Abstract
Background: Isolated methylmalonic acidemia (MMA) is managed by dietary protein restriction and medical food supplementation. Resting energy expenditure (REE) can be depressed in affected individuals for undefined reasons.
Objective: The objective was to document the spectrum of nutritional approaches used to treat patients with MMA, measure REE, and analyze the dependence of REE on body composition, biochemical, and nutritional variables.
Design: Twenty-nine patients with isolated MMA (22 mut, 5 cblA, 2 cblB; 15 males, 14 females; age range: 2–35 y) underwent evaluation. REE was measured with open-circuit calorimetry and compared with predicted values by using age-appropriate equations.
Results: Nutritional regimens were as follows: protein restriction with medical food (n = 17 of 29), protein restriction with medical food and supplemental isoleucine or valine (n = 5 of 29), or the use of natural protein alone for dietary needs (n = 7 of 29). Most mut patients had short stature and higher percentage fat mass compared with reference controls. Measured REE decreased to 74 ± 13.6% of predicted (P < 0.001) in the ≤18-y group (n = 22) and to 83 ± 11.1% (P = 0.004) in patients aged >18 y (n = 7). Linear regression modeling suggested that age (P = 0.001), creatinine clearance (P = 0.01), and height z score (P = 0.04) accounted for part of the variance of measured REE per kilogram of fat-free mass (model R2 = 0.66, P < 0.0001).
Conclusions: There is wide variation in the dietary treatment of MMA. Standard predictive equations overestimate REE in this population primarily due to their altered body composition and decreased renal function. Defining actual energy needs will help optimize nutrition and protect individuals from overfeeding. This trial is registered at clinicaltrials.gov as NCT00078078.
INTRODUCTION
Isolated methylmalonic acidemias (MMAs) are a group of autosomal recessive inborn errors of metabolism representative of metabolic disorders that feature a block near the terminal oxidation step of substrates feeding into the propionate pathway, primarily the branched chain amino acids, isoleucine and valine, but also threonine, methionine, odd chain fatty acids, and cholesterol. They are predominantly caused by deficiency of methylmalonyl-coenzyme A (-CoA) mutase (MUT), an enzyme that uses 5′-deoxyadenosylcobalamin as a cofactor to catalyze the conversion of methylmalonyl-CoA into succinyl-CoA in the mitochondrial matrix (1). Genetic defects that impair cofactor transport and metabolism (cblD variant2, cblA, cblB) also cause isolated methylmalonic acidemia. Reduced MUT activity results in the production of methylmalonic acid as well as the accretion of upstream propionyl-CoA–derived metabolites, such as 3-OH- propionic acid and 2-methylcitrate. Affected individuals display massive elevation of methylmalonic acid in the blood, urine, and other bodily fluids. Despite aggressive management of patients with MMA, mortality remains high, and survivors suffer from multisystemic disease, including poor growth, recurrent metabolic crises, chronic renal disease, neurologic problems, pancreatitis, and osteopenia (2–4).
Although nutritional management is the mainstay of therapy for MMA, clear guidelines do not exist. Conventional dietary therapy revolves around limiting the intake of propiogenic precursors—particularly isoleucine, valine, methionine, and threonine—and the provision of a high-energy diet to prevent fasting and catabolism. Supplemental amino acids, such as alanine, have been supplied in an attempt to improve growth, and, rarely, growth hormone has been administered (5–8). To date, very few studies have examined optimum dietary therapy for patients or assessed whether differing dietary regimens have effected significant outcome changes (9, 10).
In this study, we documented the nutritional approaches used to treat a cohort of well-characterized patients with isolated MMA; assessed correlations between clinical and biochemical variables, particularly resting energy expenditure (REE); and searched for treatment-related effects by using regression analysis. In addition to presenting an evidence-based overview of current dietary therapy for MMA, our investigations define the factors influencing basal energy requirements in this population and provide new insights into the underlying cause of the depressed REE that is present in most affected individuals.
SUBJECTS AND METHODS
Subjects
Patient studies were approved by the National Human Genome Research Institute Institutional Review Board as part of the National Institutes of Health (NIH) study 04-HG-0127 “Clinical and Basic Investigations of Methylmalonic Acidemia and Related Disorders” and performed in compliance with the Helsinki Declaration (clinicaltrials.gov identifier: NCT00078078; recruitment start date February 2004). Adult participants or parents of the younger patients signed written informed consent for participation. Patients were evaluated between 2004 and 2009 and were primarily self-referred with no significant overrepresentation from a particular center. There was no recruitment strategy on the basis of race, sex, nutritional status, or disease severity. Ongoing care and dietary management were provided by regional metabolic centers.
Neurologic assessment by a pediatric neurologist was included in the evaluation of every participant. All subjects were ambulatory; 14 of 29 patients had clinical or neuroradiographic evidence of a metabolic stroke. Study participants were evaluated at steady state and were without clinical symptoms of metabolic instability or evidence of ketosis or lactic acidosis. Those with a history of thyroid disease were excluded from the analysis. One patient (patient 18; age: 11 y) was on growth hormone treatment at the time of the study, whereas another (patient 25; age: 24 y) had received growth hormone between the ages of 8 and 14 y.
Metabolic and laboratory studies
The diagnosis of MMA was confirmed with cellular biochemical studies including 14C-propionate incorporation and complementation assays (laboratory of David S Rosenblatt, Division of Medical Genetics, McGill University, Montreal, Canada) or molecular genetic analysis of the MUT, MMAA, and MMAB genes (GeneDx, Gaithersburg, MD) (1). (See Table 1 footnote for definitions of MMA subtypes.) Because only 2 patients were assigned the mut− subtype, they were included in the 0 group for subsequent analysis. Routine laboratory investigations included determination of the complete blood count, serum electrolytes, pancreatic enzymes, thyroid function, lipid panel, iron studies, and numerous serum and urine metabolic measurements. When possible, 24-h urine collections were used to measure creatinine clearance (11) and calculate metabolite output.
Diet and nutritional regimens
Regional metabolic experts at specialized care centers dictated dietary management. The intake used for calculations was based on the prescribed diet and prepared in the NIH metabolic kitchen and administered to patients during the study visit.
Dietary analysis was performed on the basis of formula recipes plus detailed dietary histories and 3-d food records. Five subjects received enteral formula only with negligible oral intake (on average, <100 calories and <1 g protein/d). Of the remaining 23 subjects, 9 completed 2–6-d food records and 14 provided detailed histories of typical dietary intake. Dietary analyses and calculations were performed by using Nutrition Data System for Research, software versions 2005–2009, developed by the Nutrition Coordinating Center, University of Minnesota.
Anthropometric measurements and body composition analysis
Anthropometric measurements were expressed as age- and sex-specific z scores by using the epidemiologic software package Epi Info, version 3.5.1., based on the CDC 2002 reference database (Centers for Disease Control and Prevention, Atlanta, GA). Body mass index (BMI) z scores for patients aged >18 y were calculated by using the following equation: BMI – mean/[SEM*(square root of n)] (n = number of examined persons) (12).
