Abstract
Aims
To examine the associations of several markers of adiposity and a wide range of cardiovascular risk factors and biomarkers in pre-pubertal children.
Methods and results
Four measures of adiposity,body mass index (BMI), waist circumference, dual-energy X-ray absorptiometry (DXA)-determined fat mass, and leptin concentration, were available in up to 7589 children aged 8.8–11.7 (9.9 mean) years from the Avon Longitudinal Study of Parents and Children (ALSPAC). Thirteen per cent of boys and 18.8% of girls were overweight, and 5.3% of boys and 5% of girls were obese. Body mass index was highly correlated with waist circumference (r = 0.91), DXA fat mass (r = 0.87), and leptin concentration (r = 0.75), and all had similar associations with cardiovascular risk factors. A 1 kg/m2 greater BMI was associated with 1.4mmHg (95% CI 1.25–1.44) higher systolic blood pressure (BP). In 5002 children, a 1 kg/m2 greater BMI was associated with a 0.05 mmol/L (95% CI 0.036–0.055) higher non-high-density lipoprotein (HDL) cholesterol and 0.03 mmol/L (95% CI −0.034 to −0.025) lower HDL cholesterol. There were also graded associations with apolipoproteins A1 and B, interleukin-6, and C-reactive protein. Comparing children who were obese with those who were normal weight, the odds ratio for hypertension was 10.7 (95% CI 7.2–15.9) for boys and 13.5 (95% CI 9.4–19.5) for girls.
Conclusion
In pre-pubertal UK children, overweight/obesity is common and has broadly similar associations with BP, HDL cholesterol, and non-HDL cholesterol to those observed in adults. Future research should evaluate whether effective interventions to maintain healthy weight in childhood could have important benefits for adult cardiovascular risk.
Keywords: Adiposity, BMI, Children, Cardiovascular risk, ALSPAC
Introduction
The prevalence of overweight and obesity among adults in high-income countries has increased almost three-fold in the last two decades.1,2 Increased adiposity is associated with an adverse cardiovascular risk profile, characterized by elevated blood pressure (BP), triglycerides, low-density lipoprotein (LDL) cholesterol, and reduced high-density lipoprotein (HDL) cholesterol. Beyond a threshold of 21 kg/m2, there is a continuous relationship between body mass index (BMI) and risk of death from coronary heart disease (CHD) in middle-aged adults.3 Adiposity in adults is also correlated with other biomarkers of cardiovascular risk such as apolipoproteins A1 and B, C-reactive protein, and interleukin-6 (IL-6).4–6
The prevalence of overweight and obesity doubled in young children in the UK between 1984 and 1947,7 reflecting a similar trend in most high- and middle-income countries. Although there is some suggestion that this increase is now slowing, it is too early to be certain of this.8 If unchecked, an increase in the population burden of cardiovascular disease as these children progress to adulthood can be predicted. A recently published systematic review showed that BMI is positively related to CHD risk from childhood onwards.9 In North America, severe obesity has already led to childhood cases of metabolic syndrome and type 2 diabetes,10 and children with these disorders exhibit an extremely adverse cardiovascular risk profile. There is also evidence on increasing childhood type 2 diabetes in the UK.11 However, less is known about the relationship between adiposity and cardiovascular risk factors in general populations of children or how these relationships compare with those recently reported in adults.3
Whether BMI is an adequate measure for assessing adiposity in childhood has been debated.12,13 Waist circumference provides a simple alternative measure that may be a better marker of central adiposity, being more closely associated with visceral fat mass, but BMI has the advantage of being better validated, more accurately measured, and accepted as a primary care measure in adults. More specific measures of adiposity include dual-energy X-ray absorptiometry (DXA) or plasma leptin concentration but these require specialist equipment or a blood sample.
We measured BMI, waist circumference, DXA fat mass, and leptin concentration in up to 7589 children between 9 and 12 years of age who were born in the Avon region of England in the early 1990s and who were participating in a long-term follow up programme. We examined the relationship with a wide range of cardiovascular risk factors and biomarkers. The aim of the study was to compare the magnitude of association of the four measurements of adiposity with a range of cardiovascular risk factors in children and to place the findings in the context of a recent large-scale study of BMI and cardiovascular risk factors in adult life.3
Methods
ALSPAC cohort
ALSPAC is a large longitudinal study of the childhood determinants of adult disease. The cohort and study design are described in detail elsewhere7 (http://www.alspac.bris.ac.uk). Briefly, 15 541 pregnant women with an expected delivery date between the start of April 1991 and the end of December 1992 were enrolled. The cohort of 14 062 live-born children has been followed up initially with questionnaires through childhood, and since the age of 7 at regular clinics. Between January 2001 and January 2003, 7725 children agreed to attend the clinic.
Approval for the study was obtained from the ALSPAC Law and Ethics Committee and the local research Ethics Committee. Written informed consent/assent was obtained from both the parent/guardian and the child.
Anthropometric measurements
Current age of the child was recorded in months as they arrived at the assessment clinic. Weight and height were measured in light clothing and without shoes. Childhood development and pubertal development was assessed using Tanner stages. Weight was measured to the nearest 0.1 kg using Tanita scales (Wardworth Ltd, Bolton, UK). Height was measured to the nearest 0.1 cm using a Harpenden stadiometer (Holtain Ltd, Crymych Pembrokeshire, UK). Waist circumference was measured to the nearest 1 mm at the mid-point between the lower ribs and the pelvic bone with a flexible tape. Fat mass was assessed using DXA. A Lunar Prodigy narrow fan-beam densitometer (GE Healthcare, Bedford, UK) was used to perform a whole-body DXA scan, where bone content and lean and fat mass were measured. Overweight and obesity were defined using age- and sex-specific BMI thresholds proposed by the International Obesity Task Force,14 which correspond to the adult BMI cutoffs of 25 and 30 kg/m2 for adult overweight and obesity.
