Abstract
Background: Although dietary omega-3 (n−3) fatty acids may confer some cardiovascular benefits, it is unclear whether these nutrients may also unfavorably affect risk of type 2 diabetes (T2D).
Objective: We evaluated whether dietary omega-3 fatty acids and fish consumption were associated with increased risk of T2D.
Design: This was a prospective study of 36,328 women (mean age: 54.6 y) who participated in the Women's Health Study and who were followed from 1992 to 2008. Incident T2D was self-reported and validated primarily through the collection of supplementary information from participants. Information on omega-3 and fish intakes was obtained by using a validated food-frequency questionnaire. We used Cox proportional hazard models to estimate adjusted relative risks.
Results: During an average follow-up of 12.4 y, 2370 women developed T2D. Marine but not plant-based omega-3 fatty acids were positively associated with incident T2D. From the lowest to highest quintiles of marine omega-3 intake, the multivariable-adjusted hazard ratios (95% CIs) for T2D were 1.0 (referent), 1.17 (1.03, 1.33), 1.20 (1.05, 1.38), 1.46 (1.28, 1.66), and 1.44 (1.25, 1.65), respectively (P for trend < 0.0001). A similar association was observed with fish intake, but additional adjustment for docosahexaenoic acid led to the elimination of the association. The relation between marine omega-3 fatty acids and T2D was observed in hypertensive and nonhypertensive subjects and in women who reported infrequent fish consumption.
Conclusion: Our data suggest an increased risk of T2D with the intake of long-chain omega-3 fatty acids, especially with higher intakes (≥0.20 g omega-3/d or ≥2 servings of fish/d). The Women's Health Study was registered at clinicaltrials.gov as NCT00000479.
INTRODUCTION
Type 2 diabetes (T2D) is associated with costly medical complications and a higher risk of death. At birth, the lifetime risk of T2D ranges from 27% to 53%, depending on one's ethnicity (1). Despite advances in medical management of T2D with modern drugs, T2D remains on the rise and parallels the obesity epidemic. Therefore, it is critically important to identify relevant risk factors and protective measures to design effective preventive strategies. To this end, modifiable lifestyle factors, including diet, have been recognized to play an important role (2–5). Among dietary components, omega-3 (n−3) fatty acids have also been shown to confer some cardiac benefits (6–11). However, limited and inconsistent data have been reported on the effects of omega-3 fatty acids on glucose metabolism and insulin sensitivity.
Observational studies have reported adverse effects of high amounts of fish oils on glycemic control in subjects with T2D (12, 13). In a randomized control trial, fish-oil intervention with higher amounts of omega-3 (≈6 g omega-3/d) was also associated with increased blood glucose concentrations and decreased insulin sensitivity in T2D patients (14). However, Woodman et al (15) reported no effect of 4 g purified eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)/d on insulin sensitivity or fasting insulin concentrations in a 6-wk trial of T2D patients. Given the short durations and higher amounts of omega-3 used in these trials, it remains unclear whether EPA and DHA, especially when consumed in small amounts, have long-term effects on glucose metabolism. It is not known whether omega-3 fatty acids confer higher risk of T2D in the general population. Data from the Atherosclerosis Risk in Communities (ARIC) study (16) showed no association between marine omega-3 fatty acids and T2D risk, though it is unclear whether results were similar in men and women. Likewise, data from the Kouopio Ischemic Heart Disease Risk factor study reported no association between polyunsaturated fatty acids and incident diabetes (17); however, the Koupio study was a small study of men only in whom 34 cases of diabetes occurred after a short follow-up time (4 y). In contrast, in the Iowa Women's Health Study (18), a modest increased risk of T2D was observed only in the fifth quintile of marine omega-3 fatty acids, which suggested a threshold effect. Recent data from 3 prospective cohorts (the Nurses’ Health Study I and II and the Health Professionals Follow-Up Study) were suggestive of a modest increased risk of T2D with consumption of higher amounts of long-chain omega-3 and fish in women but not in men (19). However, it is unclear whether the fish-diabetes relation is mediated by long-chain omega-3. It remains important to determine whether consumption of small to moderate amounts of total and individual omega-3 adversely affects risk of T2D. Thus, we sought to prospectively examine the association between dietary omega-3 fatty acids [including α-linolenic acid (ALA)] and fish consumption and incident diabetes in the Women's Health Study.