Whole-body composition in grams of fat or fat mass and fat-free (or lean) mass (FFM) was measured by using dual-energy X-ray absorptiometry (DXA; Hologic Delphi A; Hologic, Bedford, MA). Values were compared with mean (±SD) values for the reference child and adolescent models of body composition, matched for age, sex, and ethnicity (13–15).
REE
REE was measured by open-circuit indirect calorimetry (Deltatrac equipment; VIASYS, Yorba Linda, CA) performed with the use of a respiratory metabolic cart. Subjects consumed their habitual diet during the days before the study. Before each test, the calorimeter was calibrated with the use of a reference gas mixture obtained from the manufacturer. Respiratory variables were recorded when steady state had been achieved, typically between 0400 and 0800 while the child was supine in bed. The indirect calorimetry study was performed after 12 h of fasting in adult individuals. However, 17 patients received feedings by gastrostomy tube throughout the night and were not fasted to avoid disruption of their usual regimen.
The modified Weir equation [3.9(VO2) + 1.1(VCO2)] × 1.44 = REE (kcal/d) was used to calculate energy equivalency from oxygen consumption and carbon dioxide production (16). Predicted values for REE in patients aged ≤18 y were assessed by using the Schofield, WHO/FAO/UNU, and Fleisch equations (see Supplemental Table 1 under “Supplemental data” in the online issue). Predicted values for REE in patients >18 y old were assessed by using the Harris-Benedict and Mifflin–St Jeor equations (17–21) (Supplemental Table 1 under “Supplemental data” in the online issue). A correction for amino acid oxidation, using total urine nitrogen from 24-h collections, did not change the calculated respiratory quotient compared with what was measured during indirect calorimetry (not presented).
Data analysis
The results are presented as means ± SDs. Statistical analyses were performed by using SPSS version 17 (SPSS Inc, Chicago, IL) or SAS version 9 (Cary, NC) software. The paired t test was used for comparisons of measured REE and predicted REE, and an independent t test was used to compare values between females and males. Pearson's correlation coefficient was used to evaluate bivariate correlations. A linear regression was performed with REE and REE per kilogram of FFM as dependent variables by using SPSS (SPSS Inc). A P value <0.05 was considered significant.
RESULTS
Demographic, enzymatic, and anthropometric characteristics
Characteristics of the 29 participants (22 mut, 5 cblA, 2 cblB) who completed dietary evaluation and the indirect calorimetry study are presented in Table 1. The patient cohort was primarily white, but individual patients were of Native American, Hispanic, Northern European, Middle Eastern, and Asian descent.
TABLE 1.
Patient characteristics1
| Patient no. | Age | Age at DX | Sex | Class | Transplant | Mutation 1 | Mutation 2 |
| y | mo | ||||||
| 1 | 2 | 23 | M | cblA | p.Y129X | p.T198SfsX6 | |
| 2 | 3 | 0.25 | F | mut0 | p.R228X | p.R369H | |
| 3 | 4 | 0.25 | F | mut0 | p.G670R | p.R403X | |
| 4 | 6 | Prenatal | F | mut0 | p.R108H | p.G623R | |
| 5 | 6 | 0.3 | M | mut0 | L&K | p.E224X | p.R228X |
| 6 | 6 | 0.25 | M | mut0 | c.753+2T→A, splice | p.R581X | |
| 7 | 7 | 0.3 | F | mut0 | p.G642R | p.G642R2 | |
| 8 | 7 | 3 | M | mut0 | p.P615R | p.L434HfsX3 | |
| 9 | 7 | 0.25 | M | mut0 | L | p.R369H | p.S594RfsX11 |
| 10 | 8 | 11 | F | cblB | p.R186W | p.R186W2 | |
| 11 | 8 | Prenatal | F | mut− | p.M186V | p.R369H | |
| 12 | 9 | 0.75 | M | mut0 | p.R369C | p.R403X | |
| 13 | 10 | 0.13 | F | mut0 | p.L11TfsX38 | p.V553GfsX17 | |
| 14 | 10 | 0.1 | F | mut0 | p.A191E | N/A | |
| 15 | 10 | 12 | M | mut0 | p.R108C | p.R108C3 | |
| 16 | 10 | 0.25 | M | mut0 | p.R228X | c.1332+1delG, splice | |
| 17 | 11 | 0.5 | F | mut0 | p.R108H | p.G623R | |
| 18 | 11 | 0.06 | M | mut0 | L | p.R369H | p.R369H3 |
| 19 | 13 | 12 | M | cblA | p.R145X | p.R145X2 | |
| 20 | 17 | 6 | M | cblA | p.E147G | p.P151AfsX19 | |
| 21 | 18 | 0.1 | M | mut0 | p.A191E | p.N219Y | |
| 22 | 18 | 39 | F | cblB | L&K | p.R186W | p.R186W2 |
| 23 | 20 | 6 | M | mut0 | p.R581X | p.R581X3 | |
| 24 | 21 | 0.06 | F | mut0 | L&K | p.R369H | p.R369H2 |
| 25 | 24 | 0.3 | M | mut0 | p.N219Y | p.H350Y | |
| 26 | 25 | 0.25 | F | mut0 | N/A | N/A | |
| 27 | 31 | 0.25 | F | mut− | L&K | p.E276X | p.R369H |
| 28 | 33 | 36 | F | cblA | K | p.R145X | p.R145X2 |
| 29 | 35 | 13 | M | cblA | p.R145X | p.R145X3 |
Demographic characteristics and enzymatic and mutation information for the 29 patients studied are listed by age. The class of methylmalonic acidemia is assigned on the basis of the results of the 14C-propionate incorporation assay, which indirectly measures the activity of the MUT (methylmalonyl-coenzyme A) enzyme. 0 was assigned if enzymatic activity was nondetectable, and − was assigned if residual enzymatic activity was present. Designation of the enzymatic block to a particular complementation group (cblA, B) was made with heterokaryon rescue, in which the cell line from the affected individual is mixed with a panel of established cell lines of known status (eg, cblA, cblB). DX, diagnosis; L, liver-only transplant; K, kidney-only transplant; L&K, liver and kidney transplant; N/A: not available.
No known consanguinity.
Known consanguinity.
The time of diagnosis was highly variable and ranged from the prenatal period up to 39 mo; evaluation at our center was between the ages of 2 and 35 y (mean ± SD: 13.4 ± 9.08 y). Seven patients (5 mut, 1 cblA, 1 cblB) had received organ transplants [combined liver and kidney transplant (n = 4), liver transplant (n = 2), or kidney transplant (n = 1)].
