Abstract
In plants, AMT/MEP/Rh superfamily mediates high affinity ammonium uptake. AMT/MEP transporters form a trimeric complex, which requires a productive interaction between subunits in order to be functional. The AMT/MEP C-terminal domain is highly conserved in more than 700 AMT homologs from cyanobacteria to higher plants with no cases found to be lacking this domain. AMT1;1 exists in active and inactive states, probably controlled by the spatial positioning of the C-terminus. Ammonium triggers the phosphorylation of a conserved threonine residue (T460) in the C-terminus of AMT1;1 in a time- and concentration-dependent manner. The T460 phosphorylation level correlates with a decrease of root ammonium uptake. We propose that ammonium-induced phosphorylation modulates ammonium uptake as a general mechanism to protect against ammonium toxicity.
Key words: arabidopsis, nitrogen, ammonium transport, AMTs, phosphorylation
Plant-available nutrient pools in soils are highly variable and influenced by drought, flooding, oxygen concentration or pH of the soil. Survival of sessile organisms relies on rapid acclimation to these environmental changes. Under conditions of nutrient limitation, plants optimize morphological and physiological parameters: root surface area can increase, the metabolic conversion, storage and translocation are modified, transporters' nature and density on the plasma membrane can be altered.1 To be able to adjust these parameters and maintain optimal nutrient cellular concentration, plants use sensing systems, which monitor both the external and intracellular substrate concentrations.
About 70% of all ions acquired by plants contain nitrogen (N); N is therefore quantitatively the most important nutrient. Although ammonium is toxic at high concentrations, some species like Arabidopsis thaliana favor ammonium uptake over nitrate as a nitrogen source.2 The ammonium transporter/methylamine permease/rhesus (AMT/MEP/Rh) superfamily mediates high-affinity uptake of ammonium in bacteria, fungi and plants. Crystal structures of bacterial AMT reveal a trimeric complex with each monomer built from 11 transmembrane spanning helices. Each of the three subunits has a pore that allows passage of ammonium. The 11th helix is connected to a cytosolic C-terminus that interacts physically with its own cytosolic loops and connects with several loops of the adjacent subunit.3
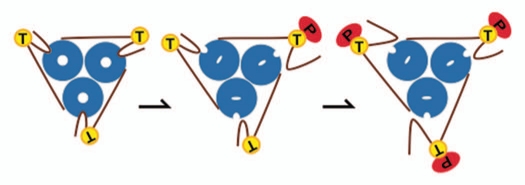
Phylogenetic analyses show that the cytosolic C-terminus is conserved in more than 700 AMT homologs, including archaebacteria, cyanobacteria, fungi and plants. Most modifications, e.g., mutations of the AMT1;1 C-terminus lead to inactive forms of the transporter when tested in yeast, suggesting that the precise positioning of the C-terminus is critical for transport activity. Threonine-460 (T460) is highly conserved among the plant AMTs, and in silico modeling locates T460 between the domain that interacts with the loops of his own monomer and the domain that connects the neighboring subunit. Replacement of T460 for an aspartate leads to loss of the activity of the transporter. Interestingly, coexpression of the defective T460D transporter with a wild-type functional transporter inhibits ammonium uptake by the wild-type protein.4 A screen identified suppressor mutants that regained activity; suppressor mutations in defined residues in the pore or the intracellular loops suggest that the interaction of the C-terminus both with its own monomer and its neighbor modulates the passage of the substrate through the channel.4–6 Interestingly, the pore mutation Q57H uncouples transport from the dependence on the C-terminal regulation. Ammonium permeability increases 10-fold and yeast cells expressing the Q57H mutant become sensitive to ammonium.5 Recently, Kustu's group speculated that the C-terminus promotes the oscillation of transmembrane helix 5, thereby affecting transport activity. In addition, threonine T460 was found to be phosphorylated in a phosphoproteomic study of cell cultures grown on high levels of ammonium.7 Considered together, these results are consistent with a model in which T460 phosphorylation induces a conformational change and that a single phosphorylation event in the C-terminus of one monomer is sufficient for cooperative closure of the trimer (Fig. 1).4
Figure 1.