SUBJECTS AND METHODS
Study participants
Subjects were participants in the Women's Health Study, a completed randomized, double-blind, placebo-controlled trial designed to study the effects of low-dose aspirin and vitamin E on the primary prevention of cardiovascular disease and cancer in 1992–2004. At the completion of the trial, participants have been prospectively followed up. Detailed descriptions of the Women's Health Study have been published (20–22). Of the 39,876 female subjects aged ≥45 y at entry (1992–1995), we excluded women with self-reported diabetes at baseline (n = 1148), missing data on omega-3 fatty acids (as a consequence of missing items on a food-frequency questionnaire to allow nutrient computation) or fish consumption (n = 1339), and missing data on key covariates, including exercise, smoking, alcohol consumption, hypertension, menopausal status, and body mass index (BMI; n = 1061). Thus, a total sample of 36,328 women from the parent study was analyzed for the current project. Each woman signed an informed consent form, and the Institutional Review Board at Brigham and Women's Hospital approved the study protocol.
Ascertainment of T2D
The ascertainment of T2D and other endpoints was achieved by asking women to report these items on annual follow-up questionnaires. All cases of self-reports of T2D were validated by using the American Diabetes Association criteria by primarily obtaining additional information with a telephone interview and supplemental questionnaire (23, 24). The positive predictive value for self-reported T2D in this cohort by using these validation procedures was 91% (23).
Exposure assessment
Dietary omega-3 fatty acids were derived from a single, baseline 128-food-frequency questionnaire, which was previously validated (25, 26). ALA, EPA, and DHA were individually adjusted for energy intake by using the residual method (27). For total marine omega-3 fatty acids, we used the sum of energy-adjusted EPA, DHA, and docosapentaenoic acid. For each fatty acid, we created quintiles and 4 indicator variables for analyses. Fish consumption was obtained through 4 items on the food-frequency questionnaire. Participants were asked to report their average consumption of canned tuna (3–4 oz), dark-meat fish (3–5 oz), other fish (3–5 oz), and shrimp, lobster, and scallops as a main dish over the past year. Possible responses included never or <1 time/mo, 1–3 times/mo, 1 time/wk, 2–4 times/wk, 5–6 times/wk, 1 time/d; 2–3 times/d, 4–5 times/d, and ≥6 times/d. We converted individual responses into servings per day by using the midpoint for each response category. We summed the frequency of consumption of canned tuna, dark fish, other fish, and shrimp, lobster, and scallops as a main dish to obtain a fish variable and created quintiles of fish consumption.
Ascertainment of other covariates
Demographic data were collected at baseline. Self-reported baseline weight and height were used to compute BMI (weight in kilograms divided by height in meters squared). Self-reported walking, stair climbing, and participation in 8 groups of recreational activities were obtained to estimate the energy expended on physical activity (28, 29). In addition, information on the prevalence of hypertension, hypercholesterolemia, parental history of diabetes, menopausal status, hormone therapy, smoking, and alcohol consumption was obtained at baseline.
Statistical analyses
We calculated person-time of follow-up from baseline until the first occurrence of 1) T2D, 2) death, or 3) censoring date, which was the date of receipt of the last follow-up questionnaire for the current study. We used Cox proportional hazard models to compute multivariable-adjusted hazard ratios with corresponding 95% CIs by using subjects in the lowest quintile of omega-3 fatty acid or fish consumption as the reference group. A parsimonious model adjusted for age, BMI, parental history of diabetes, smoking (never, former, and current smoker), exercise (quintiles of metabolic equivalent task hours per week), alcohol intake (4 categories), and menopausal status (pre- or postmenopausal or uncertain). A final multivariable model also controlled for red-meat intake and quintiles of energy intake, linoleic acid, ALA, dietary magnesium, trans and saturated fats, cereal fiber, and glycemic index (quintiles for all of these variables). To examine whether the relation between fish consumption and diabetes was mediated by EPA or DHA, we evaluated the risk of diabetes associated with reported fish intake while adjusting for EPA or DHA in the multivariable model. The P value for the linear trend was obtained by fitting a continuous variable that assigned the median value for each exposure category in a Cox regression model.
In secondary analyses, we examined possible effect modification by prevalent hypertension (yes or no) and infrequent compared with regular fish consumption. We also conducted sensitivity analyses by excluding subjects with <2 y of follow-up or by restricting follow-up to the first 5 y. Last, we analyzed omega-3 as a continuous variable. All analyses were completed with SAS, version 9.1 (SAS Institute, Cary, NC). The significance level was set at 0.05.