The diversity of the patient group is supported by the spectrum of mutations that is present (Table 1). In the mut group (n = 22), there were 23 different mutations identified. The majority of mut patients had missense mutations [16 of 22 patients (25 of 44 alleles)]. The most common mutation was p.R369H, which was present in 6 individuals. Eleven of 22 mut patients harbored at least one nonsense or frameshift mutation (14 of 44 alleles). Seventeen mut patients were compound heterozygotes, and none shared an identical genotype with the exception of 2 sisters (patients 4 and 17). Five individuals were apparently homozygous for a single mutation, and, of these, 3 originated from consanguineous unions. Both patients with cblB and 3 of 5 patients with cblA were homozygous for the common mutations in MMAB (p.R186W) and MMAA (p.R145X), respectively.
Height-for-age, weight-for-age, and BMI-for-age, expressed as z scores (mean ± SD), are presented for the mut patients in Figure 1A and in more detail for all subtypes in Supplemental Table 2 under “Supplemental data” in the online issue. Patients aged ≤20 y in the mut group had a mean z score of 0.01 ± 1.2 for weight, −1.04 ± 1.2 for height, and 0.77 ± 1.1 for BMI (n = 18). Four females and 3 males aged ≤20 y had a BMI-for-age in the 85th–95th percentile (classified as overweight), and 1 female and 3 males aged ≤20 y had a BMI-for-age above the 95th percentile (classified as obese). The mean BMI (in kg/m2) for all patients aged >20 y (n = 6) was 24.2 ± 6.2, with z scores between −1.56 and 0.9 SD (mean = −0.49). Women had higher values than did men (26.5 ± 6.5 compared with 19.5 ± 0.63, respectively; P = 0.12); 2 subjects were considered obese with a BMI >30 (22). BMI-for-age z score correlated significantly with age (r = −0.555, P = 0.002), with the highest values observed in the younger age group (Supplemental Figure 1, B and C, under “Supplemental data” in the online issue).
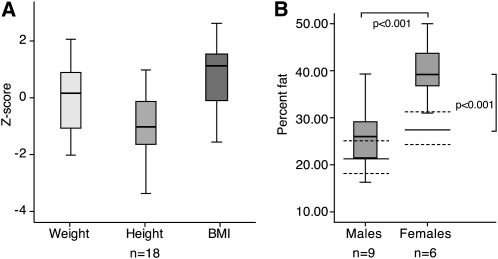
FIGURE 1.
A: Weight-, height-, and BMI-for-age z scores for patients in the mut (methylmalonyl-coenzyme A) group of methylmalonic acidemia aged ≤20 y (n = 18) are depicted. Boxes represent the middle 50% of all cases per variable, whereas the remaining 50% is contained between the box and whiskers on each side. The single line inside each box represents the median of the entire data set. The location of this line suggests the skewness in the distribution, when noticeably shifted away from the center, as is the case particularly for the BMI-for-age z score in the mut subgroup. B: Percentage fat mass in males and females aged ≤18 y with the mut subtype of methylmalonic acidemia is depicted. Percentage fat mass was significantly higher in female patients with the mut subtype than in males (P < 0.001, t test). Female patients also had significantly higher percentage fat mass compared with the highest value for white females in the reference population [27.6 ± 6.1% (n = 46), depicted as solid lines ± dotted lines (P < 0.001, t test)] (14). Statistical analyses were performed by using SPSS version 17 (SPSS Inc, Chicago, IL).
Fat mass (Figure 1B), measured by DXA, ranged between 13% and 50% (mean ± SD: 30.8 ± 10%). Mean values in patients aged ≤18 y were compared with age-, sex-, and ethnicity-matched control reference data (14, 15). Percentage fat mass was significantly higher in females than in males with the mut subtype [39.78 ± 7.26 (n = 6) compared with 27.3 ± 7.3 (n = 9), respectively; P < 0.001]. Females with the mut subtype had also significantly higher percentage fat mass compared with the highest value for white females in the reference population [27.6 ± 6.1 (n = 46), P < 0.001] (Figure 1B).
Although patients with the mut and cblB type had lower mean height z scores than did patients with cblA, consistent with the milder phenotype of the vitamin B-12–responsive cblA patients, and girls in the mut group had lower average height (−1.49 ± 1.4 compared with −0.76 ± 0.9) and higher BMI (1.07 ± 0.9 compared with 0.58 ± 1.2) z scores compared with males, these differences were not statistically significant (Supplemental Table 2 under “Supplemental data” in the online issue). Transplanted patients had significantly greater height z scores than did nontransplanted patients (mean ± SD: −0.006 ± 0.99 compared with −1.028 ± 1.47, respectively; P = 0.054) (data not presented). Patient 5 (Table 1), who received a liver and kidney transplant at the age of 5 y, had a height and weight at age 6 y that were greater than the 60th percentile. Patient 18, who received a liver transplant at age 19 mo and who began growth hormone replacement therapy 1 y before the NIH visit at age 11 y, had a height z score of −0.13 and a BMI z score of 1.29. Patient 25, who was 24 y old during the NIH evaluation, received growth hormone between 8 and 14 y of age, and his height and BMI z scores were −1.7 and −1.4, respectively.
Laboratory and metabolic studies
Biochemical variables are presented in Table 2. Plasma methylmalonic acid concentrations ranged between 36 and 6129 μmol/L, plasma glycine between 211 and 1179 μmol/L, and daily urinary output of methylmalonic acid between 21 and 7308 μmol · kg−1 · d−1.
TABLE 2.
Biochemical variables (n = 29)1
| Subgroups | Plasma methylmalonic acid | Plasma glycine | Cr/Cl | Urine methylmalonic acid | Methylmalonic acid output |
| μmol/L | μmol/L | mL · min−1 · 1.73 m−2 | mmol/mol Cr | μmol · kg−1 · d−1 | |
| mut | |||||
| All nontransplanted patients (n = 18) | 1434 ± 1807 (84–6129) | 576 ± 289 (211–1179) | 68 ± 30 (14–115) | 5232 ± 4843 (559–16,543) | 3460 ± 2336 (390–7308) |
| Age groups | |||||
| 2–9 y (n = 8) | 626 ± 455 (139–1380) | 539 ± 261 (211–861) | 78 ± 15 (59–103) | 5624 ± 6329 (684–16,543) | 4165 ± 3064 (996–7308) |
| 10–18 y (n = 6) | 2020 ± 2176 (151–5328) | 598 ± 352 (288–1179) | 66 ± 35 (32–115) | 7045 ± 4650 (2970–14,439) | 3533 ± 2451 (839–6815) |
| >18 y (n = 4) | 2317 ± 2698 (84–6129) | 618 ± 321 (339–1066) | 49 ± 43 (14–104) | 2478 ± 1893 (559–4391) | 2151 ± 1876 (390–4522) |
| Transplanted patients (n = 4) | 120 ± 81 (45–231) | 454 ± 111 (364–602) | 86 ± 15 (64–96) | 1025 ± 616 (314 ± 1401) | 375 ± 333 (93–822) |
| cblA | |||||
| All patients (n = 5) | 89 ± 77 (36–223) | 412 ± 167 (302–702) | 97 ± 31 (45–132) | 728 ± 459 (180–1206) | 684 ± 883 (21–1688) |
| cblB | |||||
| All patients (n = 2) | 665 (290–1020) | 445 (380–508) | 56 (51–61) | 3722 (1289–6156) | 1618 (1328–1908) |
All values are means ± SDs; ranges in parentheses. Reference ranges: plasma methylmalonic acid (≤0.04 μmol/L), plasma glycine (127–341 μmol/L), urine methylmalonic acid [<3 mmol/mol creatinine (Cr)]. Cr/Cl, creatinine clearance; mut, methylmalonyl-coenzyme A.
