Abstract
Aims
Patients with diabetes mellitus (DM) have high platelet reactivity and are at increased risk of ischaemic events and bleeding post-acute coronary syndromes (ACS). In the PLATelet inhibition and patient Outcomes (PLATO) trial, ticagrelor reduced the primary composite endpoint of cardiovascular death, myocardial infarction, or stroke, but with similar rates of major bleeding compared with clopidogrel. We aimed to investigate the outcome with ticagrelor vs. clopidogrel in patients with DM or poor glycaemic control.
Methods and results
We analysed patients with pre-existing DM (n = 4662), including 1036 patients on insulin, those without DM (n = 13 951), and subgroups based on admission levels of haemoglobin A1c (HbA1c; n = 15 150). In patients with DM, the reduction in the primary composite endpoint (HR: 0.88, 95% CI: 0.76–1.03), all-cause mortality (HR: 0.82, 95% CI: 0.66–1.01), and stent thrombosis (HR: 0.65, 95% CI: 0.36–1.17) with no increase in major bleeding (HR: 0.95, 95% CI: 0.81–1.12) with ticagrelor was consistent with the overall cohort and without significant diabetes status-by-treatment interactions. There was no heterogeneity between patients with or without ongoing insulin treatment. Ticagrelor reduced the primary endpoint, all-cause mortality, and stent thrombosis in patients with HbA1c above the median (HR: 0.80, 95% CI: 0.70–0.91; HR: 0.78, 95% CI: 0.65–0.93; and HR: 0.62, 95% CI: 0.39–1.00, respectively) with similar bleeding rates (HR: 0.98, 95% CI: 0.86–1.12).
Conclusion
Ticagrelor, when compared with clopidogrel, reduced ischaemic events in ACS patients irrespective of diabetic status and glycaemic control, without an increase in major bleeding events.
Keywords: Acute coronary syndromes, Diabetes, Ticagrelor, Clopidogrel, Mortality, Myocardial infarction
See page 2971 for the editorial comment on this article (doi:10.1093/eurheartj/ehq347)
Introduction
Patients with diabetes mellitus (DM) and acute coronary syndromes (ACS) are particularly at high risk for recurrent cardiovascular (CV) events including death.1–3 Although clopidogrel combined with aspirin has been used successfully to prevent thrombotic events in patients with ACS,4–6 patients with DM, when compared with those without, have consistently been shown to have higher on-treatment platelet reactivity and worse clinical outcomes.7–11 The mechanisms leading to poor response to clopidogrel in patients with DM are not fully elucidated but are likely multifactorial including genetic, metabolic, cellular, and clinical factors.7,12,13 More recent work suggests that reduced generation of the active clopidogrel metabolite may contribute to poor clopidogrel responsiveness in patients with DM.14,15 Prasugrel, a third generation thienopyridine with more favourable pharmacokinetic and pharmacodynamic profile than clopidogrel, has been shown to overcome these limitations and was associated with a numerically lower rate of the primary composite ischaemic endpoint in patients with DM in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38 (TRITON–TIMI 38) study at the expense of an increase in major bleeds.16
Ticagrelor is an oral non-thienopyridine P2Y12 inhibiting agent with a reversible and direct action on the receptor that provides faster, greater, and more consistent platelet inhibition than clopidogrel.17 The PLATelet inhibition and patient Outcomes (PLATO) trial showed that ticagrelor was superior to clopidogrel for the prevention of CV death, myocardial infarction (MI), or stroke without a significant increase in major bleeding in a broad population of patients with ACS.18 Establishing the clinical impact of ticagrelor vs. clopidogrel in patients with DM was a pre-specified aim of the PLATO trial and is reported here.
Methods
The PLATO trial randomized 18 624 patients with ST-segment elevation or non-ST-segment elevation ACS, with onset during the previous 24 h to ticagrelor or clopidogrel as soon as possible after admission. Details of study design, patients, outcome definitions, and results have been published.18,19
Ticagrelor was given in a loading dose of 180 mg followed by 90 mg twice daily. Patients randomized to clopidogrel were given a maintenance dose of 75 mg daily. Those who were clopidogrel naïve received a 300-mg loading dose. An additional 300-mg dose was allowed pre PCI. All patients received acetylsalicylic acid unless intolerant. The randomized treatment continued for a minimum of 6 to a maximum of 12 months with a median duration of study treatment of 9.1 months.
The primary efficacy variable was time to first occurrence of any event from the composite of death from vascular causes, MI, or stroke. The primary safety variable was the time to first occurrence of any PLATO-defined major bleeding.19 Diabetic status and whether or not patients were on insulin treatment were assessed at the time of randomization. Venous blood samples were obtained via a direct venous puncture after randomization in a non-fasting state. After centrifugation serum was frozen at −20°C in aliquots and sent for central laboratory analysis of glucose and haemoglobin A1c (HbA1c) concentration.
Statistical analyses
Patient characteristics were compared by DM status using χ2 and Wilcoxon's rank-sum tests. We pre-specified to report treatment effects for primary and secondary efficacy and safety events by DM status, the median of serum glucose (6.8 mmol/L), and the median of percentage HbA1c (6.0%). The Kaplan–Meier estimates were plotted by treatment group, dichotomized HbA1c, and DM status. The Cox proportional hazards models were used to investigate univariable relationships between diabetes-related variables (DM, serum glucose, and HbA1c as continuous variables, DM type, or insulin treatment) and endpoint. The interactions between treatment group and diabetes-related variables were evaluated with the addition of treatment and the treatment-by-diabetes variable interaction. The multivariable Cox regression models were fitted for the primary efficacy endpoint, the primary safety endpoint, and all-cause mortality. Forward and backward selections were used with the following covariates: age, sex, prior MI, heart failure, hypertension, smoking, height, weight, previous percutaneous coronary intervention, coronary artery bypass surgery, ST elevation or left bundle branch block on ECG at entry, estimated creatinine clearance, heart rate, peripheral artery disease, prior tachyarrhythmia, blood pressure, and prior angina pectoris. The models were repeated with the inclusion of DM, HbA1c, or glucose and the randomized treatment-by-diabetes variable interaction was allowed to enter if significant. Subgroups based on intended treatment strategy, ST elevation at entry, and creatinine clearance were tested using the Cox models with the inclusion of the three-way interaction of DM, treatment, and subgroup.
