Abstract
Mechanical resistance to the gravitational force is a principal gravity response in plants distinct from gravitropism. In the final step of gravity resistance, plants increase the rigidity of their cell walls. Here we discuss the role of cortical microtubules, which sustain the function of the cell wall, in gravity resistance. Hypocotyls of Arabidopsis tubulin mutants were shorter and thicker than the wild-type, and showed either left-handed or right-handed helical growth at 1 g. The degree of twisting phenotype was intensified under hypergravity conditions. Hypergravity also induces reorientation of cortical microtubules from transverse to longitudinal directions in epidermal cells. In tubulin mutants, the percentage of cells with longitudinal microtubules was high even at 1 g, and it was further increased by hypergravity. The left-handed helical growth mutants had right-handed microtubule arrays, whereas the right-handed mutant had left-handed arrays. Moreover, blockers of mechanoreceptors suppressed both the twisting phenotype and reorientation of microtubules in tubulin mutants. These results support the hypothesis that cortical microtubules play an essential role in maintenance of normal growth phenotype against the gravitational force, and suggest that mechanoreceptors are involved in signal perception in gravity resistance. Space experiments will confirm whether this view is applicable to plant resistance to 1 g gravity, as to the resistance to hypergravity.
Key words: cortical microtubules, gravity, gravity resistance, hypergravity, mechanoreceptor, microgravity, tubulin mutants
Gravity Resistance in Plants
The development of a response to resist the gravitational force has played an important role in the transition of plant ancestors from an aquatic environment to a terrestrial environment about 450 million years ago and in the consequent establishment of land plants.1,2 Nevertheless, the presence of this gravity response has not been properly recognized for long, and its mechanism has been often confused with that of gravitropism. We have termed this response ‘gravity resistance’, and examined its mechanism mainly using hypergravity conditions produced by centrifugation.3,4 Plant protoplasts are surrounded by well-developed cell walls, which is the major source of mechanical strength for plant body. Therefore, the cell wall may be responsible for gravity resistance. Actually, we have obtained evidence supporting this hypothesis.2–4 Hypergravity has been shown to increase the cell wall rigidity in various plant materials. Hypergravity also caused an increase in cell wall thickness and a polymerization of certain matrix polysaccharides, such as xyloglucans in dicotyledons and 1,3,1,4-β-glucans in monocotyledonous Gramineae. Furthermore, hypergravity has been shown to increase the apoplastic pH of various materials.5,6 Thus, plants increase the rigidity of their cell walls in response to the gravitational force, via modifications to the cell wall metabolism and apoplastic environment.
Role of Cortical Microtubules in Gravity Resistance
Cortical microtubules give the cytoplasm structural stability and sustain various functions of the cell wall. The expression of most α- and β-tubulin genes was upregulated by hypergravity in Arabidopsis hypocotyls.7,8 In the epidermis of the growing region of azuki bean epicotyls, cells with transverse cortical microtubules were predominant at 1 g. Hypergravity induces reorientation of cortical microtubules from transverse to longitudinal directions.9 In addition, hypergravity increased transiently the expression of γ-tubulin and katanin genes,10,11 which are assumed to be responsible for reorientation of cortical microtubules.12 These results suggest that cortical microtubules are involved in gravity resistance.2,13 To confirm this point, we investigated the changes in cell morphology and orientation of cortical microtubule arrays in hypocotyls of Arabidopsis tubulin mutants grown under hypergravity conditions.14
We used three mutants, tua3(D205N), tua4(S178Δ) and tua6(A281T)15,16 in the study. Wild-type hypocotyls grew almost straight irrespective of gravity conditions. Hypocotyls of tubulin mutants showed either left-handed (tua3 and tua4) or right-handed (tua6) helical growth at 1 g, and the degree of twisting phenotype was intensified under hypergravity conditions at 300 g.14 On the other hand, hypergravity greatly stimulated reorientation of cortical microtubules from transverse to longitudinal directions in wild-type, without any preference to the left or right direction. In the left-handed helical growth mutants, the frequency distribution was dispersed with a bias toward right-handed microtubule arrays at 1 g. The percentage of cells with right-handed microtubule arrays was greatly increased by hypergravity. The frequency distribution of microtubule orientation was dispersed with a bias toward left-handed arrays in the right-handed helical growth mutant at 1 g, which was further stimulated by hypergravity. There was a close correlation between the alignment angle of epidermal cell files and the alignment of cortical microtubules.14 These results indicate that cortical microtubules play an essential role in maintenance of normal growth phenotype against the gravitational force.