RESULTS
The mean age of women at baseline was 54.6 ± 7.0 y. The median intake (interquartile range) of ALA, EPA, DHA, and total marine omega-3 fatty acids in this population was 1.11 g ALA/d (0.92–1.34 ALA/d), 0.04 g EPA/d (0.02–0.08 g EPA/d), 0.12 g DHA/d (0.08–0.19 g DHA/d), and 0.18 g total marine omega-3 fatty acids/d (0.11–0.30 g total marine omega-3 fatty acids/d). The baseline characteristics of the study participants according to dietary omega-3 fatty acids are presented in Tables 1 and 2. A higher intake of omega-3 was associated with more physical activity and a higher intake of cereal fiber, fruit and vegetables, magnesium, and cholesterol. In contrast, omega-3 intake was inversely associated with the consumption of red meat, nuts, and trans and saturated fats.
TABLE 1.
Baseline characteristics of 36,328 women according to quintiles (Q) of dietary α-linolenic acid and eicosapentaenoic acid1
|
α-Linolenic acid (median) |
Eicosapentaenoic acid (median) |
|||||||
| Characteristic | Q1: 0.79 g/d | Q3: 1.11 g/d | Q5: 1.59 g/d | P | Q1: 0.01 g/d | Q3: 0.03 g/d | Q5: 0.12 g/d | P |
| n | 7423 | 7338 | 7261 | 7344 | 9399 | 7055 | ||
| Age (y) | 54.1 ± 6.92 | 54.5 ± 6.9 | 55.3 ± 7.3 | <0.0001 | 54.5 ± 7.3 | 54.3 ± 6.8 | 55.1 ± 7.0 | <0.0001 |
| White (%) | 93.1 | 95.6 | 96.8 | <0.0001 | 96.6 | 96.7 | 91.3 | <0.0001 |
| BMI (kg/m2) | 25.6 ± 4.9 | 25.9 ± 4.9 | 26.1 ± 5.1 | <0.0001 | 25.9 ± 5.1 | 26.0 ± 4.9 | 25.6 ± 4.7 | <0.0001 |
| Exercise (MET-h/wk) | 14.7 ± 19.3 | 14.4 ± 17.5 | 14.2 ± 17.4 | 0.010 | 12.2 ± 16.1 | 13.8 ± 17.2 | 17.9 ± 20.7 | <0.0001 |
| Fruit and vegetables (servings/d) | 5.32 ± 3.05 | 6.20 ± 3.20 | 6.39 ± 3.45 | <0.0001 | 5.41 ± 3.11 | 5.73 ± 2.97 | 6.61 ± 3.41 | <0.0001 |
| Nuts (servings/d) | 0.26 ± 0.46 | 0.27 ± 0.39 | 0.27 ± 0.39 | 0.11 | 0.30 ± 0.51 | 0.24 ± 0.35 | 0.22 ± 0.31 | <0.0001 |
| Red meat (servings/d) | 0.72 ± 0.59 | 0.74 ± 0.52 | 0.67 ± 0.51 | <0.0001 | 0.78 ± 0.61 | 0.71 ± 0.51 | 0.56 ± 0.45 | <0.0001 |
| Energy intake (kcal/d) | 1701 ± 522 | 1756 ± 537 | 1701 ± 532 | <0.0001 | 1715 ± 508 | 1667 ± 525 | 1648 ± 504 | <0.0001 |
| Dietary magnesium (mg)3 | 337 ± 80 | 339 ± 71 | 334 ± 75 | <0.0001 | 323 ± 76 | 335 ± 71 | 361 ± 76 | <0.0001 |
| trans Fatty acids (g/d)3 | 2.07 ± 1.01 | 2.28 ± 1.00 | 2.46 ± 1.18 | <0.0001 | 2.51 ± 1.17 | 2.33 ± 1.03 | 1.91 ± 0.90 | <0.0001 |
| Saturated fatty acids (g/d)3 | 19.4 ± 5.5 | 19.7 ± 4.7 | 19.9 ± 4.5 | <0.0001 | 20.7 ± 5.4 | 19.8 ± 4.5 | 18.2 ± 4.5 | <0.0001 |
| Dietary cholesterol (mg/d)3 | 222 ± 77 | 228 ± 71 | 221 ± 71 | <0.0001 | 204 ± 76 | 231 ± 65 | 242 ± 73 | <0.0001 |
| Cereal fiber (g/d)3 | 4.36 ± 1.44 | 4.54 ± 1.24 | 4.69 ± 1.29 | <0.0001 | 4.45 ± 1.36 | 4.47 ± 1.23 | 4.73 ± 1.37 | <0.0001 |
| Parental history of diabetes (%) | 24.3 | 24.8 | 25.8 | 0.19 | 24.7 | 25.0 | 24.9 | 0.70 |