Schematic representation of the regulation of AMT1;1 trimer. The homotrimer (blue) is represented with the C-terminus (brown line) connecting the neighboring subunit. In absence of phosphorylation of T460, the pores are in open conformation, enabling ammonium to enter the cell. A single phosphorylation event in the C-terminus of one the monomers (red phosphate), induces a conformational change which is sufficient for cooperative closure of the trimer. Multiple phosphorylation events could potentially maintain the complex closed.
The key question was whether T460 phosphorylation is regulated and thereby modulates AMT activity. To identify potential signals that elicit the phosphorylation in planta, we produced a phosphospecific antiserum, which specifically recognizes a peptide carrying the phosphorylated form of T460. In the absence of ammonium in the growth medium, phosphorylation was undetectable. Addition of ammonium triggered rapid phosphorylation of T460 in a time- and concentration-dependent manner. Monovalent cations, such as potassium, sodium or the analog of ammonium, methylammonium, were ineffective in eliciting the phosphorylation. Neither glutamine, a downstream product of ammonium assimilation nor ammonium intracellular accumulation by L-methionine sulfoximine, triggered phosphorylation. External ammonium levels as low as 50 µM (∼Km of the AMT1;1 transporter) induced phosphorylation, suggesting that an unknown extracellular high affinity sensor or the transporter itself could be involved in the sensing.
The hypothesis of sensing extracellular ammonium levels through the transporter is supported by the finding that bacterial and fungal AMTs function as transceptors, responsible for both transport and ammonium sensing.
Phosphorylation of T460 in response to an increase in external ammonium correlates with a reduction of ammonium uptake, consistent with the model of a mechanism where phosphorylation modulates the uptake of ammonium into the root. It had been previously observed that prior to a decrease of RNA, the ammonium uptake was diminished, suggesting a post-translational regulation.8 However, this is the first time that the reduction of the uptake is explained mechanistically. Thus, phosphorylation triggered by an increase in ammonium supply in the medium appears to function as a negative modulator that restricts ammonium uptake, potentially to prevent ammonium toxicity.
In E. coli, ammonium uptake by AmtB is regulated through the recruitment of the soluble protein GlnK to the plasma membrane. Depending on the nitrogen demand, trimeric GlnK interacts with the cytosolic face of the trimeric transporter, blocking the entrance of ammonium. The binding of 2-oxoglutarate and ATP allosterically regulates GlnK, thus integrating the cell's energy status and the amount of available precursors for ammonium assimilation. In the E. coli AmtB, only the highly conserved domain of the C-terminus is present. In contrast, the plant C-terminus is extended. Deletion of the non-conserved part has no obvious effect on transport activity when the plant proteins are expressed in yeast. GlnK is a PII protein and in plants, the PII proteins identified so far are located in the chloroplast. Thus, possibly, the extended C-terminus in the plant AMTs could bind to proteins that serve similar functions as GlnK in E. coli. Using mass spectrometry-based proteomic analysis we identified multiple phosphorylation sites in the non-conserved domain of the C-terminus in addition to T460, supporting the idea that this domain is involved in regulating AMT function in planta. Two of the phosphorylated residues, S488 and S490, were regulated in response to ammonium re-supply, while S475 was not. These additional phosphorylation sites could integrate information on nitrogen status, cells' energy supply or availability of precursors for assimilation of ammonium. Given the role of its bacterial counterparts in signaling, it is conceivable that plant AMTs are involved in sensing and signal integration as well.
