as other proteins.18 Upon GTP bindings, ROPs undergo transient S-acylation and consequent partitioning into lipid rafts.24 Lipid rafts are sterol-rich membrane domains. The involvement of sterols in PIN polarization has been shown.25 ICR1 can interact with the exocyst vesicle tethering subunit SEC3A and ROPs can recruit ICR1-SEC3A complexes to the plasma membrane. In addition, ectopic expression of ICR1 results in cellular deformation similar to that induced by constitutively active ROP mutants. Constitutively active ROPs affect the organization of actin filament and microtubules.16,17,26 Thus, the ICR1 overexpression-induced cell deformation is likely associated with reorganization of the cytoskeleton.18 Collectively, ICR1 may regulate PINs recruitment to polar domains in the plasma membrane19 through its interaction with the exocyst complex. In addition, ICR 1 may affect ROP localization in the membrane and consequently actin organization. The requirement for actin in the recruitment of PIN s to the plasma membrane has been shown.11
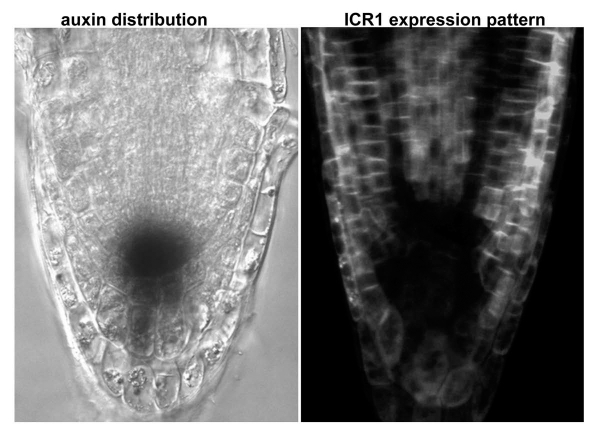
Figure 2.
Post-trasncriptional repression of ICR 1 expression around the root meristem coincides with the site of the stable auxin maximum. (Left) Nomarsky differential interference contrast image showing the distribution of auxin at the root tip (darker region) as detected with the auxin sensitive marker DR5::GUS. (Right) ICR1 promoter-driven expression of GFP fused to the genomic ICR1 gene. The image is a projection stack of multiple confocal scans taken through the middle of the root.
To define the role of ICR1 in plant development, we examined auxin distribution and the development of embryos, root columella cells and lateral roots in icr1 mutant plants. In addition, the expression pattern of three transcriptional regulators that define the root stem cell niche; WUSCHEL related Homeobox 5 (WOX5) SCARECRAW (SCR) and SHORTROOT (SHR),20–22 was examined in icr1 mutant embryos and roots. Collectively, these analyses indicated that in icr1 mutants the basic genetic framework that regulates embryo and root development is present and that the root meristem collapse, the altered organ development, and changes in cell identities can be attributed primarily to the compromised auxin distribution.
Immuno-localization and GFP fusion proteins showed basal to apical shift as well as reduced recruitment to the plasma membrane of PIN1 and PIN2 in icr1 mutants. BFA treatments caused accumulation of PIN1 and PIN2 in BFA compartments in both wild type and icr1 mutants. However, BFA washout treatments showed that recruitment of PIN2 to the plasma membrane was slower in icr1. Interestingly, ICR1 did not accumulate in BFA bodies, in-line with previous data showing that its recruitment to the plasma membrane is ROP-dependent.18 Analysis of exocytosis using a marker called secGFP23 showed that secretion is compromised in icr1 mutants. Collectively, these data indicated that the Rab5/Ara7-dependent endocytic recycling of PINs is unaffected in icr1. The data further suggested that ICR1 likely functions in parallel to the BFA-sensitive ARF-GEF GNOM in PINs recruitment to the membrane (Fig. 1). It would now be interesting to examine if and how the ROP-ICR1-exocyst machinery regulates PINs recruitment. Recruitment of PINs to the membrane is actin-dependent11 and ROPs are central regulators of actin organization and dynamics.16,17 It is therefore also possible that ICR1-ROP complexes affect polarized recruitment of PINs through actin (Fig. 1).