All analyses were performed according to the intention-to-treat principle, utilizing SAS® version 9.1. A two-sided P-value of 0.05 was regarded statistically significant for overall treatment differences.
Results
Patients
Among the 18 624 patients randomized in the PLATO study 4662 (25%) were reported as having DM by the investigators (Table 1). Patients with DM more often had multiple CV risk factors. The majority (96%) was reported as type 2. Prior to randomization, almost one-fourth of the diabetic patients were on long-term insulin treatment, and more than half were treated with insulin and 84% were treated with any anti-diabetic medications during the initial hospitalization (Table 2). Furthermore, patients with DM were less often intended for an invasive treatment strategy and underwent coronary angiography and PCI less often but had coronary artery bypass surgery performed more often during the course of the study. At discharge more patients with DM were diagnosed with non-ST-elevation ACS and fewer with ST-elevation MI compared with patients without DM.
Table 1.
Patient characteristics at baseline
| Characteristic | Diabetes (n = 4662) | No diabetes (n = 13 951) | P-value |
|---|---|---|---|
| Age [median (25th–75th percentile)] | 64 (56–72) | 61 (53–70) | <0.0001 |
| Age ≥75 years [% (n)] | 17.4 (809) | 14.8 (2067) | <0.0001 |
| Gender, women [% (n)] | 34.8 (1624) | 26.2 (3660) | <0.0001 |
| Body weight [median (25th–75th percentile)] | 81 (70–93) | 79 (70–89) | <0.0001 |
| Body weight < 60 kg [% (n)] | 5.9 (274) | 7.4 (1038) | 0.0011 |
| BMI [median (25th–75th percentile)] | 28.7 (25.7–32.0) | 27.0 (24.5–29.8) | <0.0001 |
| Waist circumference [median (25th–75th percentile)] | 102 (93–110) | 97 (89–105) | <0.0001 |
| Race [% (n)] | <0.0001 | ||
| Black | 2.1 (98) | 0.9 (131) | |
| Caucasian | 89.4 (4169) | 92.5 (12 898) | |
| Oriental | 6.8 (319) | 5.6 (777) | |
| Other | 1.6 (76) | 1.0 (145) | |
| CV risk factors [% (n)] | |||
| Habitual smoker | 24.8 (1156) | 39.6 (5522) | <0.0001 |
| Hypertension | 81.6 (3802) | 60.1 (8381) | <0.0001 |
| Dyslipidaemia | 59.7 (2782) | 42.3 (5907) | <0.0001 |
| History [% (n)] | |||
| Angina pectoris | 54.0 (2517) | 41.9 (5841) | <0.0001 |
| Myocardial infarction | 27.0 (1261) | 18.4 (2563) | <0.0001 |
| Congestive heart failure | 9.4 (440) | 4.4 (610) | <0.0001 |
| Percutaneous coronary intervention | 18.2 (847) | 11.8 (1645) | <0.0001 |
| Coronary artery bypass graft | 10.0 (464) | 4.6 (642) | <0.0001 |
| Transient ischaemic attack | 3.4 (157) | 2.5 (342) | 0.0011 |
| Non-haemorrhagic stroke | 5.8 (269) | 3.2 (453) | <0.0001 |
| Peripheral arterial disease | 9.2 (431) | 5.1 (713) | <0.0001 |
| Chronic renal disease | 7.8 (362) | 3.0 (423) | <0.0001 |
| Treatment [% (n)] | |||
| OL clopidogrel dose ≥ 600 mg before randomization | 9.0 (421) | 13.2 (1846) | <0.0001 |
| Total clopidogrel (OL + IP) dose ≥ 600 mg before randomization to 24 h after first dose | 16.4 (765) | 22.1 (3080) | <0.0001 |
| Randomized to ticagrelor | 49.9 (2326) | 50.2 (6999) | 0.7445 |
| Planned invasive | 66.7 (3109) | 73.8 (10 289) | <0.0001 |
| Baseline labs [median (25th–75th percentile)] | |||
| Glucose (mmol/L) | 9.8 (7.2–13.2) | 6.4 (5.6–7.7) | <0.0001 |
| Haemoglobin A1c (%) | 7.6 (6.7–9.0) | 5.8 (5.6–6.1) | <0.0001 |
| Creatinine clearance (mL/min) | 76.4 (58.0–96.6) | 81.6 (64.7–99.6) | <0.0001 |
| First central troponin Ia (µmol/L) | 2.1 (0.2–11.7) | 2.1 (0.2–12.0) | 0.2857 |
OL, open label; IP, investigational product.
aAdvia Centaur TnI-Ultra Immunoassay (Siemens).
Table 2.
Medication and procedures during study and final diagnosis according to diabetes status
| Characteristic | Diabetes (n = 4662) | No diabetes (n = 13 951) | P-value |
|---|---|---|---|
| Medications from index event to end of hospitalization [% (n)] | |||
| Aspirin | 97.1 (4525) | 97.2 (13 549) | 0.5984 |
| Beta-blockers | 83.7 (3899) | 86.4 (12 042) | <0.0001 |
| ACE inhibitors and/or angiotensin receptor blockers | 89.8 (4186) | 81.0 (11 287) | <0.0001 |
| Cholesterol lowering (statin) | 93.5 (4359) | 94.0 (13 099) | 0.2394 |
| Ca-channel blockers | 29.7 (1385) | 18.9 (2638) | <0.0001 |
| Diuretics | 49.4 (2301) | 33.7 (4692) | <0.0001 |
| Glycoprotein IIb/IIIa inhibitors | 24.0 (1119) | 28.1 (3918) | <0.0001 |
| Insulin | 55.2 (2573) | 5.5 (766) | <0.0001 |
| Any anti-diabetic medication | 84.2 (3924) | 6.4 (886) | <0.0001 |
| Procedures [% (n)] | |||
| Coronary angiography before discharge | 77.4 (3607) | 82.9 (11 562) | <0.0001 |
| Coronary angiography during study | 82.9 (3863) | 86.8 (12 116) | <0.0001 |
| PCI before discharge | 53.9 (2514) | 63.4 (8849) | <0.0001 |
| PCI during study | 58.0 (2703) | 66.5 (9274) | <0.0001 |
| Stenting | 54.1 (2520) | 62.9 (8769) | <0.0001 |
| With bare-metal stent only | 33.0 (1538) | 45.0 (6275) | <0.0001 |
| With ≥1 drug-eluting stent | 21.1 (982) | 17.9 (2494) | <0.0001 |
| CABG before discharge | 6.4 (300) | 4.8 (670) | <0.0001 |
| CABG during study | 13.2 (617) | 9.2 (1282) | <0.0001 |
| Final diagnosisa [% (n)] | <0.0001 | ||
| STEMI | 28.7 (1336) | 40.8 (5690) | |
| NSTEMI | 47.6 (2217) | 41.1 (5738) | |
| Unstable angina pectoris | 20.9 (975) | 15.3 (2137) | |
| Other | 2.8 (130) | 2.6 (359) | |
PCI, percutaneous coronary intervention; CABG, coronary artery bypass surgery.