Creatinine clearances ranged between 14 and 132 mL · min−1 · 1.73 m−2. A progressive decrease in creatinine clearance associated with a decrease in methylmalonic acid urinary output and an increase in plasma methylmalonic acid concentrations was observed in patients with the mut subgroup in the different age groups, reflecting the decreasing filtration of methylmalonic acid in patients with progressive renal failure. As expected, plasma concentration of methylmalonic acid correlated negatively with creatinine clearance in the mut group (r = −0.746, P < 0.001). Three patients, >20 y of age, had a creatinine clearance <30 mL · min−1 · 1.73 m−2; a liver and kidney transplant recipient had a creatinine clearance of 64 mL · min−1 · 1.73 m−2 9 y after transplant. No significant correlations were observed between biochemical variables and any of the dietary indexes.
Dietary and nutritional management
Patients were treated with highly variable dietary regimens (Table 3). Five patients received only formula and had negligible oral dietary intake, 18 subjects received a combination of formula and diet, and 6 subjects only received food orally. Energy intake ranged between 23 and 86 kcal · kg−1 · d−1; and total protein intake, expressed as natural (complete) protein plus free amino acids in medical foods, ranged between 0.38 and 2.94 g · kg−1 · d−1. Natural (complete) protein intake ranged between 0.29 and 2.12 g · kg−1 · d−1 [33–265% of Recommended Dietary Allowance (RDA)]. As a subgroup, the transplant recipients had total protein intakes ranging between 0.50 and 1.06 g · kg−1 · d−1 with natural (complete) protein intake accounting for between 0.29 and 0.85 g · kg−1 · d−1 (36–89% of RDA). Eighteen patients were treated with medical foods that provided from 0.21 to 1.95 g · kg−1 · d−1 of synthetic amino acids. Daily propiogenic precursor load from these diets ranged between 0.28 and 2.66 mmol/kg. Five patients received valine supplementation (68–170 mg/d), including one who had received a combined liver and kidney transplantation, and 2 received additional isoleucine (48–340 mg/d).
TABLE 3.
Dietary characteristics1
| Subgroups | Total protein | Complete protein | Incomplete protein equivalent | Total energy | Provided kcal/ measured REE |
| g · kg−1 · d−1 | g · kg−1 · d−1 | g · kg−1 · d−1 | kcal/d | ||
| mut | |||||
| All patients (n = 21) | 1.33 ± 0.722 | 0.75 ± 0.32 | 0.58 ± 0.57 | 1725 ± 581 | 1.98 ± 0.5 |
| Age groups | |||||
| 2–9 y (n = 10) | 1.50 ± 0.67 | 0.84 ± 0.17 | 0.72 ± 0.63 | 1453 ± 263 | 2.12 ± 0.6 |
| 10–18 y (n = 7) | 1.46 ± 0.81 | 0.82 ± 0.46 | 0.64 ± 0.60 | 1780 ± 511 | 1.83 ± 0.4 |
| >18 y (n = 5) | 0.69 ± 0.26 | 0.48 ± 0.18 | 0.23 ± 0.26 | 2307 ± 904 | 1.91 ± 0.4 |
| cblA | |||||
| All patients (n = 5) | 1.32 ± 0.72 | 1.0 ± 0.63 | 0.31 ± 0.52 | 1665 ± 871 | 1.68 ± 0.3 |
| cblB | |||||
| All patients (n = 1) | 0.67 | 0.67 | 0 | 1409 | 1.49 |
Dietary characteristics of the 29 patients are grouped by methylmalonic acidemia class and age for the patients in the mut (methylmalonyl-coenzyme A) group. REE, resting energy expenditure.
Mean ± SD (all such values).
REE
Mean measured REE for the ≤18-y group was 78 ± 13.6%, 71 ± 12.2%, and 75 ± 12.7% of the REE predicted based on the Fleisch, WHO/FAO/UNU, and Schofield equations, respectively (P < 0.001 for each mean measured compared with predicted REE value by paired t test comparisons), as depicted in Figure 2A and Table 4. Similarly, in the group aged >18 y, measured REE was 78 ± 11% predicted by the Harris-Benedict equation (P = 0.004) (Figure 2B).
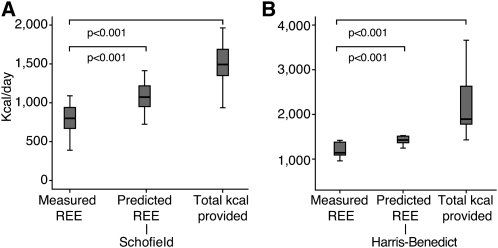
FIGURE 2.
Measured and predicted resting energy expenditure (REE) and total kilocalories provided are presented in kilocalories per day for the entire patient population. A: Measured REE compared with predicted REE based on the Schofield equation (17) and total kilocalories per day provided to patients ≤18 y of age (n = 22). B: The same variables for patients >18 y of age in which predicted REE is calculated by the Harris-Benedict equation (20) (n = 7). Measured REE was lower than predicted by any of the equations as well as from the actual total calories provided in both age groups (P < 0.001 for both paired comparisons between measured REE and the values derived from each of the predictive equations as well as measured REE compared with total daily kilocalories provided, paired t test). Statistical analyses were performed by using SPSS version 17 (SPSS Inc, Chicago, IL).
TABLE 4.