| Glycated hemoglobin (%) | 5.03 ± 0.37 | 5.02 ± 0.35 | 5.04 ± 0.37 | 0.17 | 5.04 ± 0.36 | 5.03 ± 0.38 | 5.02 ± 0.38 | 0.06 |
| Current smoking (%) | 14.9 | 11.7 | 13.4 | <0.0001 | 13.9 | 14.4 | 11.3 | <0.0001 |
| Current drinking (%) | 55.3 | 57.7 | 53.7 | <0.0001 | 44.9 | 57.8 | 65.0 | <0.0001 |
| Postmenopausal (%) | 52.2 | 54.1 | 57.4 | <0.0001 | 53.4 | 53.1 | 56.7 | <0.0001 |
| HT use (%) | 40.6 | 42.8 | 43.8 | <0.0001 | 39.8 | 43.1 | 44.5 | <0.0001 |
| Hypertension (%) | 23.9 | 23.9 | 25.7 | 0.05 | 23.2 | 24.3 | 25.8 | 0.005 |
MET-h, metabolic equivalent task hours; HT, hormone therapy. P values were derived from ANOVA (continuous variables) or chi-square test (categorical variables).
Mean ± SD (all such values).
Energy adjusted.
TABLE 2.
Baseline characteristics of 36,328 women according to quintiles (Q) of dietary docosahexaenoic acid and total omega-3 fatty acids1
| Docosahexaenoic acid (median) |
Total marine omega-3 (median)2 |
|||||||
| Characteristic | Q1: 0.04 g/d | Q3: 0.12 g/d | Q5: 0.27 g/d | P | Q1: 0.07 g/d | Q3: 0.18 g/d | Q5: 0.43 g/d | P |
| n | 6984 | 6737 | 7588 | 7148 | 6661 | 7151 | ||
| Age (y) | 54.5 ± 7.33 | 54.4 ± 7.0 | 54.9 ± 6.9 | <0.0001 | 54.5 ± 7.3 | 54.4 ± 6.9 | 54.9 ± 6.9 | <0.0001 |
| White (%) | 96.5 | 96.2 | 91.9 | <0.0001 | 96.6 | 96.2 | 91.5 | <0.0001 |
| BMI (kg/m2) | 25.8 ± 5.0 | 25.9 ± 4.9 | 25.9 ± 4.9 | 0.19 | 25.8 ± 5.0 | 26.0 ± 4.9 | 25.8 ± 4.9 | 0.06 |
| Exercise (MET-h/wk) | 12.3 ± 16.5 | 14.2 ± 17.3 | 17.7 ± 20.5 | <0.0001 | 12.2 ± 16.4 | 14.1 ± 17.6 | 17.9 ± 20.7 | <0.0001 |
| Fruit and vegetables (servings/d) | 5.34 ± 3.20 | 5.96 ± 3.06 | 6.72 ± 3.53 | <0.0001 | 5.32 ± 3.16 | 5.93 ± 3.08 | 6.76 ± 3.52 | <0.0001 |
| Nuts (servings/d) | 0.30 ± 0.52 | 0.26 ± 0.37 | 0.22 ± 0.32 | <0.0001 | 0.30 ± 0.52 | 0.26 ± 0.38 | 0.22 ± 0.31 | <0.0001 |
| Red meat (servings/d) | 0.78 ± 0.63 | 0.73 ± 0.53 | 0.55 ± 0.45 | <0.0001 | 0.79 ± 0.63 | 0.72 ± 0.53 | 0.55 ± 0.45 | <0.0001 |
| Energy intake (kcal/d) | 1712 ± 553 | 1745 ± 539 | 1662 ± 515 | <0.0001 | 1705 ± 546 | 1727 ± 552 | 1662 ± 512 | <0.0001 |
| Dietary magnesium (mg)4 | 321 ± 79 | 336 ± 69 | 365 ± 77 | <0.0001 | 321 ± 79 | 336 ± 71 | 365 ± 77 | <0.0001 |
| trans Fatty acids (g/d)4 | 2.55 ± 1.20 | 2.30 ± 1.01 | 1.89 ± 0.89 | <0.0001 | 2.56 ± 1.20 | 2.30 ± 1.02 | 1.87 ± 0.88 | <0.0001 |
| Saturated fatty acids (g/d)4 | 20.8 ± 5.5 | 19.7 ± 4.5 | 18.1 ± 4.5 | <0.0001 | 20.9 ± 5.5 | 19.6 ± 4.5 | 18.0 ± 4.5 | <0.0001 |
| Dietary cholesterol (mg/d)4 | 190 ± 65 | 229 ± 70 | 251 ± 74 | <0.0001 | 192 ± 67 | 231 ± 70 | 250 ± 74 | <0.0001 |
| Cereal fiber (g/d)4 | 4.43 ± 1.36 | 4.49 ± 1.24 | 4.78 ± 1.39 | <0.0001 | 4.43 ± 1.37 | 4.48 ± 1.23 | 4.77 ± 1.39 | <0.0001 |
| Parental history of diabetes (%) | 24.6 | 24.5 | 25.6 | 0.43 | 24.6 | 24.6 | 25.1 | 0.94 |
| Glycated hemoglobin (%) | 5.04 ± 0.39 | 5.03 ± 0.35 | 5.03 ± 0.37 | 0.63 | 5.04 ± 0.37 | 5.03 ± 0.38 | 5.02 ± 0.38 | 0.32 |