aThe type of ACS defined using the final diagnosis of index event.
Baseline characteristics, medications, and procedures were well matched between the randomized treatment groups (Supplementary material online, Tables S6 and S7).
Outcomes in relation to diabetes status
Diabetes mellitus was strongly associated with all evaluated ischaemic and bleeding endpoints (Tables 3 and 4). Also after adjustment for other significant clinical and laboratory predictors of outcome in multivariable analyses, DM was significantly associated with higher incidences of the primary composite outcome, mortality, and major bleeding. Baseline levels of serum glucose and HbA1c analysed as continuous variables were also significantly associated with the evaluated ischaemic and bleeding endpoints. Haemoglobin A1c had a stronger association with the primary composite endpoint (χ2: 38, P = 0.001 vs. 30, P = 0.001) and all-cause mortality (χ2: 31, P = 0.001 vs. 22, P = 0.001) but a weaker association with major bleeding (χ2: 21, P = 0.001 vs. 25, P = 0.001) than diabetes status (data not shown).
Table 3.
Association of diabetes-related variables with endpoints
| Characteristica | χ2 | HR (95% CI) | P-value |
|---|---|---|---|
| Efficacy endpoints | |||
| CV death, MI, or stroke | |||
| Diabetes | 108.78 | 1.66 (1.51–1.82) | <0.0001 |
| Baseline glucose (truncated)b | 67.22 | 1.12 (1.09–1.15) | <0.0001 |
| Baseline HbA1c (truncated)b | 90.72 | 1.30 (1.23–1.37) | <0.0001 |
| All-cause death | |||
| Diabetes | 78.87 | 1.84 (1.61–2.10) | <0.0001 |
| Baseline glucose (truncated)b | 83.47 | 1.21 (1.16–1.25) | <0.0001 |
| Baseline HbA1c (truncated)b | 75.14 | 1.40 (1.30–1.51) | <0.0001 |
| MI | |||
| Diabetes | 44.09 | 1.53 (1.35–1.73) | <0.0001 |
| Baseline glucose (truncated)b | 9.92 | 1.06 (1.02–1.10) | 0.0016 |
| Baseline HbA1c (truncated)b | 34.85 | 1.24 (1.15–1.33) | <0.0001 |
| Definite stent thrombosisc | |||
| Diabetes | 1.91 | 1.26 (0.91–1.77) | 0.1673 |
| Baseline glucose (truncated)b | 12.68 | 1.19 (1.08–1.31) | 0.0004 |
| Baseline HbA1c (truncated)b | 5.20 | 1.24 (1.03–1.50) | 0.0226 |
| Safety endpoints | |||
| Major bleeding | |||
| Diabetes | 48.13 | 1.41 (1.28–1.55) | <0.0001 |
| Baseline glucose | 14.91 | 1.03 (1.01–1.04) | 0.0001 |
| Baseline HbA1c | 20.39 | 1.07 (1.04–1.11) | <0.0001 |
| Non-CABG major bleeding | |||
| Diabetes | 14.50 | 1.38 (1.17–1.62) | 0.0001 |
| Baseline glucose | 9.78 | 1.04 (1.01–1.06) | 0.0018 |
| Baseline HbA1c | 0.03 | 1.00 (0.95–1.06) | 0.8676 |
| CABG-related major bleeding | |||
| Diabetes | 34.16 | 1.42 (1.26–1.60) | <0.0001 |
| Baseline glucose | 5.49 | 1.02 (1.00–1.04) | 0.0191 |
| Baseline HbA1c | 28.65 | 1.10 (1.06–1.14) | <0.0001 |
aGlucose and HbA1c values are treated as linear for the range of values for the safety endpoints.
bFor efficacy outcomes, glucose values <5 and >10 are treated as 5 and 10, respectively. For efficacy outcomes, HbA1c values >8 are treated as 8.
cOf the 11 289 patients who received a stent, 2520 had DM.
Table 4.