Involvement of Mechanoreceptors in Gravity Resistance
Gadolinium ions have been used as blockers of mechanosensitive ion channels in various materials.17,18 The blockers are capable of nullifying hypergravity-induced modifications to growth anisotropy and cell wall rigidity.19,20 In our recent study, we found that gadolinium ions decreased the alignment angle of epidermal cell files and that of cortical microtubules of tubulin mutants at 1 g.14 Moreover, hypergravity had no effects on the alignment angles of cell files or cortical microtubules in the presence of gadolinium ions. These results suggest that mechanoreceptors are involved in signal perception in gravity resistance (Fig. 1). Plasma membrane proteins MCA1 and MCA2 have been identified as a candidate for the Ca2+-permeable mechanosensitive channel in Arabidopsis.21–23 We are now examining, with null and overexpressing mutants of MCA1 and MCA2, whether they are responsible for gravity signal perception in gravity resistance.
Figure 1.
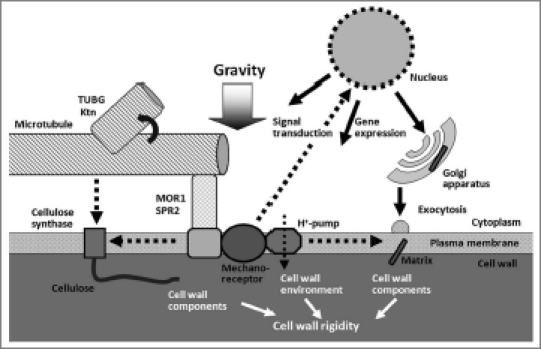
Mechanism of gravity resistance in plants. The gravity signal is perceived by the mechanoreceptors located on the plasma membrane, and then transformed and transduced into the cells. The transduced signal induces the expression of diverse genes and influences the structure and function of various cellular components. Cortical microtubules, in concert with the plasma membrane, regulate the cell wall metabolism as well as apoplastic environment, leading to an increase in the cell wall rigidity.
Mechanism of Resistance to 1 g Gravity
In our recent study, we obtained an interesting result that blockers of mechanosensitive ion channels suppressed helical growth as well as reorientation of cortical microtubules, from transverse to longitudinal directions with a bias toward the left or the right direction, of Arabidopsis tubulin mutants grown not only at 300 g but also at 1 g.14 Namely, the blockers are capable of nullifying phenotypes of the mutants at 1 g. The result suggests that tubulin mutants are hypersensitive to the gravitational force and the effects of gravity are saturated at lower doses. It is then expected that under microgravity condition in space, the defects of growth in tubulin mutants are rescued and they grow and develop more or less normally.24 To confirm this possibility, we carried out a space experiment termed Resist Wall on the International Space Station. Unfortunately, no plants developed to the expected developmental stage because of serious anomalies of water supply system.25 Another space experiment related to this topic, Space Seed, is now underway. We are also preparing for the next experiment named Resist Tubule on the International Space Station. These experiments will confirm whether this view is applicable to plant resistance to 1 g gravity, as to the resistance to hypergravity.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 17510159), and a Grant for Ground-based Research for Space Utilization from the Japan Space Forum (to T.H.).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11706
References
- 1.Hoson T. Development of the anti-gravitational system in land plants and its implication for the interaction between plants and other organisms. Biol Sci Space. 2003;17:54–56. doi: 10.2187/bss.17.54. [DOI] [PubMed] [Google Scholar]
- 2.Hoson T. The mechanism and significance of gravity resistance in plants. J Gravit Physiol. 2006;13:97–100. [Google Scholar]
- 3.Hoson T, Soga K. New aspects of gravity responses in plant cells. Int Rev Cytol. 2003;229:209–244. doi: 10.1016/s0074-7696(03)29005-7. [DOI] [PubMed] [Google Scholar]
- 4.Hoson T, Saito Y, Soga K, Wakabayashi K. Signal perception, transduction and response in gravity resistance. Another graviresponse in plants. Adv Space Res. 2005;36:1196–1202. [Google Scholar]
- 5.Soga K, Wakabayashi K, Hoson T, Kamisaka S. Hypergravity-induced increase in the apoplastic pH and its possible involvement in suppression of β-glucan breakdown in maize seedlings. Aust J Plant Physiol. 2000;27:967–972. [PubMed] [Google Scholar]
- 6.Soga K, Wakabayashi K, Hoson T, Kamisaka S. Changes in the apoplastic pH are involved in regulation of xyloglucan breakdown of azuki bean epicotyls under hypergravity conditions. Plant Cell Physiol. 2000;41:509–514. doi: 10.1093/pcp/41.4.509. [DOI] [PubMed] [Google Scholar]