| Current smoking (%) | 14.4 | 13.3 | 11.6 | <0.0001 | 14.5 | 13.8 | 11.4 | <0.0001 |
| Current drinking (%) | 45.8 | 57.5 | 61.9 | <0.0001 | 45.6 | 56.7 | 63.1 | <0.0001 |
| Postmenopausal (%) | 53.3 | 54.1 | 56.3 | <0.0001 | 53.5 | 53.7 | 56.2 | <0.0001 |
| HT use (%)4 | 40.2 | 42.2 | 44.3 | <0.0001 | 40.1 | 42.7 | 44.3 | <0.0001 |
| Hypertension (%) | 23.1 | 24.3 | 26.2 | 0.0002 | 23.0 | 24.8 | 26.3 | 0.0001 |
MET-h, metabolic equivalent task hours; HT, hormone therapy. P values were derived from ANOVA (for continuous variables) or chi-square test (for categorical variables).
Includes eicosapentaenoic acid, docosahexaenoic acid, and docosapentaenoic acid.
Mean ± SD (all such values).
Energy adjusted.
Dietary omega-3 and risk of T2D
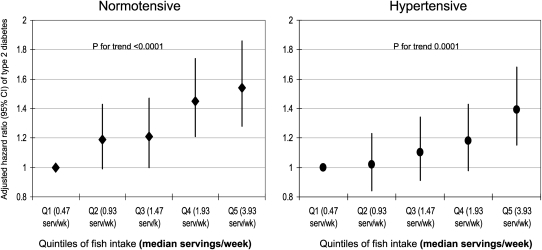
During a mean follow-up of 12.4 y, 2370 (6.5%) new cases of T2D occurred in this population. Dietary intake of ALA was not associated with incident diabetes (Table 3). In contrast, a higher intake of marine omega-3 fatty acids was associated with incident T2D. Compared with the lowest quintile of EPA, the risk of T2D was 8% (−6% to 24%), 25% (11–42%), 30% (13–49%), and 38% (21–59%) higher in the second, third, fourth, and fifth quintiles of EPA, respectively, in a model that was controlled for age, BMI, parental history of diabetes, smoking, exercise, alcohol intake, menopausal status, red-meat intake, and quintiles of energy intake, linoleic acid, ALA, dietary magnesium, trans fat, saturated fat, cereal fiber, and glycemic index (P for linear trend < 0.0001; Table 3). Corresponding values for DHA were 21% (6–38%), 21% (6–39%), 46% (28–68%), and 52% (33–75%) higher, respectively (P for linear trend < 0.0001; Table 3). For total long-chain omega-3 fatty acids (EPA, docosapentaenoic acid, and DHA), corresponding increased risk of T2D was 17% (3–33%), 20% (5–38%), 46% (28–66%), and 44% (25–65%) higher, respectively (P for trend < 0.0001; Table 3). These findings persisted after stratification by fish intake. For example, in women who reported infrequent fish consumption (<1/wk), multivariable-adjusted hazard ratios (95% CIs) for T2D were 1.0, 1.21 (1.03, 1.42), 1.27 (1.01, 1.60), 1.11 (0.79, 1.57), and 1.76 (1.02, 3.04) from the lowest to the highest quintiles of total long-chain omega-3 fatty acids, respectively (P for trend = 0.015). In women who reported fish consumption of >1/wk, corresponding hazard ratios (95% CIs) were 1.0, 0.99 (0.74, 1.33), 1.03 (0.78, 1.38), 1.31 (0.99, 1.74), and 1.28 (0.96, 1.70), respectively (P for trend = 0.0002). Likewise, a similar association was observed when the relation between fish intake and T2D was stratified by prevalent hypertension at baseline, and no significant interaction was shown between fish intake and hypertension (P = 0.74; Figure 1).