Outcome in relation to diabetes status and glucose metabolic control
| n | Overall | Ticagrelor | Clopidogrel | HR (95% CI) | P-value (interaction) | |
|---|---|---|---|---|---|---|
| CV death, myocardial infarction, or stroke | ||||||
| No diabetes | 13 951 | 9.3 (1219) | 8.4 (555) | 10.2 (664) | 0.83 (0.74–0.93) | 0.49 |
| Diabetes | 4662 | 15.2 (659) | 14.1 (309) | 16.2 (350) | 0.88 (0.76–1.03) | |
| Glucose < 6.8 mmol/L | 7604 | 8.9 (630) | 8.0 (284) | 9.7 (346) | 0.83 (0.71–0.98) | 0.52 |
| Glucose ≥ 6.8 mmol/L | 7646 | 12.8 (925) | 11.7 (428) | 14.0 (497) | 0.85 (0.74–0.96) | |
| HbA1c < 6.0% | 7260 | 8.6 (593) | 8.2 (288) | 9.0 (305) | 0.93 (0.79–1.09) | 0.24 |
| HbA1c ≥ 6.0% | 7890 | 12.8 (947) | 11.4 (419) | 14.2 (528) | 0.80 (0.70–0.91) | |
| All-cause death | ||||||
| No diabetes | 13 951 | 4.3 (564) | 3.7 (246) | 5.0 (318) | 0.77 (0.65–0.91) | 0.66 |
| Diabetes | 4662 | 7.9 (341) | 7.0 (153) | 8.7 (188) | 0.82 (0.66–1.01) | |
| Glucose < 6.8 mmol/L | 7604 | 3.6 (252) | 3.1 (110) | 4.1 (142) | 0.79 (0.62–1.01) | 0.38 |
| Glucose ≥ 6.8 mmol/L | 7646 | 6.9 (492) | 6.0 (218) | 7.8 (274) | 0.79 (0.66–0.94) | |
| HbA1c < 6.0% | 7260 | 3.8 (256) | 3.4 (114) | 4.2 (142) | 0.79 (0.62–1.01) | 0.71 |
| HbA1c ≥ 6.0% | 7890 | 6.5 (475) | 5.6 (206) | 7.4 (269) | 0.78 (0.65–0.93) | |
| Myocardial infarction | ||||||
| No diabetes | 13 951 | 5.6 (731) | 5.0 (329) | 6.2 (402) | 0.81 (0.70–0.94) | 0.32 |
| Diabetes | 4662 | 8.7 (366) | 8.4 (175) | 9.1 (191) | 0.92 (0.75–1.13) | |
| Glucose < 6.8 mmol/L | 7604 | 5.9 (415) | 5.5 (192) | 6.2 (223) | 0.87 (0.72–1.06) | 0.84 |
| Glucose ≥ 6.8 mmol/L | 7646 | 7.2 (500) | 6.4 (227) | 7.9 (273) | 0.82 (0.68–0.97) | |
| HbA1c < 6.0% | 7260 | 5.4 (369) | 5.1 (179) | 5.8 (190) | 0.92 (0.75–1.13) | 0.47 |
| HbA1c ≥ 6.0% | 7890 | 7.5 (540) | 6.8 (241) | 8.2 (299) | 0.81 (0.68–0.96) | |
| Definite stent thrombosis | ||||||
| No diabetes | 8766 | 1.5 (130) | 1.3 (53) | 1.8 (77) | 0.68 (0.48–0.97) | 0.89 |
| Diabetes | 2518 | 2.0 (47) | 1.6 (18) | 2.4 (29) | 0.65 (0.36–1.17) | |
| Glucose < 6.8 mmol/L | 4383 | 1.1 (48) | 1.2 (25) | 1.0 (23) | 1.07 (0.61–1.89) | 0.45 |
| Glucose ≥ 6.8 mmol/L | 4882 | 1.9 (89) | 1.5 (33) | 2.4 (56) | 0.60 (0.39–0.93) | |
| HbA1c < 6.0% | 4592 | 1.4 (62) | 1.4 (30) | 1.4 (32) | 0.91 (0.55–1.50) | 0.51 |
| HbA1c ≥ 6.0% | 4636 | 1.7 (74) | 1.3 (28) | 2.0 (46) | 0.62 (0.39–1.00) | |
| Major bleeding, PLATO defined | ||||||
| No diabetes | 13 798 | 10.4 (1298) | 10.8 (674) | 10.0 (624) | 1.08 (0.97–1.20) | 0.21 |
| Diabetes | 4621 | 14.4 (592) | 14.1 (287) | 14.8 (305) | 0.95 (0.81–1.12) | |
| Glucose < 6.8 mmol/L | 7604 | 10.7 (734) | 11.0 (370) | 10.4 (364) | 1.04 (0.90–1.20) | 0.35 |
| Glucose ≥ 6.8 mmol/L | 7646 | 11.5 (790) | 12.0 (412) | 11.1 (378) | 1.09 (0.94–1.25) | |
| HbA1c < 6.0% | 7260 | 9.8 (647) | 10.9 (357) | 8.8 (290) | 1.22 (1.05–1.43) | 0.08 |
| HbA1c ≥ 6.0% | 7890 | 12.4 (874) | 12.3 (428) | 12.6 (446) | 0.98 (0.86–1.12) | |
| Non-CABG-related major bleeding, PLATO defined | ||||||
| No diabetes | 13 798 | 3.8 (461) | 4.1 (253) | 3.4 (208) | 1.22 (1.01–1.46) | 0.69 |
| Diabetes | 4621 | 5.2 (207) | 5.5 (109) | 4.9 (98) | 1.13 (0.86–1.49) | |
| Glucose < 6.8 mmol/L | 7604 | 3.7 (243) | 3.9 (126) | 3.4 (117) | 1.10 (0.86–1.42) | 0.97 |
| Glucose ≥ 6.8 mmol/L | 7646 | 4.4 (297) | 4.9 (168) | 3.9 (129) | 1.30 (1.03–1.64) | |
| HbA1c < 6.0% | 7260 | 3.5 (228) | 4.2 (132) | 2.9 (96) | 1.36 (1.05–1.77) | 0.47 |
| HbA1c ≥ 6.0% | 7890 | 4.5 (307) | 4.8 (163) | 4.2 (144) | 1.16 (0.93–1.46) | |
| CABG-related major bleeding, PLATO defined | ||||||
| No diabetes | 13 798 | 7.0 (871) | 6.8 (430) | 7.1 (441) | 0.97 (0.85–1.11) | 0.51 |
| Diabetes | 4621 | 9.9 (402) | 9.3 (189) | 10.4 (213) | 0.90 (0.74–1.09) | |
| Glucose < 6.8 mmol/L | 7604 | 7.4 (510) | 7.4 (252) | 7.4 (258) | 1.00 (0.84–1.19) | 0.32 |
| Glucose ≥ 6.8 mmol/L | 7646 | 7.6 (515) | 7.5 (254) | 7.7 (261) | 0.97 (0.81–1.15) | |
| HbA1c < 6.0% | 7260 | 6.6 (436) | 6.9 (230) | 6.3 (206) | 1.10 (0.91–1.33) | 0.31 |
| HbA1c ≥ 6.0% | 7890 | 8.4 (591) | 8.0 (278) | 8.8 (313) | 0.91 (0.77–1.07) | |
| Major bleeding, TIMI defined | ||||||
| No diabetes | 13 798 | 7.3 (910) | 7.6 (476) | 7.0 (434) | 1.10 (0.96–1.25) | 0.10 |
| Diabetes | 4621 | 9.5 (385) | 9.0 (181) | 9.9 (204) | 0.90 (0.74–1.10) | |
| Glucose < 6.8 mmol/L | 7604 | 7.4 (508) | 7.7 (261) | 7.2 (247) | 1.09 (0.91–1.29) | 0.07 |
| Glucose ≥ 6.8 mmol/L | 7646 | 7.9 (537) | 7.9 (269) | 7.9 (268) | 1.00 (0.84–1.18) | |
| HbA1c < 6.0% | 7260 | 6.9 (454) | 7.5 (247) | 6.3 (207) | 1.18 (0.98–1.42) | 0.05 |
| HbA1c ≥ 6.0% | 7890 | 8.4 (593) | 8.2 (287) | 8.7 (306) | 0.96 (0.82–1.13) | |
CV, cardiovascular; CABG, coronary artery bypass grafting; TIMI, thrombolysis in myocardial infarction.