- 7.Yoshioka R, Soga K, Wakabayashi K, Takeba G, Hoson T. Hypergravity-induced changes in gene expression in Arabidopsis hypocotyls. Adv Space Res. 2003;31:2187–2193. doi: 10.1016/s0273-1177(03)00243-6. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto S, Saito Y, Kumasaki S, Soga K, Wakabayashi K, Hoson T. Upregulation of tubulin genes and roles of microtubules in hypergravityinduced growth modifications in Arabidopsis hypocotyls. Adv Space Res. 2007;39:1176–1181. [Google Scholar]
- 9.Soga K, Wakabayashi K, Kamisaka S, Hoson T. Hypergravity induces reorientation of cortical microtubules and modifies growth anisotropy in azuki bean epicotyls. Planta. 2006;224:1485–1494. doi: 10.1007/s00425-006-0319-8. [DOI] [PubMed] [Google Scholar]
- 10.Soga K, Kotake T, Wakabayashi K, Kamisaka S, Hoson T. Transient increase in the transcript levels of γ-tubulin complex genes during reorientation of cortical microtubules by gravity in azuki bean (Vigna angularis) epicotyls. J Plant Res. 2008;121:493–498. doi: 10.1007/s10265-008-0179-3. [DOI] [PubMed] [Google Scholar]
- 11.Soga K, Kotake T, Wakabayashi K, Kamisaka S, Hoson T. The transcript level of katanin gene is increased transiently in response to changes in gravitational conditions in azuki bean epicotyls. Biol Sci Space. 2009;23:23–28. [Google Scholar]
- 12.Murata T, Sonobe S, Baskin TI, Hyodo S, Hasezawa S, Nagata T, et al. Microtubule-dependent microtubule nucleation based on recruitment of γ-tubulin in higher plants. Nat Cell Biol. 2005;10:961–968. doi: 10.1038/ncb1306. [DOI] [PubMed] [Google Scholar]
- 13.Hoson T, Soga K, Wakabayashi K. Role of the cell wall-sustaining system in gravity resistance in plants. Biol Sci Space. 2009;23:131–136. [Google Scholar]
- 14.Matsumoto S, Kumasaki S, Soga K, Wakabayashi K, Hashimoto T, Hoson T. Gravity-induced modifications to development in hypocotyls of Arabidopsis tubulin mutants. Plant Physiol. 2010;152:918–926. doi: 10.1104/pp.109.147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto T. Molecular genetic analysis of left-right handedness in plants. Philos Trans Royal Soc Lond B Biol Sci. 2002;357:799–808. doi: 10.1098/rstb.2002.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida T, Kaneko Y, Iwano M, Hashimoto T. Helical microtubule arrays in a collection of twisting tubulin mutants of Arabidopsis thaliana. Proc Natl Acad Sci USA. 2007;104:8544–8549. doi: 10.1073/pnas.0701224104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding JP, Pickard BG. Mechanosensory calcium-selective cation channels in epidermal cells. Plant J. 1993;3:83–110. doi: 10.1111/j.1365-313x.1993.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 18.Fasano JM, Massa GD, Gilroy S. Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul. 2002;21:71–88. doi: 10.1007/s003440010049. [DOI] [PubMed] [Google Scholar]
- 19.Soga K, Wakabayashi K, Kamisaka S, Hoson T. Graviperception in growth inhibition of plant shoots under hypergravity conditions produced by centrifugation is independent of that in gravitropism and may involve mechanoreceptors. Planta. 2004;218:1054–1061. doi: 10.1007/s00425-003-1187-0. [DOI] [PubMed] [Google Scholar]
- 20.Soga K, Wakabayashi K, Kamisaka S, Hoson T. Mechanoreceptors rather than sedimentable amyloplasts perceive the gravity signal in hypergravity-induced inhibition of root growth in azuki bean. Funct Plant Biol. 2005;32:175–179. doi: 10.1071/fp04145. [DOI] [PubMed] [Google Scholar]
- 21.Kanzaki M, Nagasawa M, Kojima I, Sato C, Naruse K, Sokabe M, Iida H. Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science. 1999;285:882–886. doi: 10.1126/science.285.5429.882. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA. 2007;104:3639–3644. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamanaka T, Nakagawa Y, Mori K, Nakano M, Imamura T, Kataoka H, et al. MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis. Plant Physiol. 2010;152:1284–1296. doi: 10.1104/pp.109.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoson T, Matsumoto S, Soga K, Wakabayashi K, Hashimoto T, Sonobe S, et al. The outline and significance of the Resist Wall experiment: Role of microtubule-membrane-cell wall continuum in gravity resistance in plants. Biol Sci Space. 2007;21:56–61. [Google Scholar]
- 25.Hoson T, Matsumoto S, Soga K, Wakabayashi K, Hashimoto T, Sonobe S, et al. Growth and cell wall properties in hypocotyls of Arabidopsis tua6 mutant under microgravity conditions in space. Biol Sci Space. 2009;23:71–76. [Google Scholar]