TABLE 3.
Type 2 diabetes by omega-3 fatty acids1
| Type of omega-3 | No. of cases | Crude | Model 1 | Model 2 |
| α-Linolenic acid | ||||
| Q1 (0.79 g/d) | 468 | 1.0 | 1.0 | 1.0 |
| Q2 (0.96 g/d) | 424 | 0.95 (0.83,1.08)2 | 0.94 (0.83,1.08) | 0.94 (0.82,1.09) |
| Q3 (1.11 g/d) | 470 | 1.00 (0.88,1.14) | 0.99 (0.87,1.12) | 0.98 (0.85,1.14) |
| Q4 (1.29 g/d) | 505 | 1.09 (0.96,1.24) | 1.01 (0.89,1.15) | 1.00 (0.86,1.17) |
| Q5 (1.59 g/d) | 503 | 1.09 (0.96,1.23) | 0.96 (0.85,1.09) | 1.01 (0.85,1.21) |
| P for trend | — | 0.04 | 0.86 | 0.67 |
| Eicosapentaenoic acid | ||||
| Q1 (0.01 g/d) | 450 | 1.0 | 1.0 | 1.0 |
| Q2 (0.02 g/d) | 370 | 1.04 (0.90,1.19) | 1.07 (0.93,1.22) | 1.08 (0.94,1.24) |
| Q3 (0.03 g/d) | 646 | 1.12 (0.99,1.26) | 1.20 (1.06,1.35) | 1.25 (1.11,1.42) |
| Q4 (0.08 g/d) | 447 | 1.08 (0.95,1.24) | 1.26 (1.11,1.44) | 1.30 (1.13,1.49) |
| Q5 (0.12 g/d) | 457 | 1.07 (0.94,1.21) | 1.26 (1.10,1.44) | 1.38 (1.21,1.59) |
| P for trend | — | 0.57 | 0.0005 | <0.0001 |
| Docosahexaenoic acid | ||||
| Q1 (0.04 g/d) | 400 | 1.0 | 1.0 | 1.0 |
| Q2 (0.09 g/d) | 524 | 1.13 (1.00,1.29) | 1.19 (1.04,1.35) | 1.21 (1.06,1.38) |
| Q3 (0.12 g/d) | 424 | 1.09 (0.95,1.25) | 1.16 (1.01,1.33) | 1.21 (1.06,1.39) |
| Q4 (0.17 g/d) | 481 | 1.21 (1.06,1.38) | 1.36 (1.19,1.56) | 1.46 (1.28,1.68) |
| Q5 (0.17 g/d) | 541 | 1.25 (1.10,1.43) | 1.35 (1.19,1.54) | 1.52 (1.33,1.75) |
| P for trend | — | 0.0005 | <0.0001 | <0.0001 |
| Total marine omega-3 | ||||
| Q1 (0.07 g/d) | 419 | 1.0 | 1.0 | 1.0 |
| Q2 (0.13 g/d) | 521 | 1.10 (0.97,1.26) | 1.15 (1.02,1.31) | 1.17 (1.03,1.33) |
| Q3 (0.18 g/d) | 428 | 1.09 (0.95,1.25) | 1.14 (1.00,1.31) | 1.20 (1.05,1.38) |
| Q4 (0.28 g/d) | 514 | 1.19 (1.05,1.35) | 1.37 (1.20,1.56) | 1.46 (1.28,1.66) |
| Q5 (0.43 g/d) | 488 | 1.17 (1.03,1.33) | 1.29 (1.13,1.47) | 1.44 (1.25,1.65) |
| P for trend | — | 0.015 | <0.0001 | <0.0001 |
Q, quintile. Model 1 was adjusted for age, BMI, parental history of diabetes, smoking (never, former, or current), exercise (quintiles of metabolic equivalent task hours per week), alcohol intake (4 categories), and menopausal status (not sure or pre- or postmenopausal) by using Cox proportional hazard models. Model 2 was adjusted for age, BMI, parental history of diabetes, smoking (never, former, or current), exercise (quintiles of metabolic equivalent task hours per week), alcohol intake (4 categories), menopausal status (not sure or pre- or postmenopausal), red-meat intake, and quintiles of energy intake, linoleic acid, α-linolenic acid, dietary magnesium, trans fat, saturated fat, cereal fiber, and glycemic index by using Cox proportional hazard models.