The pattern and regulation of ICR1 expression are remarkably interesting and complement that analysis of the icr1 mutant phenotype (Fig. 2). ICR1 is expressed in tissues that transport auxin but its expression is suppressed at the site of the stable auxin maximum at the root tip. Furthermore, ICR1 subcellular localization becomes progressively polarized away from auxin maximum. The ICR1 promoter contains and auxin response element and its mRNA expression is quickly induced by auxin. The suppression of ICR1 expression at the site of stable auxin maximum (Fig. 2) is likely regulated by a post-transcriptional mechanism, since expression of other reporters under regulation of the ICR1 promoter was detected at this domain. Taken together, these data indicated that expression levels and subcellular localization of ICR1 play a central role in regulation of asymmetric auxin distribution. The stable auxin maximum around the root stem cell niche may facilitate a positive feedback loop that maintains the repression of ICR1 expression and its distribution in the immediate proximal and subtending cells. The results from our work18,19 suggest that ICR1 functions as an auxin modulated scaffold that facilitates compartmentalization of ROPs and other proteins such as the exocyst complex. This polarization machinery is required asymmetric auxin distribution.
Acknowledgements
Work described in this paper was supported by grants from Israel Science Foundation (ISF-312/07), US-Israel Binational Research and Development fund (BARD-IS-4032-07) and the Deutschland-Israel Program (DIP-H.3.1) to S.Y.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11644
References
- 1.Kleine-Vehn J, Friml J. Polar targeting and endocytic recycling in auxin-dependent plant development. Annu Rev Cell Dev Biol. 2008;24:447–473. doi: 10.1146/annurev.cellbio.24.110707.175254. [DOI] [PubMed] [Google Scholar]
- 2.Mockaitis K, Estelle M. Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol. 2008;24:55–80. doi: 10.1146/annurev.cellbio.23.090506.123214. [DOI] [PubMed] [Google Scholar]
- 3.Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, et al. Local, effluxdependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 4.Berleth T, Sachs T. Plant morphogenesis: long-distance coordination and local patterning. Curr Opin Plant Biol. 2001;4:57–62. doi: 10.1016/s1369-5266(00)00136-9. [DOI] [PubMed] [Google Scholar]
- 5.Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 6.Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- 7.Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- 8.Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, et al. PIN proteins perform a ratelimiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 9.Wisniewska J, Xu J, Seifertova D, Brewer PB, Ruzicka K, Blilou I, et al. Polar PIN localization directs auxin flow in plants. Science. 2006;312:883. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- 10.Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, et al. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport and auxin-dependent plant growth. Cell. 2003;112:219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 11.Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 12.Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, et al. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol. 2007;17:520–527. doi: 10.1016/j.cub.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 13.Dhonukshe P, Tanaka H, Goh T, Ebine K, Mahonen AP, Prasad K, et al. Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature. 2008;456:962–966. doi: 10.1038/nature07409. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Valdez-Taubas J, Pelham HR. Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr Biol. 2003;13:1636–1640. doi: 10.1016/j.cub.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Yalovsky S, Bloch D, Sorek N, Kost B. Regulation of membrane trafficking, cytoskeleton dynamics and cell polarity by ROP/RAC GTPases. Plant Physiol. 2008;147:1527–1543. doi: 10.1104/pp.108.122150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z. Cell polarity signaling in Arabidopsis. Annu Rev Cell Dev Biol. 2008;24:551–575. doi: 10.1146/annurev.cellbio.23.090506.123233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, et al. A Novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol. 2007;17:947–952. doi: 10.1016/j.cub.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 19.Hazak O, Bloch D, Poraty L, Sternberg H, Zhang J, Friml J, et al. A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS Biol. 2010;8:1000282. doi: 10.1371/journal.pbio.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, et al. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development. 2004;131:657–668. doi: 10.1242/dev.00963. [DOI] [PubMed] [Google Scholar]
- 21.Sabatini S, Heidstra R, Wildwater M, Scheres B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev. 2003;17:354–358. doi: 10.1101/gad.252503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446:811–814. doi: 10.1038/nature05703. [DOI] [PubMed] [Google Scholar]
- 23.Zheng H, Kunst L, Hawes C, Moore I. A GFP-based assay reveals a role for RHD3 in transport between the endoplasmic reticulum and Golgi apparatus. Plant J. 2004;37:398–414. doi: 10.1046/j.1365-313x.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 24.Sorek N, Poraty L, Sternberg H, Bar E, Lewinsohn E, Yalovsky S. Activation status-coupled transient S acylation determines membrane partitioning of a plant Rho-related GTPase. Mol Cell Biol. 2007;27:2144–2154. doi: 10.1128/MCB.02347-06. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Men S, Boutte Y, Ikeda Y, Li X, Palme K, Stierhof YD, et al. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat Cell Biol. 2008;10:237–244. doi: 10.1038/ncb1686. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z, Fu Y. ROP/RAC GTPase signaling. Curr Opin Plant Biol. 2007;10:490–494. doi: 10.1016/j.pbi.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]