Measurement of cardiovascular risk factors
Blood pressure was recorded in the right arm in the seated position using a Dinamap 9301 Vital Signs Monitor (Morton Medical, London, UK), and the mean of two values were used for analysis. A child-size cuff (upper arm circumference 12–19 cm) or a small adult-size cuff (17–25 cm) was used depending on the size of the upper arm circumference. Non-fasting blood samples were taken using standard procedures, with samples immediately spun and frozen at −80°C. The measurements were assayed in 2008 after a median of 7.5 years in storage with no previous freeze–thaw cycles during this period. Plasma lipids (total cholesterol, triglycerides, and HDL cholesterol) were performed by modification of the standard Lipid Research Clinics Protocol using enzymatic reagents for lipid determination. Apolipoprotein A1 and B were measured by immunoturbidimetric assays (Hitachi/Roche). Leptin was measured by an in-house enzyme-linked immunosorbent assay (ELISA) validated against commercial methods. Adiponectin and IL-6 were measured by ELISA (R&D systems, Abingdon, UK), and C-reactive protein was measured by automated particle-enhanced immunoturbidimetric assay (Roche UK, Welwyn Garden City, UK). All assay coefficients of variation were <5%. Non-HDL cholesterol was calculated as total cholesterol minus HDL cholesterol.
The amount of weekly vigorous physical activity was assessed by a self-completion questionnaire at 9 years of age, asking how many times a week the child participated in a range of activities including running, dance, gymnastics, netball, swimming, and aerobics.
Definitions of hypertension, hypertriglyceridaemia, hypercholesterolaemia, and low-high-density lipoprotein cholesterol
Hypertension was defined as systolic or diastolic BP above the 95th percentile, using age- and height-specific cut-points defined by the National High Blood Pressure Education Program Working Group on High Blood Pressure.15 Levels of triglycerides >1.7 mmol/L were considered hypertriglyceridaemia, whereas HDL-cholesterol was defined as low if <1.03 mmol/L, as recommended by the International Diabetes Federation (IDF).16
Statistical analysis
We conducted all analyses using Stata 10. We assessed the distribution of continuous variables and conducted log transformations for DXA, leptin, triglycerides, C-reactive protein, and IL-6, which were not normally distributed. We used the Pearson correlation coefficient and linear regression analysis to evaluate the associations among adiposity measures (BMI, waist circumference, leptin, and DXA) and of these measures with cardiovascular risk factors. To facilitate comparison of the association of adiposity measures with cardiovascular risk factors, we estimated the difference in each risk factor for a standard deviation difference in each adiposity measure. We used logistic regression analysis to estimate odds ratios for hypertension, hypertriglyceridaemia, and low-HDL cholesterol in overweight and obese children in comparison with children of normal weight. Analyses were restricted to participants with complete data on the marker of interest, and results are presented separately for boys and girls.
Results
More than 13 600 children were invited to attend the clinic between January 2001 and January 2003 and 7725 agreed. Among these, 7589 (98%) had a valid BMI and waist circumference, 7220 (93%) DXA measurement, 7481 (97%) BP, and 5002 (65%) blood sample measurements. Eighty-two per cent of boys and 55% of girls were in the pre-pubertal stage (Tanner scale I) and 99% of boys and 90% of girls were in Tanner scale I or II. Children who attended the clinic were higher educated, had older mothers, and lived in owner-occupied housing. Children who attended also had a slightly higher mean birthweight (unpublished data).
Distribution of body mass index and other adiposity measures and proportion of normal weight, overweight, and obese children
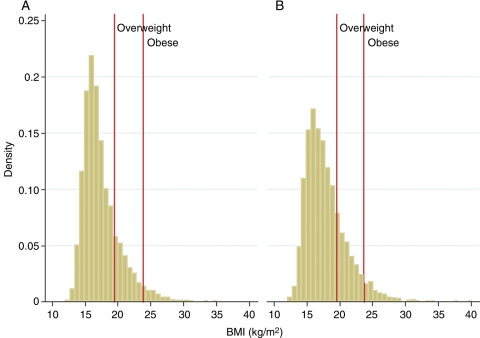
The anthropometric characteristics, and mean values of the measured indices of adiposity among the children studied (mean age 9.9 years; 51% girls), are shown in Table 1. Body mass index in both sexes exhibited a slight rightward skew in its distribution, but transformations did not improve the normality and linearity assumption. Using consensus thresholds for BMI from the International Obesity Task Force, it was found that 15.9% of the children were overweight (13% of the boys and 18.8% of the girls) and 5.2% were obese (5.3% of the boys and 5% of the girls) (Figure 1).
Table 1.