Measured and percentage predicted resting energy expenditure (REE)1
| Percentage predicted REE2 |
|||||||
| Subgroups | Measured REE3 | Measured REE/FFM3 | Fleisch | WHO/FAO/UNU | Schofield | Harris-Benedict | Mifflin–St Jeor |
| kcal/d | kcal/kg FFM | ||||||
| mut | |||||||
| All patients (n = 22) | 890 ± 248 | 38.7 ± 10.7 | 76 ± 10 | 70 ± 10 | 73 ± 10 | 75 ± 10 | 79 ± 9 |
| Age groups | |||||||
| 2–9 y (n = 10) | 714 ± 174 | 43.1 ± 5.3 | 72 ± 12 | 67 ± 11 | 71 ± 11 | ||
| 10–18 y (n = 7) | 974 ± 218 | 41.2 ± 14 | 81 ± 7 | 73 ± 7 | 76 ± 8 | ||
| >18 y (n = 5) | 1124 ± 161 | 28.7 ± 5 | 75 ± 10 | 79 ± 9 | |||
| Sex | |||||||
| Males (n = 12) | 944 ± 259 | 39.3 ± 9.5 | 77 ± 8 | 72 ± 7 | 75 ± 8 | 79 ± 14 | 81 ± 13 |
| Females (n = 10) | 825 ± 229 | 38.0 ± 12.4 | 74 ± 13 | 66 ± 12 | 71 ± 13 | 72 ± 8 | 78 ± 9 |
| cblA | |||||||
| All patients (n = 5) | 1172 ± 459 | 37.0 ± 9.0 | 87 ± 18 | 76 ± 24 | 80 ± 25 | 88 | 90 |
| Age groups | |||||||
| 2–9 y (n = 1) | 409 | 41.1 | 58 | 48 | 51 | ||
| 10–18 y (n = 2) | 1457 | 43.3 | 100 | 90 | 94 | ||
| >18 y (n = 2) | 1270 | 28.5 | 88 | 90 | |||
| Sex | |||||||
| Males (n = 3) | 1139 ± 638 | 35.7 ± 6 | 87 | 72 | 76 | 97 | 97 |
| Females (n = 2) | 1222 ± 144 | 38.9 ± 15 | 87 | 84 | 88 | 80 | 83 |
| cblB | |||||||
| All patients (n = 2) | 830 ± 155 | 36.9 ± 19 | 75 | 71 | 77 | ||
REE scores for all 29 patients are shown. Equations listed in Supplemental Table 1 under “Supplemental data” in the online issue were used to calculate predicted score (17–21). FFM, fat-free mass; mut, methylmalonyl-coenzyme A.
Values are measured REE as a percentage of predicted values ± SDs.
Values are means ± SDs.
Measured REE was also lower than the actual total energy provided in both age groups (P < 0.0001 in the ≤18-y group, and P = 0.004 in the >18-y group), with a mean ratio of total energy provided over measured REE (in kcal) for all patients of 1.91 ± 0.50 (range: 1.29–3.54). Ratios per MMA subgroup are provided in Table 3. The ratio of total energy over the predicted REE was 1.45 ± 0.32 (range: 1.01–2.39).
There was no significant difference between the predicted REE calculated by using Fleisch, WHO/FAO/UNU, or Schofield equations for the ≤18-y group (P = 0.230, one-factor ANOVA). Mean REE measurements for the patients aged >18 y using the Harris-Benedict (78 ± 11% predicted) and Mifflin–St Jeor (81 ± 10% predicted) equations were likewise very similar (P = 0.6). No differences were observed in measured or percentage predicted REE between males and females (Table 4). Similarly, there were no significant differences between transplant recipients (77 ± 6% predicted, n = 7) and nontransplanted patients (76 ± 13% predicted) (P = 0.91) (data not shown).
We sought to explore the dependency of the REE on biochemical and anthropometric variables. Linear regression analysis was performed for measured REE as well as for REE corrected for FFM as determined by DXA analysis (REE/kg FFM) (Table 4). For measured REE, a model including age, percentage FFM, and total protein intake (g · kg−1 · d−1) had an R2 of 0.588 (F statistics, P < 0.0022). Correlation coefficient P values in the model were as follows: P = 0.012 for age, P = 0.016 for percentage FFM, and P = 0.041 for total protein intake (Table 5).
TABLE 5.
Contribution of body composition, protein intake, and renal function to resting energy expenditure (REE) in children with methylmalonic acidemia1
| Variable | Parameter (β) | SE | P (variable) | 2 | P (model) |
| REE (kcal/d) | |||||
| Intercept | 208.9 | 275.1 | 0.45 | 0.58 | <0.0022 |
| Age | 14.2 | 5.2 | 0.012 | ||
| FFM percentage | 11.1 | 4.2 | 0.016 | ||
| Total protein intake (g · kg−1 · d−1) | −159.1 | 73.4 | 0.041 | ||
| REE/kg FFM (kcal/kg FFM) | |||||
| Intercept | 36.9 | 5.0 | 0.001 | 0.66 | <0.0001 |
| Age | −0.642 | 0.1 | 0.001 | ||
| Creatinine clearance | 0.120 | 0.04 | 0.013 | ||
| Height z score | −2.39 | 1.09 | 0.041 | ||
| Total protein intake (g · kg−1 · d−1) | −0.39 | 2.7 | 0.884 |
Linear regression models for measured REE and measured REE per kilogram of fat-free mass (FFM) in the whole patient population are shown. The resulting equation for REE was as follows: REE = 208.9 + (14.2 × age) + (11.1 × percentage FFM) – [159.1 × total protein intake (in g · kg−1 · d−1)]. The equation for REE/kg FFM was as follows: REE/kg FFM = 36.9 − (0.642 × age) + (0.12 × creatinine clearance) – (2.39 × height z score) – [0.39 × total protein intake (in g · kg−1 · d−1)]. Statistical analysis was performed by using SPSS version 17 (SPSS Inc, Chicago, IL).
REE per kilogram of FFM was also examined by using a regression model including age, creatinine clearance, height-for-age z score, and total protein intake (g · kg−1 · d−1) as dependent variables. This analysis revealed an R2 of 0.661 (F statistics, P < 0.0001) and the following P values: age, P = 0.001; creatinine clearance, P = 0.013; height-for-age z score, P = 0.041; and total protein intake, P = 0.884.
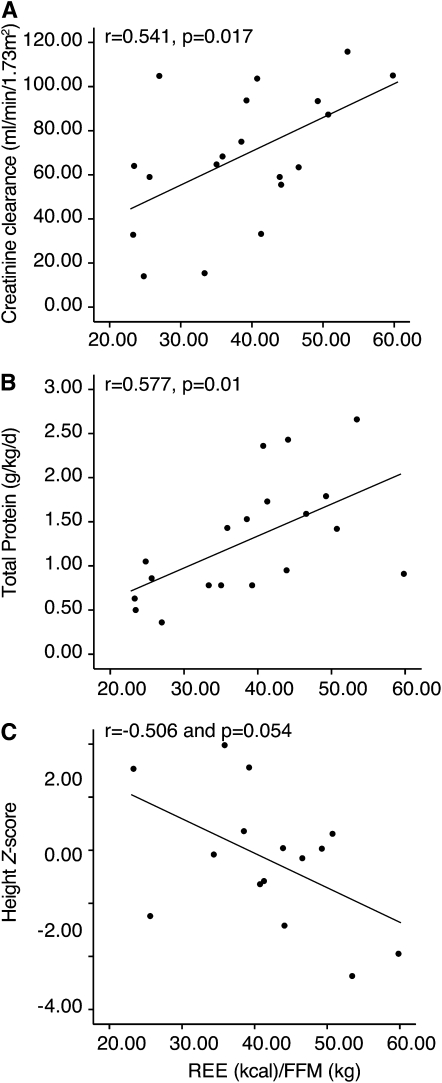
Bivariate correlations of REE per kilogram of FFM with variables including creatinine clearance (r = 0.541, P = 0.017), total protein intake (r = 0.577, P = 0.01), and height z score (r = 0.503, P = 0.056) are shown in Figure 3, A, B and C, respectively. The difference between total energy provided and measured REE expressed per kilogram of FFM correlated significantly with age (r = −0.569, P = 0.006) and was highest in the younger patients (Supplemental Figure 1A under “Supplemental data” in the online issue).