Hazard ratio; 95% CI in parentheses (all such values).
FIGURE 1.
Multivariable-adjusted hazard ratios (95% CIs) for type 2 diabetes according to fish consumption and prevalent hypertension obtained from Cox proportional hazard models. P value for interaction between hypertension and fish intake = 0.74. Q, quintile; serv, servings.
Fish consumption and risk of T2D
A higher consumption of fish was associated with a higher risk of incident T2D, especially in the fourth and fifth quintiles of fish intake (P for trend < 0.0001; Table 4). In the comparison of a median fish intake of 3.93 servings of fish/wk (highest quintile) with the lowest category of fish consumption (median: 0.47 serving of fish/wk), there was a 49% (30–70%) increased risk of T2D in a multivariable-adjusted model (Table 4). This positive association between fish consumption and T2D risk was almost eliminated on additional adjustment of DHA (which contributed >50% of total marine omega-3 FA in this population) and was minimally influenced after additional adjustment for EPA (Table 5).
TABLE 4.
Type 2 diabetes according to median fish consumption1
| Quintiles of fish consumption | No. of cases | Crude | Model 1 | Model 2 |
| 1 (0.47 servings of fish/wk) | 464 | 1.0 | 1.0 | 1.0 |
| 2 (0.93 servings of fish/wk) | 450 | 1.10 (0.97,1.25)2 | 1.11 (0.98,1.27) | 1.11 (0.98,1.27) |
| 3 (1.47 servings of fish/wk) | 402 | 1.06 (0.92,1.21) | 1.16 (1.01,1.32) | 1.17 (1.02,1.34) |
| 4 (1.93 servings of fish/wk) | 503 | 1.12 (0.99,1.27) | 1.32 (1.17,1.50) | 1.35 (1.19,1.54) |
| 5 (3.93 servings of fish/wk) | 551 | 1.38 (1.28,1.56) | 1.41 (1.25,1.60) | 1.49 (1.30,1.70) |
| P for trend | — | <0.0001 | <0.0001 | <0.0001 |
Model 1 was adjusted for age, BMI, parental history of diabetes, smoking (never, former, or current), exercise (quintiles of metabolic equivalent task hours per week), alcohol intake (4 categories), and menopausal status (not sure or pre- or postmenopausal) by using Cox proportional hazard models. Model 2 was adjusted for age, BMI, parental history of diabetes, smoking (never, former, or current), exercise (quintiles of metabolic equivalent task hours per week), alcohol intake (4 categories), menopausal status (not sure or pre- or postmenopausal), red-meat intake, and quintiles of energy intake, linoleic acid, α-linolenic acid, dietary magnesium, trans fat, saturated fat, cereal fiber, and glycemic index by using Cox proportional hazard models.
Hazard ratio; 95% CI in parentheses (all such values).
TABLE 5.
Type 2 diabetes by median fish intake and potential mediation by eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)1
| Quintiles of fish consumption | No. of cases | Full model | Full model + DHA | Full model + EPA |
| 1 (0.47 servings of fish/wk) | 464 | 1.0 | 1.0 | 1.0 |
| 2 (0.93 servings of fish/wk) | 450 | 1.11 (0.98,1.27)2 | 1.01 (0.86,1.18) | 1.08 (0.91,1.29) |
| 3 (1.47 servings of fish/wk) | 402 | 1.17 (1.02,1.34) | 1.02 (0.85,1.22) | 1.12 (0.90,1.38) |
| 4 (1.93 servings of fish/wk) | 503 | 1.35 (1.19,1.54) | 1.12 (0.92,1.38) | 1.28 (1.02,1.62) |
| 5 (3.93 servings of fish/wk) | 551 | 1.49 (1.30,1.70) | 1.19 (0.94,1.51) | 1.42 (1.11,1.80) |
| P for trend | — | <0.0001 | 0.10 | 0.002 |
Full model was adjusted for age, BMI, parental history of diabetes, smoking (never, former, or current), exercise (quintiles of metabolic equivalent task hours per week), alcohol intake (4 categories), menopausal status (not sure or pre- or postmenopausal), red-meat intake, and quintiles of energy intake, linoleic acid, α-linolenic acid, dietary magnesium, trans fat, saturated fat, cereal fiber, and glycemic index by using Cox proportional hazard models.