Outcomes in relation to diabetes status and randomized treatment
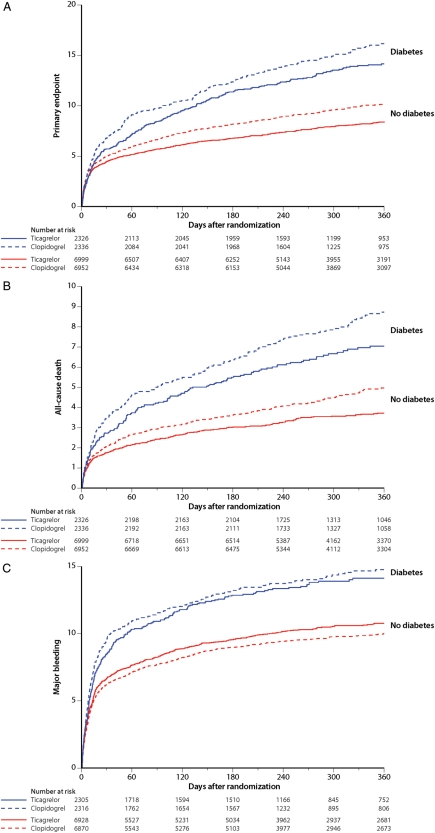
Ticagrelor significantly reduced the primary composite endpoint and also, separately, all-cause mortality, MI, and stent thrombosis in patients without DM (Figure 1A and B). In the smaller subgroup of patients with DM, this benefit was consistent with the overall trial results but did not reach nominal statistical significance. No diabetes status-by-treatment interaction was found (Table 4).
Figure 1.
Cumulative incidence of (A) the primary composite of cardiovascular death, myocardial infarction and stroke and (B) total mortality and (C) major bleeding in the ticagrelor (solid lines) and clopidogrel (dotted lines) groups in patients with diabetes at baseline (blue lines) and no diabetes (red lines).
Bleeding occurred with similar frequency in the ticagrelor and clopidogrel groups independent of DM status (Figure 1C). Interaction tests were not significant irrespective of bleeding type and definition (i.e. PLATO major, fatal or life threatening, or TIMI major). PLATO-defined major bleeding events unrelated to CABG were numerically more frequent in the ticagrelor group, whereas bleeding events related to CABG were numerically more frequent in the clopidogrel group irrespective of diabetic status and with no heterogeneity between the groups (Table 4).
Outcomes in relation to level of metabolic control and randomized treatment
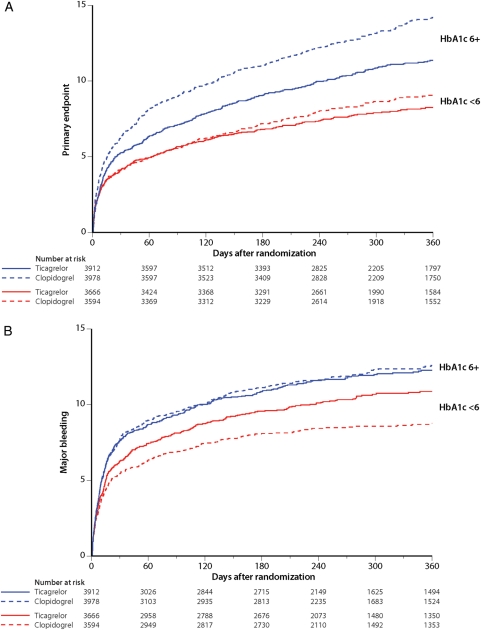
Higher serum levels of HbA1c and higher glucose levels were both strongly associated with a higher incidence of all evaluated ischaemic outcome variables and major bleeding. For patients with HbA1c levels above the median of 6%, the primary composite outcome was significantly reduced with ticagrelor vs. clopidogrel by 2.8% (20% relative), all-cause mortality by 1.8% (22% relative) but with similar major bleeding rates (Table 4, Figure 2). For patients with glucose levels above the median of 6.8 mmol/L, the primary composite endpoint was significantly reduced with ticagrelor vs. clopidogrel by 2.3% (15% relative), all-cause mortality by 1.8% (21% relative) but with a similar absolute major bleeding rate. There were no significant interactions for treatment-by-glucose or -HbA1c level. Also in patients without a diagnosis of diabetes at baseline above the median levels of glucose or HbA1c were associated with higher event rates, and the reduction in the primary endpoint and MI by ticagrelor was more pronounced in patients with levels of HbA1c above the median with significant treatment-by-HbA1c interactions (Supplementary material online, Table S8).
Figure 2.
Cumulative incidence of (A) the primary composite of cardiovascular death, myocardial infarction and stroke and (B) major bleeding in the ticagrelor (solid lines) and clopidogrel (dotted lines) groups in patients with levels of HbA1c at baseline above median of 6% (blue lines) and below median of 6% (red lines).