Measures of adiposity and cardiovascular risk factors in the study participants, by body mass index group and sex
| n | Normal weight | Overweight | Obese | Total | |
|---|---|---|---|---|---|
| Male (n) | 3063 | 487 | 199 | ||
| BMI (kg/m2) | 3749 | 16.4 (1.4) | 21 (1.1) | 25 (2.2) | 17.5 (2.8) |
| Waist circumference (cm) | 3743 | 60.41 (4.28) | 72.53 (5.17) | 83.02 (6.89) | 63.18 (7.71) |
| DXA total fat (g)a | 3570 | 5031 (0.46) | 13 162 (0.23) | 20 001(0.22) | 6134 (0.6) |
| Systolic BP (mmHg) | 3694 | 101.1 (8.4) | 107.9 (8.3) | 112 (10.4) | 102.5 (9.1) |
| Diastolic BP (mmHg) | 3694 | 56.7 (6.3) | 59 (5.8) | 61.3 (6.8) | 57.2 (6.4) |
| Total cholesterol (mmol/L) | 2543 | 4.19 (0.65) | 4.3 (0.67) | 4.21 (0.68) | 4.2 (0.65) |
| LDL cholesterol (mmol/L) | 2543 | 2.23 (0.57) | 2.4 (0.61) | 2.36 (0.57) | 2.26 (0.58) |
| HDL cholesterol (mmol/L) | 2543 | 1.47 (0.31) | 1.32 (0.28) | 1.18 (0.27) | 1.44 (0.31) |
| Triglycerides (mmol/L)a | 2543 | 0.97 (0.44) | 1.14 (0.43) | 1.32 (0.45) | 1 (0.44) |
| Leptin (ng/mL)a | 2543 | 3.86 (0.58) | 12.13 (0.53) | 21.05 (0.52) | 4.78 (0.75) |
| C-reactive protein (mg/L)a | 2543 | 0.18 (1.12) | 0.46 (1.17) | 1.03 (1.16) | 0.22 (1.21) |
| IL-6 (pg/mL)a | 2535 | 0.71 (0.89) | 1.02 (0.74) | 1.37 (0.67) | 0.77 (0.88) |
| Apolipoprotein A1 (mg/dL) | 2543 | 139.6 (20.44) | 133.4 (18.69) | 128.9 (18.14) | 138.4 (20.33) |
| Apolipoprotein B (mg/dL) | 2543 | 56.26 (12.27) | 60.28 (13.3) | 62.12 (13.44) | 57.01 (12.56) |
| Adiponectin (µg/mL) | 2541 | 12.97 (5.3) | 12.12 (5.16) | 10.3 (4.37) | 12.74 (5.24) |
| Participation in vigorous physical activity during past month (%) | |||||
| Never or less than once a week | 134 | 4.3 | 9.4 | 6.3 | 5.0 |
| One to three times a week | 1082 | 38.9 | 48.5 | 47.2 | 40.6 |
| Four to six times a week | 816 | 31.8 | 24.9 | 25.7 | 30.6 |
| Daily | 635 | 25.0 | 17.3 | 20.8 | 23.8 |
| Female (n) | 2924 | 723 | 193 | ||
| BMI (kg/m2) | 3840 | 16.6 (1.6) | 21.2 (1.2) | 26 (2.4) | 17.9 (3) |
| Waist circumference (cm) | 3837 | 59.31 (4.6) | 70.91 (4.94) | 82.41 (6.58) | 62.66 (7.99) |
| DXA total fat (g)a | 3650 | 7003 (0.39) | 14 779 (0.19) | 22 980 (0.17) | 8556 (0.51) |
| Systolic BP (mmHg) | 3787 | 101.2 (8.7) | 107.7 (9.1) | 113.9 (10.7) | 103.1 (9.6) |
| Diastolic BP (mmHg) | 3787 | 57.1 (6.3) | 59.5 (6.1) | 61.7 (5.9) | 57.7 (6.4) |
| Total cholesterol (mmol/L) | 2459 | 4.33 (0.64) | 4.37 (0.72) | 4.5 (1.12) | 4.34 (0.69) |
| LDL cholesterol (mmol/L) | 2459 | 2.42 (0.58) | 2.5 (0.69) | 2.66 (0.89) | 2.44 (0.62) |
| HDL cholesterol (mmol/L) | 2459 | 1.41 (0.29) | 1.25 (0.29) | 1.1 (0.27) | 1.36 (0.3) |
| Triglycerides (mmol/L)a | 2459 | 1.01 (0.4) | 1.22 (0.46) | 1.44 (0.49) | 1.06 (0.42) |
| Leptin (ng/mL)a | 2459 | 5.88 (0.57) | 16.31 (0.49) | 30.55 (0.4) | 7.59 (0.74) |
| C-reactive protein (mg/L)a | 2459 | 0.28 (1.1) | 0.61 (1.19) | 1.36 (1.12) | 0.34 (1.19) |
| IL-6 (pg/mL)a | 2457 | 0.87 (0.85) | 1.26 (0.71) | 1.8 (0.6) | 0.96 (0.84) |
| Apolipoprotein A1 (mg/dL) | 2459 | 135.3 (19.36) | 128.7 (18.69) | 122 (18.34) | 133.6 (19.52) |
| Apolipoprotein B (mg/dL) | 2459 | 60.54 (12.96) | 64.25 (15.39) | 68.16 (14.73) | 61.54 (13.65) |
| Adiponectin (µg/mL) | 2458 | 14.06 (5.66) | 11.97 (5.12) | 10.51 (4.63) | 13.53 (5.61) |
| Participation in vigorous physical activity during past month (%) | |||||
| Never or less than once a week | 170 | 5.0 | 7.0 | 12.9 | 5.7 |
| One to three times a week | 1697 | 56.0 | 61.5 | 62.1 | 57.3 |
| Four to six times a week | 821 | 29.1 | 24.8 | 17.9 | 27.7 |
| Daily | 273 | 10.0 | 6.7 | 7.1 | 9.2 |
aValues are arithmetic or geometric mean (SD). Overweight and obesity were defined using the International Classification Standards.14
Figure 1.
Body mass index distribution by sex : (A) males; (B) females. Lines for overweight and obese are approximations, as the cutoffs are not fixed but age–height specific.
Relationship between body mass index and other measures of adiposity
Waist circumference, DXA fat mass, and leptin concentration were all higher among overweight and obese children, defined on the basis of BMI, than among children of normal weight (Table 1). All of the adiposity measures exhibited strong linear correlations with one another over the whole range of values (Table 2).
Table 2.