FIGURE 3.
Bivariate correlations for patients in the mut (methylmalonyl-coenzyme A) subgroup with dual-energy X-ray absorptiometry data are depicted between REE (resting energy expenditure) per kilogram of FFM (fat-free mass) and creatinine clearance (mL · min−1 · 1.73 m−2; n = 19), total protein intake (g · kg−1 · d−1; n = 19), and height-for-age z score (age: ≤20 y; n = 15). Pearson's correlation coefficients and P values for each correlation are shown.
DISCUSSION
As part of an effort to document the spectrum of nutritional approaches used to manage patients with MMA, we have evaluated affected individuals in the inpatient setting to survey dietary therapy, to study the potential contribution of dietary management on patient phenotype, and to examine whether REE, a critical variable that profoundly influences the individual response to diet, is inherently abnormal in these patients or ascribable to a set of clinical or laboratory variables. The data presented in this article derive from a cohort who have been comprehensively characterized in a research environment and should serve as a valuable resource for future studies on the natural history of MMA and other disorders of organic acid metabolism.
The subjects who participated in this study were evaluated at a single site but represented multiple treatment centers, mainly in the United States; they had varying ages, anthropometric measurements, nutritional regimens, biochemical variables, and transplant status (Table 1). The largest subgroup consisted of the o class (n = 20 of 29), which is in agreement with other survey-based reports on isolated MMA (23, 24). In contrast to other single-center studies, all patients in our cohort were placed into subclasses by enzymology and in almost every instance (28 of 29 patients) causative mutations were identified. Molecular genetic subclassification will be especially important to perform as future cohorts are accrued, because it should allow the inherent heterogeneity of the population to be refined and treatment regimens to be precisely compared between centers.
The nutritional management of the patients evaluated here was very diverse and appeared to follow 3 major approaches: natural protein restriction with medical food use (n = 17 of 29); natural protein restriction with medical food and supplemental isoleucine or valine (n = 5 of 29) and the use of natural protein alone for dietary needs (n = 7 of 29). Restriction of complete (natural) protein to less than the RDA was common (n = 22 of 29) and ranged from 33% to 98% of the RDA, which averaged 70 ± 19% over the entire group. A surprising observation of this study was the administration of large supplemental doses of isoleucine solution, valine solution, or both to a subset of patients (n = 5 of 29) who were also receiving medical foods. Although some nutritional textbooks support the administration of valine and isoleucine to patients with MMA and propionic acidemia (25), older studies firmly established that isoleucine and valine were toxic and induced vomiting and ketosis when given to patients (26, 27). Clearly, the use of supplemental valine and isoleucine in MMA is an area in which further clinical research is required, especially because there have been no studies to prove the efficacy of such an approach.
The diversity of approaches used to treat similar groups of patients provided an ideal opportunity to examine whole-body energetics in MMA. The one consistent finding noted in most patients (26 of 29) was a significantly lower than predicted REE (P < 0.0001) (Figure 2, A and B) measured by the well-accepted method of indirect calorimetry and assessed by using appropriate predictive equations (28–30). A methodologic or measurement error in our reported values seems unlikely because cblC patients participating in our protocol (data not shown) and several cblA patients reported herein showed a normal REE, validating the accuracy of our method.
There have been conflicting reports on the REE in MMA. Feillet et al (31) observed, on average, a 20% reduction as compared with predicted values, with significantly lower values in females. In contrast, Van Hagen et al (32) observed no difference between measured and predicted values of REE in 2 patients with MMA and in 6 patients with propionic acidemia. These authors noted a higher relative BMI than in the patients studied by Feillet et al and suggested that the intake of synthetic amino acid mixtures and a higher energy intake may account for the discrepancy. The results of our study suggest a means to reconcile these findings. By normalizing measured REE to FFM, we predict that both patient groups would show similar results to what we have documented in our cohort.
The failure of predictive equations to accurately estimate REE has been reported in various clinical settings (33–35). Because predictive equations are based on age, weight, and height of normal historical reference populations, adjustments for significant deviations from normal body composition, such as obesity, have been proposed (36–38). FFM represents ≈40% of body weight in nonobese patients (39) and is the major determinant of the REE, explaining ≈60–80% of its variance (40–43). Although lower than predicted in most of our patients, when measured REE was adjusted for FFM in this study group (mean REE/kg FFM = 41.8 ± 9.9 kcal/kg FFM in patients aged <18 y) (Table 5), it was very similar to values observed by other authors for controls in several pediatric populations (44–46). Because most of our patients had an increased amount of fat and decreased FFM, it is highly unlikely that a control set from the normal population will be identified, and future efforts will need to use patients with obesity (38) or decreased muscle mass syndromes, such as Duchenne muscular dystrophy (47), as comparators.
To examine the dependency of REE on other factors, measured REE, as well as REE adjusted for FFM, were used to query further correlations between clinical variables. Regression modeling then defined significant associations between FFM-adjusted REE and creatinine clearance, height z score, total protein intake, and age in the mut group (Table 5 and Supplemental Figure 1 under “Supplemental data” in the online issue). Whereas a depressed REE is observed in patients with MMA, it can be largely explained by the patients having diminished FFM, with contributions likely from factors known to affect REE, such as impaired renal function and protein intake (48–50). That protein intake may influence the REE in MMA is particularly intriguing because branched-chain amino acid oxidation, a process known to liberate the bulk of circulating metabolite load in patients (51), occurs largely in the skeletal muscle beds (52, 53), the dominant component of FFM. Examining organ-specific contributions to REE (54) may yield further insight into the mechanisms by which the REE is altered in this patient population.
The increased fat mass and BMI present in several patients in this study agrees with observations made by previous authors (10, 31, 32). The present article and that of Feillet et al (31) provide direct measures of the low basal energy requirements in this patent population and lend support to the observations by Thomas et al (55) that some patients with organic acidemias can achieve normal growth with an energy intake of as low as 53% of the RDA. Although the fat mass percentage was higher in our female population compared with males, this was not associated with a lower measured REE, as was reported by Feillet et al (31). Thus, the depressed REE does not explain the higher than expected fat mass of females in our study and suggests that, in some patients, abnormal body composition may be multifactorial. The total energy provided to patients in this study was ≈2-fold greater (mean: 1.91; range: 1.29–3.54; Table 3) than the measured REE. This ratio would correspond to the energy demands of heavy physical activity (56), yet none of the patients had more than mild to moderate activity levels. Whether the higher energy intake (Supplemental Figure 1A under “Supplemental data” in the online issue) and increased BMI in the younger age group (Supplemental Figure 1, B and C, under “Supplemental data” in the online issue) represent a trend toward increased use of gastrostomy tube feeding, supplemental medical food, or both in recent years is uncertain, but these factors are likely contributory to the abnormal body habitus seen in the patient population. Taken together, obesity can be influenced by multiple factors in this disorder, including decreased physical activity, continuous overnight feeding, excess energy intake to avoid catabolic state, and possibly other factors intrinsic to disease-related pathophysiology, such as mitochondrial dysfunction (57).