The outcome measures were also consistent across various subgroups of patients with DM with no interactions for the type of ACS (ST-elevation MI or non-ST-elevation ACS), initial intended treatment strategy (non-invasive or invasive treatment), and degree of renal function (data not shown).
Outcomes in relation to type of diabetes and randomized treatment
Insulin-treated patients had higher rates of all evaluated endpoints when compared with diabetic patients not on insulin (Table 5). The treatment effects of ticagrelor vs. clopidogrel were consistent with the overall trial results with no treatment-by-diabetes type (insulin-/non-insulin-treated or type 1/type 2) interaction. Thus, DM patients on insulin had a numerical 5.1% absolute (22% relative) reduction in the primary composite outcome. No significant difference was found in all-cause mortality or major bleeding (Table 5).
Table 5.
Outcome in randomized groups in relation to type of diabetes
| n | Overall | Ticagrelor | Clopidogrel | HR (95% CI) | P-value (interaction) | |
|---|---|---|---|---|---|---|
| CV death, MI, or stroke | ||||||
| Diabetes, no insulina | 3625 | 13.7 (468) | 13.1 (225) | 14.2 (243) | 0.93 (0.78–1.12) | 0.30 |
| Diabetes, insulina | 1036 | 20.3 (190) | 17.7 (84) | 22.8 (106) | 0.78 (0.58–1.03) | |
| Diabetes, type 1 | 209 | 14.4 (28) | 12.4 (13) | 16.4 (15) | 0.78 (0.37–1.63) | 0.73 |
| Diabetes, type 2 | 4451 | 15.2 (631) | 14.2 (296) | 16.1 (335) | 0.89 (0.76–1.04) | |
| All-cause death | ||||||
| Diabetes, no insulina | 3625 | 7.0 (238) | 6.2 (105) | 7.8 (133) | 0.79 (0.61–1.03) | 0.66 |
| Diabetes, insulina | 1036 | 10.9 (102) | 10.0 (48) | 11.7 (54) | 0.88 (0.60–1.30) | |
| Diabetes, type 1 | 209 | 3.9 (8) | 4.6 (5) | 3.1 (3) | 1.53 (0.37–6.41) | 0.39 |
| Diabetes, type 2 | 4451 | 8.1 (333) | 7.2 (148) | 9.0 (185) | 0.81 (0.65–1.00) | |
| Major bleeding | ||||||
| Diabetes, no insulina | 3593 | 14.2 (458) | 13.8 (217) | 14.7 (241) | 0.91 (0.76–1.09) | 0.28 |
| Diabetes, insulina | 1027 | 15.2 (134) | 15.1 (70) | 15.1 (64) | 1.12 (0.80–1.58) | |
| Diabetes, type 1 | 208 | 14.8 (29) | 18.0 (19) | 11.1 (10) | 1.79 (0.83–3.86) | 0.08 |
| Diabetes, type 2 | 4412 | 14.4 (563) | 13.9 (268) | 14.9 (295) | 0.92 (0.78–1.09) | |
CV, cardiovascular; MI, myocardial infarction.
aOn insulin vs. no insulin treatment before index event.
Discussion
The present study confirms the increased risk of adverse ischaemic events, mortality, and bleeding associated with DM in patients treated for ACS as shown in several other clinical trials and databases.1 In the present study, patients with DM had more often several high-risk criteria including reduced renal function, and less often underwent angiography and PCI when compared with non-diabetic patients. Despite good adherence to guidelines concerning pharmacological therapies and invasive procedures in patients with DM, the mortality was 80% higher than in patients without DM. The risk of MI, stent thrombosis, and major bleeding were also considerably higher. Furthermore, patients with insulin-treated diabetes had a 50% higher mortality rate compared with DM patients not on insulin.
We demonstrated that a more potent and consistent inhibition of platelet aggregation with ticagrelor reduced ischaemic events and mortality with no significant increase in overall major bleeding complications. Non-CABG-related major bleeding events were, however, more frequent than in the clopidogrel group. These findings were consistent among both diabetic and non-diabetic patients.
Prasugrel compared with clopidogrel administered after angiography reduced the primary endpoint of CV death, MI, or stroke by 4.8% (30% relative) in diabetic patients in a subgroup analysis of the TRITON–TIMI 38 trial.17 Ticagrelor compared with clopidogrel administered prior to, or at the time of, randomization with higher doses in the PLATO trial reduced the primary endpoint in diabetic patients by 2.1% (23% relative) that did not reach nominal statistical significance. Similar to the subgroup analysis of prasugrel vs. clopidogrel in the TRITON–TIMI 38 trial, there was no statistically significant interaction for the primary outcome by DM status or by diabetes type. However, patients with above the median levels of HbA1c at randomization experienced a significant 2.8% absolute (30% relative) reduction in the primary composite endpoint with ticagrelor vs. clopidogrel. All-cause death was numerically reduced with ticagrelor in patients with diabetes and significantly in patients with above the median levels of HbA1c or glucose with no significant diabetes-by-randomized group interactions. Furthermore, in patients with above the median levels of HbA1c or glucose ticagrelor significantly reduced the rate of MI with 1.8% (22 and 21% relative). In the TRITON–TIMI 38 trial, there was a numerical reduction in CV death and a significant reduction in MI with 5% (40% relative) with prasugrel vs. clopidogrel in diabetic patients. The relatively lower reduction in MI with ticagrelor in the PLATO trial compared with prasugrel in the TRITON–TIMI 38 trial may be explained by the higher average loading dose of clopidogrel in the clopidogrel arm and pre-treatment with clopidogrel in half of the patients in the ticagrelor arm.19 Furthermore, the TRITON–TIMI 38 trial results depend on early periprocedural MI determined by enzyme changes alone, detection of which was facilitated by delay of subject enrolment until after coronary angiography. PLATO enrolled patients soon after the index event, making early MI detection more difficult. Thus, any apparent difference in MI results between trials likely results from study design rather than actual outcome. Therefore, any comparison between PLATO and TRITON regarding early ischaemic events should be performed with caution.