Correlations between different measures of adiposity and cardiovascular risk factors and biomarkers
| BMI |
Waist circumference |
DXA total fat |
Leptin |
|||||
|---|---|---|---|---|---|---|---|---|
| Correlation coefficient | P-value | Correlation coefficient | P-value | Correlation coefficient | P- value | Correlation coefficient | P- value | |
| Adiposity measures | ||||||||
| BMI | 1 | |||||||
| Waist circumference | 0.91 | <0.001 | 1 | |||||
| DXA total fata | 0.87 | <0.001 | 0.83 | <0.001 | 1 | |||
| Leptina | 0.75 | <0.001 | 0.7 | <0.001 | 0.81 | <0.001 | 1 | |
| Cardiovascular risk factors and biomarkers | ||||||||
| Systolic BP | 0.42 | <0.001 | 0.41 | <0.001 | 0.41 | <0.001 | 0.35 | <0.001 |
| Diastolic BP | 0.22 | <0.001 | 0.22 | <0.001 | 0.23 | <0.001 | 0.21 | <0.001 |
| Total cholesterol | 0.07 | <0.001 | 0.04 | 0.004 | 0.11 | <0.001 | 0.14 | <0.001 |
| Triglyceridesa | 0.22 | <0.001 | 0.23 | <0.001 | 0.21 | <0.001 | 0.26 | <0.001 |
| LDL cholesterol | 0.13 | <0.001 | 0.09 | <0.001 | 0.18 | <0.001 | 0.18 | <0.001 |
| HDL cholesterol | −0.28 | <0.001 | −0.29 | <0.001 | −0.28 | <0.001 | −0.24 | <0.001 |
| Apolipoprotein A1 | −0.19 | <0.001 | −0.2 | <0.001 | −0.19 | <0.001 | −0.12 | <0.001 |
| Apolipoprotein B | 0.18 | <0.001 | 0.14 | <0.001 | 0.22 | <0.001 | 0.2 | <0.001 |
| C-reactive proteina | 0.41 | <0.001 | 0.38 | <0.001 | 0.44 | <0.001 | 0.41 | <0.001 |
| IL-6a | 0.23 | <0.001 | 0.23 | <0.001 | 0.25 | <0.001 | 0.24 | <0.001 |
| Adiponectin | −0.16 | <0.001 | −0.15 | <0.001 | −0.06 | <0.001 | −0.04 | 0.005 |
Values are Pearson correlation coefficients.
aLog-transformed.
Relationship between overweight and obesity and cardiovascular risk factors and biomarkers
Body mass index, waist circumference, log DXA total fat mass, and log leptin concentration were all linearly associated with cardiovascular risk factors and biomarkers, and the magnitudes of the associations were generally similar. However, lean DXA mass had a slightly weaker association with the other risk factors and a negative relationship with total cholesterol (Table 3).
Table 3.
Change in risk factors for an SD difference in the adiposity measurements
| BMI (kg/m2) | Waist circumference (cm) | Leptina (ng/mL) | DXA total fata (g) | DXA central fata (g) | DXA lean massa (g) | |
|---|---|---|---|---|---|---|
| Males (n= 2414) | ||||||
| Systolic BP (mmHg) | 3.8 | 3.7 | 3.1 | 3.5 | 3.5 | 3.4 |
| Diastolic BP (mmHg) | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 0.9 |
| Total cholesterol (mmol/L) | 0.04 | 0.03 | 0.08 | 0.05 | 0.05 | −0.06 |
| Triglycerides (mmol/L)a | 0.09 | 0.09 | 0.10 | 0.06 | 0.07 | 0.02 |
| LDL cholesterol (mmol/L) | 0.08 | 0.06 | 0.08 | 0.08 | 0.08 | (−0.03) |
| HDL cholesterol (mmol/L) | −0.08 | −0.08 | −0.06 | −0.06 | −0.07 | −0.05 |
| Leptin (ng/mL)a | 0.60 | 0.57 | — | 0.58 | 0.58 | 0.27 |
| C-reactive protein (mg/L)a | 0.49 | 0.48 | 0.44 | 0.45 | 0.46 | 0.15 |
| IL-6 (pg/mL)a | 0.17 | 0.18 | 0.16 | 0.16 | 0.17 | 0.05 |
| Alipoprotein A1 (mg/dL) | −3.03 | −3.11 | −1.07 | −2.32 | −2.33 | −2.55 |
| Alipoprotein B (mg/dL) | 2.09 | 1.8 | 1.9 | 1.8 | 2.0 | −0.96 |
| Adiponectin (µg/mL) | −0.58 | −0.46 | (−0.01) | (−0.07) | (−0.19) | −0.62 |
| Females (n = 2331) | ||||||
| Systolic BP (mmHg) | 3.8 | 3.7 | 3.8 | 4.7 | 4.6 | 3.5 |
| Diastolic BP (mmHg) | 1.3 | 1.3 | 1.5 | 1.7 | 1.7 | 0.9 |
| Total cholesterol (mmol/L) | 0.04 | 0.03 | 0.09 | 0.06 | 0.07 | −0.04 |
| Triglycerides (mmol/L)a | 0.11 | 0.12 | 0.13 | 0.12 | 0.12 | 0.07 |
| LDL cholesterol (mmol/L) | 0.06 | 0.05 | 0.09 | 0.09 | 0.09 | (−0.03) |
| HDL cholesterol (mmol/L) | −0.09 | −0.10 | −0.08 | −0.10 | −0.10 | −0.06 |
| Leptin (ng/mL)a | 0.60 | 0.55 | — | 0.70 | 0.68 | 0.29 |
| C-reactive protein (mg/L)a | 0.47 | 0.46 | 0.51 | 0.58 | 0.58 | 0.10 |
| IL-6 (pg/mL)a | 0.20 | 0.21 | 0.21 | 0.22 | 0.22 | 0.07 |
| Apolipoprotein A1 (mg/dL) | −3.9 | −4.5 | −2.5 | −4.2 | −4.4 | −3.4 |
| Apolipoprotein B (mg/dL) | 2.20 | 2.00 | 2.50 | 2.70 | 3.00 | (−0.34) |
| Adiponectin (µg/mL) | −1.24 | −1.26 | −0.79 | −1.14 | −1.24 | −1.19 |
Linear regression coefficients for one z-score increase in adiposity measurements adjusted by age.
Analysis was restricted to 2414 males and 2331 females with all valid adiposity measurements.
Values in parentheses are statistically not significant (P ≥ 0.05).
aLog-transformed.