Until better treatment options become available, optimizing dietary management remains the primary means to improve outcome in patients with MMA. The results of this study show that predictive equations are not a reliable guide to the energy requirements of individuals with MMA, especially because significant deviations from normal growth and body composition are present. Future studies to examine whole-body metabolism with stable isotopes may be useful to delineate the factors determining energy requirements and nutritional needs in this patient population.
Supplementary Material
Acknowledgments
We thank Isa Bernardini, Roxanne Fischer, and Richard Hess for excellent laboratory assistance; the nurses and respiratory therapists of the NIH Clinical Research Center for their help with performing the indirect calorimetry studies; the patients and families for participating in the protocol; Elizabeth Moylan and Nancy Sebring for assistance with diet assessments, the NIH health technicians for food record analyses; and Laura N Venditti (Biomedical Statistical Consulting, Wynnewood, PA) for statistical advice.
The authors’ responsibilities were as follows—NSH: data collection and analysis and manuscript writing; IM: analysis of DXA data, statistical analysis, figure and table formatting, and manuscript writing; JCG: collection of dietary data and analysis, nutritional consultation of the patients, coordination of indirect calorimetry studies, and manuscript writing; JS: genetic counseling and molecular genetic studies for patients enrolled in the protocol, coordinator of NIH study 04-HG-0127, and manuscript writing; and CPV: study design, data collection and analysis, manuscript writing, administrative support, and supervision. No conflicts of interest were identified for any author.
REFERENCES
- 1.Manoli I, Venditti CP. Methylmalonic acidemia. GeneReviews at GeneTests: Medical Genetics Information Resource [database online]. 16 August 2005 (updated 28 September 2010). Seattle, WA: University of Washington, 2010.
- 2.Horster F, Garbade SF, Zwickler T, et al. Prediction of outcome in isolated methylmalonic acidurias: combined use of clinical and biochemical parameters. J Inherit Metab Dis 2009;32:630–9 [DOI] [PubMed] [Google Scholar]
- 3.Horster F, Baumgartner MR, Viardot C, et al. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut-, cblA, cblB). Pediatr Res 2007;62:225–30 [DOI] [PubMed] [Google Scholar]
- 4.Shevell MI, Matiaszuk N, Ledley FD, Rosenblatt DS. Varying neurological phenotypes among muto and mut- patients with methylmalonylCoA mutase deficiency. Am J Med Genet 1993;45:619–24 [DOI] [PubMed] [Google Scholar]
- 5.Wolff JA, Kelts DG, Algert S, Prodanos C, Nyhan WL. Alanine decreases the protein requirements of infants with inborn errors of amino acid metabolism. J Neurogenet 1985;2:41–9 [DOI] [PubMed] [Google Scholar]
- 6.Al-Owain M, Freehauf C, Bernstein L, Kappy M, Thomas J. Growth hormone deficiency associated with methylmalonic acidemia. J Pediatr Endocrinol Metab 2004;17:239–43 [DOI] [PubMed] [Google Scholar]
- 7.Kao CH, Liu MY, Liu TT, et al. Growth hormone therapy in neonatal patients with methylmalonic acidemia. J Chin Med Assoc 2009;72:462–7 [DOI] [PubMed] [Google Scholar]
- 8.Yannicelli S. Nutritional management of patients with inherited disorders of organic acid metabolism. Acosta PB. Nutrition management of patients with inherited metabolic disorders. Sudbury, MA: Jones and Bartlett Publishers, 2010:283–308 [Google Scholar]
- 9.Yannicelli S, Acosta PB, Velazquez A, et al. Improved growth and nutrition status in children with methylmalonic or propionic acidemia fed an elemental medical food. Mol Genet Metab 2003;80:181–8 [DOI] [PubMed] [Google Scholar]
- 10.Touati G, Valayannopoulos V, Mention K, et al. Methylmalonic and propionic acidurias: management without or with a few supplements of specific amino acid mixture. J Inherit Metab Dis 2006;29:288–98 [DOI] [PubMed] [Google Scholar]
- 11.Walter JH, Michalski A, Wilson WM, Leonard JV, Barratt TM, Dillon MJ. Chronic renal failure in methylmalonic acidaemia. Eur J Pediatr 1989;148:344–8 [DOI] [PubMed] [Google Scholar]
- 12.McDowell MA, Fryar CD, Hirsch R, Ogden CL. Anthropometric reference data for children and adults: U.S. population, 1999-2002. Adv Data 2005;1–5 [PubMed] [Google Scholar]
- 13.Ellis KJ, Shypailo RJ, Abrams SA, Wong WW. The reference child and adolescent models of body composition: a contemporary comparison. Ann N Y Acad Sci 2000;904:374–82 [DOI] [PubMed] [Google Scholar]
- 14.Ellis KJ, Abrams SA, Wong WW. Body composition reference data for a young multiethnic female population. Appl Radiat Isot 1998;49:587–8 [DOI] [PubMed] [Google Scholar]
- 15.Ellis KJ. Body composition of a young, multiethnic, male population. Am J Clin Nutr 1997;66:1323–31 [DOI] [PubMed] [Google Scholar]
- 16.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 1985;39(suppl 1):5–41 [PubMed] [Google Scholar]
- 18.FAO/WHO/UNU Energy and protein requirements: report of a joint FAO/WHO/UNU expert consultation. Geneva, Switzerland: World Health Organization, 1985 [PubMed] [Google Scholar]
- 19.Fleisch A. [Le metabolisme basal standard et sa determination au moyen du “metabocalculator”.] Helv Med Acta 1951;18:23–44 (in French) [PubMed] [Google Scholar]
- 20.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA 1918;4:370–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7 [DOI] [PubMed] [Google Scholar]
- 22.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. National Health Statistics Reports. June 25, 2010. Hyattsville, MD: National Center for Health Statistics, 2010:1–8 [PubMed] [Google Scholar]
- 23.Worgan LC, Niles K, Tirone JC, et al. Spectrum of mutations in mut methylmalonic acidemia and identification of a common Hispanic mutation and haplotype. Hum Mutat 2006;27:31–43 [DOI] [PubMed] [Google Scholar]
- 24.Ledley FD, Rosenblatt DS. Mutations in mut methylmalonic acidemia: clinical and enzymatic correlations. Hum Mutat 1997;9:1–6 [DOI] [PubMed] [Google Scholar]
- 25.Yannicelli S. Nutrition management of patients with inherited disorders of organic acid metabolism. Sudbury, MA: Jones and Bartlett Publishers, 2010 [Google Scholar]