No significant interactions for diabetes status with the respective primary composite outcomes were found in the CURE trial (clopidogrel vs. placebo in unstable angina) or in the CURRENT OASIS 7 trial20 (evaluating high- vs. low-dose clopidogrel in patients undergoing PCI). Furthermore, neither of these trials could demonstrate statistically significant differences between the randomized treatment arms concerning the primary composite endpoint or any of the secondary outcome events.
Given high platelet reactivity levels in diabetic patients,9 it remains an open question whether a higher dose of ticagrelor could have resulted in greater clinical benefit in terms of reduction in ischaemic events in the current trial. However, predicted steady-state plasma exposure of ticagrelor and its active metabolite were not different in patients with or without DM (AZ internal data). Nevertheless, very high levels of platelet inhibition may not be sufficient for adequate protection against ischaemic events in patients with DM. The prothrombotic condition that DM constitutes21 may require anti-thrombin or other long-term anti-coagulation therapy for a more general prevention of CV events among these high-risk patients.
The overall major bleeding rate was 4% higher (40% relative) in patients with vs. without DM. Still in patients with DM, there was no significant difference in major bleeding rates between ticagrelor and clopidogrel neither in patients with DM nor in patients with a poor glycaemic control. No significant differences in bleeding rates (irrespective of type or severity) were observed between the randomized treatment groups among patients with DM or with poor glycaemic control on admission.
Limitations
Although pre-specified, the present study is a subgroup analysis of the PLATO trial with its inherent limitations. The DM cohort of 4600 patients was not powered for showing a difference in the primary outcome between the randomized groups. However, the results were consistent with the overall trial results, and the analyses based on diabetes status and levels of glucose and HbA1c were pre-specified in the statistical analysis plan before any statistical analyses were performed. Furthermore, randomization was not stratified by diabetes status, type of diabetes, or level of glycaemic control. Still, baseline characteristics were well balanced between the randomized groups in patients with DM.
Conclusions
This pre-specified substudy from the PLATO trial showed that DM and higher levels of glucose and HbA1c were strongly associated with all evaluated ischaemic and bleeding endpoints and with higher risks of the primary outcome and mortality in patients on insulin treatment. Ticagrelor compared with clopidogrel reduced the primary composite outcome of CV death, MI, or stroke. Furthermore, total mortality and stent thrombosis were also reduced without any significant increase in overall major bleeding. These effects were seen irrespective of diabetic status, insulin treatment, and glycaemic control.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by AstraZeneca. Funding to pay the Open Access publication charges for this article was provided by Uppsala Clinical Research Center.
Conflict of interest: S.J.: research grants and advisory board fees from AstraZeneca, honoraria from AstraZeneca, Bristol-Myers Squibb, Schering-Plough, Merck, and Eli Lilly. D.J.A., Honoraria/Lectures: Bristol-Myers Squibb, sanofi-aventis, Eli Lilly and Company, Daiichi Sankyo, Inc.; Honoraria/Advisory board: Bristol-Myers Squibb, sanofi-aventis, Eli Lilly and Company, Daiichi Sankyo, Inc., Astra Zeneca, The Medicines Company, Portola Pharmaceuticals, Novartis, Arena Pharmaceuticals, Evolva Pharmaceuticals, Merck; Research Grants: GlaxoSmithKline, Otsuka, Accumetrics, Eli Lilly and Company, Daiichi Sankyo, Inc., The Medicines Company, AstraZeneca, Eisai, Portola Pharmaceutical, Schering-Plough, Johnson and Johnson, Bristol-Myers Squibb, sanofi-aventis. J.H.C.: consulting fees from Eli Lilly. D.E. and S.H.: research grants from AstraZeneca, Bristol-Myers Squibb, Pfizer, and Bayer; consultant fees from sanofi-aventis, Pfizer, and AstraZeneca. Dr Katus: consulting and lecture fees from AstraZeneca. F.K.: advisory board fees from AstraZeneca and Boehringer Ingelheim; consulting fees from AstraZeneca, Boehringer Ingelheim, and sanofi-aventis; grant support from Merck Sharp & Dohme (MSD) and Perseus Proteomics Inc.; lecture fees from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Pfizer, and sanofi-aventis. N.C.N.: consulting/advisory board fees from AstraZeneca, sanofi-aventis, and Schering-Plough; lecture fees from AstraZeneca, Eli Lilly, and sanofi-aventis. J.S. and R.F.S.: research grants from AstraZeneca, Dynabyte, Eli Lilly/Daiichi Sankyo alliance, and Schering-Plough; honoraria from AstraZeneca, Eli Lilly/Daiichi Sankyo alliance, Medscape, Novartis, GlaxoSmithKline, and Schering-Plough; consultant fees from AstraZeneca, Eli Lilly/Daiichi Sankyo alliance, Schering-Plough, Teva, Novartis, sanofi-aventis/Bristol-Myers Squibb, and The Medicines Company; travel support from AstraZeneca, Eli Lilly/Daiichi Sankyo alliance, and Schering-Plough. L.W.: research grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, and Schering-Plough; honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Schering-Plough, and Eli Lilly; consultant fees from Regado Biotechnologies, Athera Biotechnologies, Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, and Eli Lilly; lecture fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly. S.R.S.: no conflicts of interest. J.M.: employee of AstraZeneca and having equity ownership in AstraZeneca.
Supplementary Material
Acknowledgements
The complete list of PLATO investigators and main study committees has been published previously.