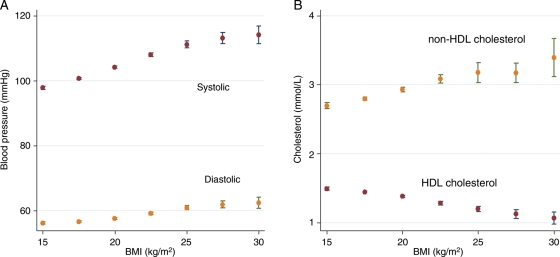
In males, a 1 kg/m2 higher BMI was associated with a 1.35mmHg (95% CI 1.25–1.44) higher systolic BP, 0.5 mmHg (95% CI 0.42–0.57) higher diastolic BP, a 0.05 mmol/L (95% CI 0.036–0.055) higher non-HDL-cholesterol concentration and a 0.03 mmol/L (95% CI −0.034 to −0.025) lower HDL-cholesterol concentration. The differences in these risk factors for a 1 kg/m2 difference in BMI are similar in magnitude to the associations observed in the Prospective Studies Collaboration analysis of data from 900 000 adults3 (Table 4, Figure 2). The same difference in BMI in boys was also associated with an apolipoprotein B concentration higher by 0.76 mg/dL (95% CI 0.58–0.95), a 6% higher IL-6 concentration (95% CI 5–7), an 18% higher C-reactive protein concentration (95% CI 16–19), and an apolipoprotein A1 concentration lower by 1.12 mg/dL (95% CI −1.42 to −0.82). Girls showed similar associations of BMI with risk factors to those in boys, with the exception of adiponectin, which, for a 1kg/m2 higher BMI, was 0.21 mg/mL lower in boys (95% CI −0.29 to −0.13) and 0.3 mg/mL lower in girls (95% CI −0.50 to −0.36).
Table 4.
Mean difference in risk factors for 1 kg/m2 higher body mass index, in children in ALSPAC and adults from the Prospective Studies Collaboration
| ALSPACa | Prospective Studies Collaborationb | |
|---|---|---|
| Males | ||
| Systolic BP (mmHg) | 1.35 (1.25–1.44) | 1.16 |
| Diastolic BP (mmHg) | 0.5 (0.42–0.57) | 0.98 |
| Total cholesterol (mmol/L) | 0.016 (0.007–0.026) | |
| Non-HDL cholesterol (mmol/L) | 0.05 (0.036–0.055) | 0.10 |
| HDL cholesterol (mmol/L) | −0.03 (−0.034 to −0.025) | −0.03 |
| Females | ||
| Systolic BP (mmHg) | 1.36 (1.27–1.45) | 1.04 |
| Diastolic BP (mmHg) | 0.47 (0.40–0.54) | 0.66 |
| Total cholesterol (mmol/L) | 0.016 (0.007–0.025) | |
| Non-HDL cholesterol (mmol/L) | 0.05 (0.039–0.057) | 0.08 |
| HDL cholesterol (mmol/L) | −0.03 (−0.036 to −0.028) | −0.03 |
aLinear regression coefficients adjusted by age from 3694 males and 3787 females for BP and 2543 males and 2459 females for cholesterol.
bData from the Prospective Studies Collaboration BMI and cause-specific mortality in 900000 adults: collaborative analyses of 57 prospective studies.3 Analyses were adjusted for age, smoking status, and study.
Figure 2.
Vascular risk factors vs. body mass index. (A) Blood pressure mean and confidence intervals for body mass index groups of 2.5 kg/m2. (B) Blood cholesterol mean and confidence intervals for body mass index groups of 2.5 kg/m2.
Overweight boys and girls were three times more likely to be above thresholds for childhood hypertension [odds ratio 3.3 (95% CI 2.2–4.8) in boys and 3.5 (95% CI 2.5–4.7) in girls], about twice more likely to have hypertriglyceridaemia [odds ratio 1.6 (95% CI 1.2–2.3) in boys and 2.4 (95% CI 1.8–3.2) in girls] and about three times more likely to have low-HDL cholesterol [odds ratio 2.5 (95% CI 1.7–3.6) in boys and 3.1 (95% CI 2.4–4.1) in girls] compared with normal-weight children. Being obese was associated with an even higher risk, with an odds ratio for hypertension of 10.7 (95% CI 7.2–15.9) in boys and 13.5 (95% CI 9.4–19.5) in girls; an odds ratio for hypertriglyceridaemia of 3.7 (95% CI 2.4–5.7) in boys and 5.9 (95% CI 3.9–8.9) in girls; and an odds ratio for low-HDL cholesterol of 7.5 (95% CI 4.9–11.6) in boys and 10.2 (95% CI 6.8–15.3) in girls (Table 5).
Table 5.
Prevalence and odds ratios for hypertension and hyperlipidaemia in overweight and obese boys and girls
| Normal weight | Over weight | Obese | Total | ||
|---|---|---|---|---|---|
| Males | |||||
| Hypertension (n= 3694) | % | 2.8 | 8.6 | 23.4 | 4.6 |
| Odds ratios (95% CI) | 1 | 3.3 (2.2–4.8) | 10.7 (7.2–15.9) | ||
| High tryglicerides (≥1.7 mmol/L) (n= 2543) | % | 10.62 | 16.29 | 30.63 | 12.19 |
| Odds ratios (95% CI) | 1 | 1.6 (2.2–4.8) | 3.7 (2.4–5.7) | ||
| Low-HDL cholesterol (<1.03 mmol/L) (n= 2543) | % | 6.5 | 14.7 | 34.2 | 8.7 |
| Odds ratios (95% CI) | 1 | 2.5 (1.7–3.6) | 7.5 (4.9–11.6) | ||
| One or more risk factors | % | 17.6 | 33.7 | 57.3 | 21.3 |
| Two or more risk factors | % | 2.4 | 6.1 | 21.8 | 3.7 |
| Females | |||||
| Hypertension (n= 3790) | % | 3.3 | 10.7 | 31.8 | 6.1 |
| Odds ratios (95% CI) | 1 | 3.5 (2.5–4.7) | 13.5 (9.4–19.5) | ||
| High tryglicerides (≥1.7 mmol/L) (n= 2459) | % | 10.05 | 21.32 | 39.81 | 13.38 |
| Odds ratios (95% CI) | 1 | 2.4 (1.8–3.2) | 5.9 (3.9–8.9) | ||
| Low-HDL cholesterol (<1.03 mmol/L) (n= 2459) | % | 9 | 23.6 | 50 | 13.4 |
| Odds ratios (95% CI) | 1 | 3.1 (2.4–4.1) | 10.2 (6.8–15.3) | ||
| One or more risk factors | % | 19 | 38 | 68.2 | 24.6 |
| Two or more risk factors | % | 2.7 | 13.9 | 36.4 | 6.2 |
Hypertension was defined as systolic or diastolic BP above the 95th percentile, using age- and height-specific cutoff for the 95th percentile as defined by the National High Blood Pressure Education Program Working Group on High Blood Pressure Pediatrics.15 Analyses are unadjusted.