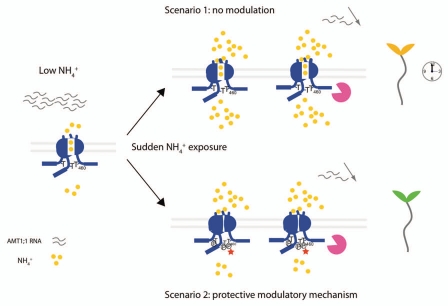
Under low nitrogen situations, plants need to be in a physiological state where they rapidly sense the re-supply of ammonium, conditions that also require efficient retrieval of nitrogen. Consistent with this possible role, high affinity ammonium transporters are expressed in root hairs and are located in the root plasma membrane. We propose that once an optimal cellular ammonium concentration is reached, a rapid regulatory mechanism prevents ammonium entrance to prevent against toxic effects of ammonium excess. This first level of regulation is achieved by a rapid negative modulation system mediated by phosphorylation of AMT in response to an increase in the substrate supply. Subsequently, a second level of regulation leads to endocytosis of the transporter or proteolysis as well as downregulation of RNA levels (Fig. 2).8
Figure 2.
Illustration of the benefice of a protective modulatory mechanism. At low ammonium concentrations, AMT1;1 expression is upregulated; ammonium is retrieved from the soil. When the cell experiences a sudden increase in ammonium (Scenario 1), the regulation of AMT1;1 transcript levels and the proteolysis of the transporter may not be not fast enough to prevent over-accumulation of ammonium leading to toxicity. In Scenario 2, the rapid ammonium-dependent phosphorylation prevents over-accumulation of the substrate, thus preventing ammonium toxicity.
Thus, ammonium uptake is subtly controlled transcriptionally and post-translationally. Trans-regulation controlled by extracellular ammonium and potentially other factors may provide the means for protecting against ammonium toxicity and fine-tuning accumulation. The next steps in the characterization of ammonium nutrition will be to determine specifically how plants sense ammonium levels: i.e., whether they use an ammonium sensor at the cell surface, or whether they use AMTs as transceptors. Important questions will be how other parameters such as nitrate supply, energy status and acceptor molecule availability are perceived and integrated into decisions regarding the cellular and system responses. A major aim will be to identify the kinases involved in phosphorylation.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11696
References
- 1.Glass A. Nutrient Absorption by Plant Roots: Regulation of Uptake to Match Plant Demand. New York: Marcel Dekker, Inc; 2002. [Google Scholar]
- 2.Gazzarrini S, Lejay L, Gojon A, Ninnemann O, Frommer WB, von Wirén N. Three functional transporters for constitutive, diurnally regulated and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell. 1999;11:937–948. doi: 10.1105/tpc.11.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade SL, Dickmanns A, Ficner R, Einsle O. Crystal structure of the archaeal ammonium transporter Amt-1 from Archaeoglobus fulgidus. Proc Natl Acad Sci USA. 2005;102:14994–14999. doi: 10.1073/pnas.0506254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loqué D, Lalonde S, Looger LL, von Wirén N, Frommer WB. A cytosolic trans-activation domain essential for ammonium uptake. Nature. 2007;446:195–198. doi: 10.1038/nature05579. [DOI] [PubMed] [Google Scholar]
- 5.Loqué D, Mora SI, Andrade SL, Pantoja O, Frommer WB. Pore mutations in ammonium transporter AMT1 with increased electrogenic ammonium transport activity. J Biol Chem. 2009;284:24988–24995. doi: 10.1074/jbc.M109.020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuhäuser B, Dynowski M, Mayer M, Ludewig U. Regulation of NH4+ transport by essential cross talk between AMT monomers through the carboxyl tails. Plant Physiol. 2007;143:1651–1659. doi: 10.1104/pp.106.094243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nühse TS, Stensballe A, Jensen ON, Peck SC. Phosphoproteomics of the Arabidopsis plasma membrane and a new phosphorylation site database. Plant Cell. 2004;16:2394–2405. doi: 10.1105/tpc.104.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawat SR, Silim SN, Kronzucker HJ, Siddiqi MY, Glass AD. AtAMT1 gene expression and NH4+ uptake in roots of Arabidopsis thaliana: evidence for regulation by root glutamine levels. Plant J. 1999;19:143–52. doi: 10.1046/j.1365-313x.1999.00505.x. [DOI] [PubMed] [Google Scholar]