References
- 1.Norhammar A, Malmberg K, Diderholm E, Lagerqvist B, Lindahl B, Ryden L, Wallentin L. Diabetes mellitus: the major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits of revascularization. J Am Coll Cardiol. 2004;43:585–591. doi: 10.1016/j.jacc.2003.08.050. doi:10.1016/j.jacc.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 2.Norhammar A, Malmberg K, Ryden L, Tornvall P, Stenestrand U, Wallentin L. Under utilisation of evidence-based treatment partially explains for the unfavourable prognosis in diabetic patients with acute myocardial infarction. Eur Heart J. 2003;24:838–844. doi: 10.1016/s0195-668x(02)00828-x. doi:10.1016/S0195-668X(02)00828-X. [DOI] [PubMed] [Google Scholar]
- 3.Lim HS, Blann AD, Lip GY. Soluble cd40 ligand, soluble p-selectin, interleukin-6, and tissue factor in diabetes mellitus: relationships to cardiovascular disease and risk factor intervention. Circulation. 2004;109:2524–2528. doi: 10.1161/01.CIR.0000129773.70647.94. doi:10.1161/01.CIR.0000129773.70647.94. [DOI] [PubMed] [Google Scholar]
- 4.Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH, Investigators C. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS) Circulation. 2000;102:624–629. doi: 10.1161/01.cir.102.6.624. [DOI] [PubMed] [Google Scholar]
- 5.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaeffer JW, Smith EE, 3rd, Steward DE, Theroux P, Gibbons RJ, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Smith SC, Jr American College of Cardiology/American Heart Association Task Force on Practice G. ACC/AHA guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction–2002: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee on the management of patients with unstable angina) Circulation. 2002;106:1893–1900. doi: 10.1161/01.cir.0000037106.76139.53. doi:10.1161/01.CIR.0000037106.76139.53. [DOI] [PubMed] [Google Scholar]
- 6.Van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E, Fox KA, Julian D, Lengyel M, Neumann FJ, Ruzyllo W, Thygesen C, Underwood SR, Vahanian A, Verheugt FW, Wijns W Task Force on the Management of Acute Myocardial Infarction of the European Society of C. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The task force on the management of acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2003;24:28–66. doi: 10.1016/s0195-668x(02)00618-8. [DOI] [PubMed] [Google Scholar]
- 7.Angiolillo DJ, Bernardo E, Sabate M, Jimenez-Quevedo P, Costa MA, Palazuelos J, Hernandez-Antolin R, Moreno R, Escaned J, Alfonso F, Banuelos C, Guzman LA, Bass TA, Macaya C, Fernandez-Ortiz A. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2007;50:1541–1547. doi: 10.1016/j.jacc.2007.05.049. doi:10.1016/j.jacc.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 9.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Sabate M, Jimenez-Quevedo P, Hernandez R, Moreno R, Escaned J, Alfonso F, Banuelos C, Costa MA, Bass TA, Macaya C. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430–2435. doi: 10.2337/diabetes.54.8.2430. doi:10.2337/diabetes.54.8.2430. [DOI] [PubMed] [Google Scholar]
- 10.Angiolillo DJ, Shoemaker SB, Desai B, Yuan H, Charlton RK, Bernardo E, Zenni MM, Guzman LA, Bass TA, Costa MA. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the optimizing antiplatelet therapy in diabetes mellitus (OPTIMUS) study. Circulation. 2007;115:708–716. doi: 10.1161/CIRCULATIONAHA.106.667741. doi:10.1161/CIRCULATIONAHA.106.667741. [DOI] [PubMed] [Google Scholar]
- 11.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. doi:10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 12.Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, Teng R, Antonino MJ, Patil SB, Karunakaran A, Kereiakes DJ, Parris C, Purdy D, Wilson V, Ledley GS, Storey RF. Randomized double-blind assessment of the onset and offset of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the onset/offset study. Circulation. 2009;120:2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. doi:10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 13.Angiolillo DJF-OA, Bernardo E, Alfonso F, Macaya C, Bass TA, Costa MA. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49:1505–1516. doi: 10.1016/j.jacc.2006.11.044. doi:10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 14.Brandt JT, Payne CD, Wiviott SD, Weerakkody G, Farid NA, Small DS, Jakubowski JA, Naganuma H, Winters KJ. A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolite formation. Am Heart J. 2007;153:66.e9–66.e16. doi: 10.1016/j.ahj.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Erlinge D, Varenhorst C, Braun OÖ, James S, Wintrers KJ, Jakubowski JA, Brandt JT, Sugidachi A, Siegbahn A, Wallentin L. Patients with poor responsiveness to thienopyridine treatment or with diabetes have lower levels of circulating active metabolite, but their platelets respond normally to active metabolite added ex vivo. J Am Coll Cardiol. 2008;52:1968–1977. doi: 10.1016/j.jacc.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 16.Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, Goodman SG, Corbalan R, Purdy DA, Murphy SA, McCabe CH, Antman EM. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel–thrombolysis in myocardial infarction 38. Circulation. 2008;118:1626–1636. doi: 10.1161/CIRCULATIONAHA.108.791061. doi:10.1161/CIRCULATIONAHA.108.791061. [DOI] [PubMed] [Google Scholar]
- 17.Gurbel PA, Bliden KP, Butler K, Antonino MJ, Wei C, Teng R, Rasmussen L, Storey RF, Nielsen T, Eikelboom JW, Sabe-Affaki G, Husted S, Kereiakes DJ, Henderson D, Patel DV, Tantry US. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the respond study. Circulation. 2010;121:1188–1199. doi: 10.1161/CIRCULATIONAHA.109.919456. doi:10.1161/CIRCULATIONAHA.109.919456. [DOI] [PubMed] [Google Scholar]
- 18.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. doi:10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 19.James S, Akerblom A, Cannon CP, Emanuelsson H, Husted S, Katus H, Skene A, Steg PG, Storey RF, Harrington R, Becker R, Wallentin L. Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: rationale, design, and baseline characteristics of the platelet inhibition and patient outcomes (PLATO) trial. Am Heart J. 2009;157:599–605. doi: 10.1016/j.ahj.2009.01.003. doi:10.1016/j.ahj.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Metha SR. A randomized comparison of a clopidogrel high loading and maintenance dose regimen versus standard dose and high versus low dose aspirin in 25,000 patients with acute coronary syndromes: Results of the CURRENT OASIS 7 Trial. Oral presentation at ESC 2009, Barcelona. http://www.escardio.org/congresses/esc-2009/congress-reports/Pages/706003-706004-mehta-vandewerf.aspx. (17 August 2010) [Google Scholar]
- 21.Grant PJ. Diabetes mellitus as a prothrombotic condition. J Intern Med. 2007;262:157–172. doi: 10.1111/j.1365-2796.2007.01824.x. doi:10.1111/j.1365-2796.2007.01824.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.