Normal-weight children had a higher frequency of participation in vigorous physical activity compared with overweight and obese, but no association was found between physical activity and being hypertensive, having low-HDL cholesterol, or high triglycerides, in any of the BMI categories.
Discussion
In this large study of children born in the 1990s, we identified a high prevalence of overweight and obesity, based on standardized and validated cut-point values for BMI. Obesity increased the risks of childhood hypertension over 10-fold and dyslipidaemia by more than three-fold; the risks were also substantial for overweight children.
There was an incremental, largely linear relationship of BMI with each of the risk factors and biomarkers evaluated, the shape and gradient of which were similar to that seen in adulthood. Similar magnitudes of association (also largely linear) were seen for other measurements of adiposity in this large cohort of children. The patterns of correlation among adiposity measures and biomarkers, with higher values of BP, non-HDL cholesterol, apolipoprotein B, C-reactive protein, and IL-6, and lower values of HDL cholesterol and apolipoprotein A1, also mirrored those seen in adulthood. These findings suggest that adiposity begins to promote an adverse cardiovascular and metabolic profile from early in life. The similar patterns of correlation in childhood and later life also suggest that adiposity exerts similar adverse effects on vascular risk factors from early life to adulthood and beyond.
There has been concern that BMI may be an imprecise measure of adiposity in children because it does not distinguish between lean and fat mass. A recent study in children aged 9–11 years (in which cardiovascular risk factors and biomarkers were not reported) compared the association of BMI and waist circumference index with DXA-determined total fat and lean mass and distribution of fat mass. It concluded ‘body mass index, rather than waist circumference index, would be a better screening tool for total and truncal fat mass in both sexes before puberty'.17 That study, however, did not compare associations of the three measurements of childhood adiposity with cardiovascular risk factor traits.17 We also found BMI to be well correlated with other indices of adiposity, including waist circumference, leptin concentration, and DXA fat mass. Using quartiles of the distributions of the different adiposity measures, we found that, of the overweight and obese children, who, by definition, were all in the top quartiles of BMI, 88% were in the top quartile of waist circumference, 91% were in the top quartile of DXA fat mass, and 80% were in the top quartile of leptin distribution (Supplementary material online, Table S1). Each of the measures also exhibited a broadly similar, incremental association with BP and blood lipids over the whole range of values. Given the greater ease, reliability, and feasibility18 of measuring weight and height, compared with waist circumference or DXA fat mass (or taking and analysing a blood sample for leptin), our findings support the continued use of BMI in public health surveillance and clinical practice in children.
Above a threshold value of 21 kg/m2, a higher BMI was associated with a higher risk of cardiovascular events and with both cardiovascular and non-cardiovascular mortality in a participant level meta-analysis of data from 900 000 adults (aged 15–89) from the general population.3 The association with cardiovascular events above this threshold was accompanied by a graded continuous association with several cardiovascular risk factors and biomarkers including BP and HDL and non-HDL cholesterol. These associations are strikingly similar to those we observed in children in the current study.
There are good reasons for thinking that the associations in adulthood are causal. First, surgery to reduce obesity is associated with a correction of an adverse profile of risk factors and with a reduction in cardiovascular events.19,20 Second, common genetic variants in the FTO gene that influence BMI have also been associated with alterations in BP, lipids, and inflammation markers, as well indices of glycaemic control, which are congruent with the observed associations of BMI itself.21 It has, however, been more difficult to establish a causal relationship between childhood overweight and obesity and clinical events in late life because the extended time span between the exposure and outcome imposes practical limitations. In two large record linkage studies, there was an association between higher BMI in childhood or adolescence (measured between ages 7–13 in one study22 and mean age 18 in the second23) and CHD mortality. The risk was linear across the BMI distribution, but the magnitude of the association increased with older age at BMI measurement. If the association of childhood BMI and CHD mortality is wholly or primarily explained by its association with adult BMI, interventions to reduce adiposity at any stage in the life course (determined by when interventions were likely to be most effective) might be appropriate.24 However, if greater adiposity in childhood is already having adverse effects on vascular and metabolic function, then there is a rationale for preventing or treating overweight/obesity in childhood in order to reduce future cardiovascular risk.24 Neither of these record linkage studies had information on cardiovascular risk factors or adult BMI and were therefore unable to explore these possibilities. Other studies have shown that obese children have higher BP, dyslipidaemia, higher fasting glucose and insulin, and a more atherosclerotic risk profile.10,25 Body mass index and waist circumference are also associated with higher BP in childhood, with these associations being continuous across the distribution of BMI or waist circumference.26
Body mass index in childhood tracks with a higher BMI in adulthood,27 and overweight and obesity at any age reflect preceding energy excess. All interventions of proven efficacy in reducing BMI, e.g. reduced energy diets,28,29 drugs that reduce appetite or induce fat malabsorption,30,31 bariatric surgery32 (that all reduce energy intake), or exercise regimen33 (that increases calorie usage), lead to a net energy deficit. The absolute energy deficit required to normalize BMI in adulthood is likely to be much greater than the energy deficit required to achieve normalization in childhood, because there has been less time for accumulation, arguing in favour of prevention and/or early intervention to reduce childhood overweight and obesity as a public health problem. Few obesity prevention strategies have been shown to be effective34 but strategies promoting sustained behavioural changes that encompass lower energy intake, greater physical activity, and less sedentary time, may be effective for weight reduction.35,36 Surgery and drug treatments are less desirable options in children, making research on the efficacy of interventions that promote improvements in diet and exercise in childhood a priority. It has already been shown in the ALSPAC cohort that moderate-to-higher intensity physical activity is associated with lower levels of fat mass in early adolescence.37 The optimal interventional strategy, and whether to target children with more extreme elevations in BMI, to reduce the average BMI among all children using a broader public health strategy, or both, requires evaluation.