- 26.Nyhan WL, Fawcett N, Ando T, Rennert OM, Julius RL. Response to dietary therapy in B 12 unresponsive methylmalonic acidemia. Pediatrics 1973;51:539–48 [PubMed] [Google Scholar]
- 27.Nyhan WL, Borden M, Childs B. Idiopathic hyperglycinemia: a new disorder of amino acid metabolism. II. The concentrations of other amino acids in the plasma and their modification by the administration of leucine. Pediatrics 1961;27:539–50 [PubMed] [Google Scholar]
- 28.Firouzbakhsh S, Mathis RK, Dorchester WL, et al. Measured resting energy expenditure in children. J Pediatr Gastroenterol Nutr 1993;16:136–42 [DOI] [PubMed] [Google Scholar]
- 29.Finan K, Larson DE, Goran MI. Cross-validation of prediction equations for resting energy expenditure in young, healthy children. J Am Diet Assoc 1997;97:140–5 [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez G, Moreno LA, Sarria A, Fleta J, Bueno M. Resting energy expenditure in children and adolescents: agreement between calorimetry and prediction equations. Clin Nutr 2002;21:255–60 [DOI] [PubMed] [Google Scholar]
- 31.Feillet F, Bodamer OA, Dixon MA, Sequeira S, Leonard JV. Resting energy expenditure in disorders of propionate metabolism. J Pediatr 2000;136:659–63 [DOI] [PubMed] [Google Scholar]
- 32.van Hagen CC, Carbasius Weber E, van den Hurk TA, et al. Energy expenditure in patients with propionic and methylmalonic acidaemias. J Inherit Metab Dis 2004;27:111–2 [DOI] [PubMed] [Google Scholar]
- 33.Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc 2005;105:775–89 [DOI] [PubMed] [Google Scholar]
- 34.Boullata J, Williams J, Cottrell F, Hudson L, Compher C. Accurate determination of energy needs in hospitalized patients. J Am Diet Assoc 2007;107:393–401 [DOI] [PubMed] [Google Scholar]
- 35.Thomson MA, Bucolo S, Quirk P, Shepherd RW. Measured versus predicted resting energy expenditure in infants: a need for reappraisal. J Pediatr 1995;126:21–7 [DOI] [PubMed] [Google Scholar]
- 36.Tverskaya R, Rising R, Brown D, Lifshitz F. Comparison of several equations and derivation of a new equation for calculating basal metabolic rate in obese children. J Am Coll Nutr 1998;17:333–6 [DOI] [PubMed] [Google Scholar]
- 37.Derumeaux-Burel H, Meyer M, Morin L, Boirie Y. Prediction of resting energy expenditure in a large population of obese children. Am J Clin Nutr 2004;80:1544–50 [DOI] [PubMed] [Google Scholar]
- 38.McDuffie JR, Adler-Wailes DC, Elberg J, et al. Prediction equations for resting energy expenditure in overweight and normal-weight black and white children. Am J Clin Nutr 2004;80:365–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest 1990;86:1423–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man: methods and results using a respiratory chamber. J Clin Invest 1986;78:1568–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravussin E, Bogardus C. Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr 1989;49:968–75 [DOI] [PubMed] [Google Scholar]
- 42.Goran MI, Kaskoun M, Johnson R. Determinants of resting energy expenditure in young children. J Pediatr 1994;125:362–7 [DOI] [PubMed] [Google Scholar]
- 43.Nielsen S, Hensrud DD, Romanski S, Levine JA, Burguera B, Jensen MD. Body composition and resting energy expenditure in humans: role of fat, fat-free mass and extracellular fluid. Int J Obes Relat Metab Disord 2000;24:1153–7 [DOI] [PubMed] [Google Scholar]
- 44.Hibbert JM, Hsu LL, Bhathena SJ, et al. Proinflammatory cytokines and the hypermetabolism of children with sickle cell disease. Exp Biol Med (Maywood) 2005;230:68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han JC, Balagopal P, Sweeten S, Darmaun D, Mauras N. Evidence for hypermetabolism in boys with constitutional delay of growth and maturation. J Clin Endocrinol Metab 2006;91:2081–6 [DOI] [PubMed] [Google Scholar]
- 46.Knops N, Wulffraat N, Lodder S, Houwen R, de Meer K. Resting energy expenditure and nutritional status in children with juvenile rheumatoid arthritis. J Rheumatol 1999;26:2039–43 [PubMed] [Google Scholar]
- 47.Zanardi MC, Tagliabue A, Orcesi S, Berardinelli A, Uggetti C, Pichiecchio A. Body composition and energy expenditure in Duchenne muscular dystrophy. Eur J Clin Nutr 2003;57:273–8 [DOI] [PubMed] [Google Scholar]
- 48.Avesani CM, Draibe SA, Kamimura MA, et al. Assessment of body composition by dual energy X-ray absorptiometry, skinfold thickness and creatinine kinetics in chronic kidney disease patients. Nephrol Dial Transplant 2004;19:2289–95 [DOI] [PubMed] [Google Scholar]
- 49.O'Sullivan AJ, Lawson JA, Chan M, Kelly JJ. Body composition and energy metabolism in chronic renal insufficiency. Am J Kidney Dis 2002;39:369–75 [DOI] [PubMed] [Google Scholar]
- 50.Shaw SN, Elwyn DH, Askanazi J, Iles M, Schwarz Y, Kinney JM. Effects of increasing nitrogen intake on nitrogen balance and energy expenditure in nutritionally depleted adult patients receiving parenteral nutrition. Am J Clin Nutr 1983;37:930–40 [DOI] [PubMed] [Google Scholar]
- 51.Thompson GN, Walter JH, Bresson JL, et al. Sources of propionate in inborn errors of propionate metabolism. Metabolism 1990;39:1133–7 [DOI] [PubMed] [Google Scholar]
- 52.Goldberg AL, Chang TW. Regulation and significance of amino acid metabolism in skeletal muscle. Fed Proc 1978;37:2301–7 [PubMed] [Google Scholar]
- 53.Brosnan JT, Brosnan ME. Branched-chain amino acids: enzyme and substrate regulation. J Nutr 2006;136:207S–11S [DOI] [PubMed] [Google Scholar]
- 54.Javed F, He Q, Davidson LE, et al. Brain and high metabolic rate organ mass: contributions to resting energy expenditure beyond fat-free mass. Am J Clin Nutr 2010;91:907–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas JA, Bernstein LE, Greene CL, Koeller DM. Apparent decreased energy requirements in children with organic acidemias: preliminary observations. J Am Diet Assoc 2000;100:1074–6 [DOI] [PubMed] [Google Scholar]
- 56.Torun B. Energy requirements of children and adolescents. Public Health Nutr 2005;8:968–93 [DOI] [PubMed] [Google Scholar]
- 57.Chandler RJ, Zerfas PM, Shanske S, et al. Mitochondrial dysfunction in mut methylmalonic acidemia. FASEB J 2009;23:1252–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.