Some of the limitations of our study are noteworthy. Our sample of children was representative but drawn from only one region of the British Isles. It is unclear whether the magnitude or shape of the associations of BMI and cardiovascular risk factors we observed would be the same in other regions, or in other countries with similar demography. Children who attended the clinic were more highly educated, had older mothers, and more lived in owner-occupied housing. Children who attended also had a slightly higher mean birthweight. This selective loss to follow-up may mean that we have underestimated the prevalence of overweight and obesity. Moreover, the drop-out in blood testing was slightly higher in obese and overweight children (56% of obese and 62% of overweight had valid blood results compared with 67% in normal-weight children). Although the children we studied in ALSPAC are a contemporary cohort in comparison with childhood cohorts studied previously, the analyses are nevertheless based on data obtained nearly a decade ago. Dietary and lifestyle habits are likely to have changed somewhat in the last decade and so our findings may not extrapolate directly to current British 9-year-olds.
White coat hypertension in children is recognized and our BP measurements were clinic-based. However, we have no reason to suspect that the white coat effect should differ systematically according to BMI categories and that the white coat syndrome would confound the association of BMI with high BP.
Although there was an association between levels of physical activity and being overweight/obese, this did not influence the association between obesity and the cardiovascular risk factors. But it is worth noticing that we may have used an insensitive self-reported rather than objective measure of physical activity.
In summary, we have shown that, in 9-year-old children, a higher BMI is already associated with adversely altered cardiovascular risk factors. Moreover, the associations recapitulate those seen in adulthood in their direction, shape, gradient, and pattern. The associations with LDL cholesterol, apolipoprotein B, and BP are noteworthy because these risk factors are considered to have a causal role in cardiovascular disease though the causal relevance of the other biomarkers is less certain. Although the alterations observed in childhood are occurring far in advance of the period of life at which clinical events occur, and the absolute risk of a cardiovascular event below the age of 45 years has been low, atheroma is known to accumulate from as early as the second decade of life.38
Future research should evaluate whether interventions to reduce adiposity in children may be a valuable investment in the health of future generations.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The ALSPAC study was funded by the Medical Research Council, the Wellcome Trust, the UK Department of Health, the Department of the Environment, the DFEE, the National Institutes of Health, and a variety of medical research charities and commercial companies. J.E.D. is supported by the British Heart Foundation; A.D.H. is supported by a BHF Senior Research Fellowship (FS05/125). The analyses in this paper have been conducted independently of the funders, who have not been involved in the decision to submit the paper for publication.
Conflict of interest: A.D.H. is on the editorial board of Drug and Therapeutics Bulletin, a BMJ Group Publication, has provided non-remunerated advice to GSK and London Genetics and received remuneration for speaking at educational meetings relating to risk factor management and primary prevention, most of which has been donated to charity. A.D.H. is principal applicant on an Medical Research Council research grant, with Pfizer as industrial partner. N.F. has received research grants and speakers fees from GSK, Novo Nordisk, Abbott Laboratories, Johnson and Johnson (Ethicon), and Amylin. J.E.D. has received honorarium for speaker programmes for Pfizer, Novartis, Merck, Zeneca, and Roche.
Supplementary Material
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALPSAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. We also thank Lynne Cherry and Pauline Watt for their excellent laboratory support.
References
- 1.Kipping RR, Jago R, Lawlor DA. Obesity in children. Part 1: epidemiology, measurement, risk factors and screening. BMJ. 2008;337:a1824. doi: 10.1136/bmj.a1824. doi:10.1136/bmj.a1824. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. doi:10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 3.Prospective Studies Collaborations. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. doi:10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. doi:10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83(suppl.):461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 6.Dervaux N, Wubuli M, Megnien JL, Chironi G, Simon A. Comparative associations of adiposity measures with cardiometabolic risk burden in asymptomatic subjects. Atherosclerosis. 2008;201:413–417. doi: 10.1016/j.atherosclerosis.2007.11.032. Published online ahead of print January 11, 2008 doi:10.1016/j.atherosclerosis.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 7.Chinn S, Rona RJ. Prevalence and trends in overweight and obesity in three cross-sectional studies of British Children, 1974–1994. BMJ. 2001;322:24–26. doi: 10.1136/bmj.322.7277.24. doi:10.1136/bmj.322.7277.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawlor DA, Lean M, Sattar N. ABC of obesity: obesity and vascular disease. BMJ. 2006;333:1060–1063. doi: 10.1136/bmj.333.7577.1060. doi:10.1136/bmj.333.7577.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owen CG, Whincup PH, Orfei L, Chou QA, Rudnicka AR, Wathern AK, Kaye SJ, Eriksson JG, Osmond C, Cook DG. Is body mass index before middle age related to coronary heart disease risk in later life? Evidence from observational studies. Int J Obes. 2009;33:866–877. doi: 10.1038/ijo.2009.102. doi:10.1038/ijo.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, Sherwin RS, Caprio S. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346:802–810. doi: 10.1056/NEJMoa012578. doi:10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 11.Haines L, Wan KC, Lynn R, Barrett TG, Shield JP. Rising incidence of type 2 diabetes in children in the U.K. Diabetes Care. 2007:1097–1101. doi: 10.2337/dc06-1813. doi:10.2337/dc06-1813. [DOI] [PubMed] [Google Scholar]
- 12.Savva SC, Tornaritis M, Savva ME, Kourides Y, Panagi A, Silikiotou N, Georgiou C, Kafatos A. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Relat Metab Disord. 2000;24:1453–1458. doi: 10.1038/sj.ijo.0801401. doi:10.1038/sj.ijo.0801401. [DOI] [PubMed] [Google Scholar]
- 13.Garnett SP, Baur LA, Srinivasan S, Lee JW, Cowell CT. Body mass index and waist circumference in midchildhood and adverse cardiovascular disease risk clustering in adolescence. Am J Clin Nutr. 2007;86:549–555. doi: 10.1093/ajcn/86.3.549. [DOI] [PubMed] [Google Scholar]
- 14.Cole T, Bellizzi M, Flegal K, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: an international survey. BMJ. 2000;320:1–6. doi: 10.1136/bmj.320.7244.1240. doi:10.1136/bmj.320.7226.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescent. The fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescent. Pediatrics. 2004;114(2 Suppl. 4th report):558. [PubMed] [Google Scholar]
- 16.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S. The metabolic syndrome in children and adolescents — an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 17.Cameron N, Jones LL, Griffith PL, Norris SA, Pettifor JM. How well do waist circumference and body mass index reflect body composition in pre-pubertal children? Eur J Clin Nutr. 2009;63:1065–1070. doi: 10.1038/ejcn.2009.26. doi:10.1038/ejcn.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordhamn K, Sodergren E, Olsson E, Karlstrom B, Vessby B, Berglund L. Reliability of anthropometric measurements in overweight and lean subjects: consequences for correlations between anthropometric and other variables. Int J Obes Relat Metab Disord. 2000;24:652–657. doi: 10.1038/sj.ijo.0801216. doi:10.1038/sj.ijo.0801216. [DOI] [PubMed] [Google Scholar]
- 19.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos A-K, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LMS The Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. doi:10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 20.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. doi:10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 21.Freathy RM, Timpson NJ, Lawlor DA, Pouta A, Ben-Shlomo Y, Ruokonen A, Ebrahim S, Shields B, Zeggini E, Weedon MN, Lindgren CM, Lango H, Melzer D, Ferrucci L, Paolisso G, Neville MJ, Karpe F, Palmer CN, Morris AD, Elliott P, Jarvelin MR, Smith GD, McCarthy MI, Hattersley AT, Frayling TM. Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes. 2008;57:1419–1426. doi: 10.2337/db07-1466. Published online ahead of printMarch 17, 2008. doi:10.2337/db07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. doi:10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjorge T, Engeland A, Tverdal A, Davey Smith G. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168:30–37. doi: 10.1093/aje/kwn096. doi:10.1093/aje/kwn096. [DOI] [PubMed] [Google Scholar]
- 24.Lawlor DA,, Mishra GD. Why family matters. In: Lawlor DA, Mishra GD,, editors. Family Matters: Designing, Analyzing and Understanding Family-based Studies in Life Course Epidemiology. Oxford: OUP; 2009. pp. 1–12. p. [Google Scholar]
- 25.Daniels SR. Complications of obesity in children and adolescents. Int J Obes (Lond) 2009;33(Suppl. 1):S60–S65. doi: 10.1038/ijo.2009.20. doi:10.1038/ijo.2009.20. [DOI] [PubMed] [Google Scholar]
- 26.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine-aminotransferase (ALT) and factors associated with ALT levels in adolescence: findings from the US National Health and Examination Survey 2001–2004. Gastroenterology. 2007;133:1814–1820. doi: 10.1053/j.gastro.2007.08.077. doi:10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deshmukh-Taskar P, Nicklas TA, Morales M, Yang SJ, Zakeri I, Berenson GS. Tracking of overweight status from childhood to young adulthood: the Bogalusa Heart Study. Eur J Clin Nutr. 2006;60:48–57. doi: 10.1038/sj.ejcn.1602266. doi:10.1038/sj.ejcn.1602266. [DOI] [PubMed] [Google Scholar]
- 28.Arkadianos I, Valdes AM, Marinos E, Florou A, Gill RD, Grimaldi KA. Improved weight management using genetic information to personalize a calorie controlled diet. Nutr J. 2007;6:29. doi: 10.1186/1475-2891-6-29. doi:10.1186/1475-2891-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avenell A, Brown TJ, McGee MA, Campbell MK, Grant AM, Broom J, Jung RT, Smith WC. What are the long-term benefits of weight reducing diets in adults? A systematic review of randomized controlled trials. J Hum Nutr Diet. 2004;17:317–335. doi: 10.1111/j.1365-277X.2004.00531.x. doi:10.1111/j.1365-277X.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 30.NICE. NHS National Institute for Clinical Excellence Guidance. Technology Appraisal Guidance No. 22. Guidance on the Use of Orlistat for the Treatment of Obesity. 2001. London: National Institute for Clinical Excellence.
- 31.NICE. Technology Appraisal Guidance No. 31. Guidance on the Use of Sibutramine for the Treatment of Obesity: 2001. NHS National Institute for Clinical Excellence Guidance. London: National Institute for Clinical Excellence. [Google Scholar]
- 32.Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, Nguyen NT, Li Z, Mojica WA, Hilton L, Rhodes S, Morton SC, Shekelle PG. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 33.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: a randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 34.Kipping RR, Jago R, Lawlor DA. Obesity in children. Part 2: prevention and management. BMJ. 2008;337:a1848. doi: 10.1136/bmj.a1848. doi:10.1136/bmj.a1848. [DOI] [PubMed] [Google Scholar]
- 35.Logue J, Thompson L, Romanes F, Wilson DC, Thompson J, Sattar N Guideline Development Group. Management of obesity: summary of SIGN guideline. BMJ. 2010;340:c154. doi: 10.1136/bmj.c154. doi:10.1136/bmj.c154. [DOI] [PubMed] [Google Scholar]
- 36.Han JC, Lawlor DA, Kimm SYS. Childhood obesity. Lancet. 2010;375:1737–1748. doi: 10.1016/S0140-6736(10)60171-7. doi:10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riddoch CJ, Leary SD, Ness AR, Blair SN, Deere K, Mattocks C, Griffiths A, Davey Smith G, Tilling K. Prospective associations between objective measures of physical activity and fat mass in 12–14 year old children: the Avon Longitudinal Study of Parents and Children (ALSPAC) BMJ. 2009;339:b4544. doi: 10.1136/bmj.b4544. doi:10.1136/bmj.b4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berenson GS, Srinivasan SR, Bao W, Newmann WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults: The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. doi:10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.