Hazard ratio; 95% CI in parentheses (all such values).
Sensitivity analyses
When we restricted analyses to the first 5 y of follow-up, we observed similar findings. For example, from the lowest to the highest quintiles of fish intake, multivariable-adjusted hazard ratios (95% CIs) were 1.0, 1.07 (0.84, 1.37), 1.21 (0.94, 1.55), 1.31 (1.03, 1.68), and 1.42 (1.11, 1.82), respectively (P for trend = 0.004). Similar results were seen for marine omega-3 (P for trend = 0.009). The exclusion of women with <2 y of follow-up and stratification by BMI did not alter these results (data not shown). When analyzed as continuous variables, the β coefficient (SE) and P value from the multivariable models were 0.98121 (0.27659) and 0.0004 for EPA, 0.78496 (0.16163) and <0.0001 for DHA, and 0.43879 (0.09749) and <0.0001 for total marine omega-3 fatty acids. Restriction to white women (95% of the sample) did not alter the findings.
DISCUSSION
In this prospective study, we showed that dietary marine omega-3 fatty acids (EPA and DHA) were individually associated with an increased risk of incident diabetes, especially with omega-3 intake ≥0.20 g omega-3/d or ≥2 servings of fish/d (which suggested a threshold effect). Furthermore, fish consumption was positively related to incident diabetes, and this association was attenuated after further adjustment for DHA. In contrast, the plant-based omega-3 fatty acid (ALA) was not associated with incident diabetes in this cohort.
Although omega-3 fatty acids have been shown to lower risk of coronary heart disease and cardiac deaths, limited and inconsistent data are available on the relation between omega-3 fatty acids and measures of glucose metabolism. In a cross-sectional study, a positive relation was observed between dietary ALA and fasting insulin but not fasting glucose concentrations (4), but Woodman et al (15) reported no effect of high amounts of purified EPA or DHA (4 g EPA or DHA/d) on insulin sensitivity or fasting insulin concentrations in a 6-wk trial of T2D patients. In a case-cohort study (30), neither phospholipids nor dietary omega-3 fatty acids were associated with incident diabetes. These results were similar to those from the ARIC study (16) and the Kouopio study (17) that also showed no association between marine omega-3 fatty acids or polyunsaturated fatty acids and T2D risk, respectively. In contrast, higher but not lower amounts of long-chain omega-3 fatty acids were associated with a modest increase in incident diabetes in participants of the Nurses’ Health Study I and II with a significant 25% increased risk of diabetes when the highest to the lowest quintiles of long-chain omega-3 fatty acids were compared (19). A suggestive, although not significant, increased risk of T2D with higher intakes of long-chain omega-3 fatty acids was reported in 51,529 participants of the Health Professionals Follow-Up Study (19). Our data showed increased risk of T2D with higher intakes of long-chain omega-3 fatty acids and are consistent with a threshold effect as suggested by reports from the Nurses’ Health studies (19) and the Iowa Women's Health Study (18). Specifically, relative risks were close to unity except for the fifth quintile (0.39 g omega-3/d), which showed increased risk of diabetes in the Iowa Women's Health Study (18) [adjusted hazard ratio (95% CI) of 1.20 (1.03, 1.39) compared to the lowest quintile of long-chain omega-3 fatty acids). Comparable thresholds were observed in the Nurses’ Health Study (19) (0.20–0.25 g omega-3/d) and the current study (0.18 g omega-3/d).
Long-chain omega-3 fatty acids are predominantly found in fatty fish. Hence, if the association between EPA and DHA and T2D were causal, then one would expect an increased risk of T2D with frequent intakes of fish. Data from the Nurses’ Health studies (19) reported an increased risk of T2D with fish consumption of ≥2 servings of fish/wk but not a meaningful increase below that threshold; eg, the multivariable-adjusted hazard ratios (95% CIs) were 1.0, 1.02 (0.87, 1.21), 1.12 (0.97, 1.29), 1.22 (1.04, 1.43), and 1.29 (1.05, 1.57) for fish intake of <1 time/mo, 1–3 times/mo, 1 time/wk, 2–4 times/wk, and ≥5 times/wk, respectively. These results were consistent with our findings of which higher (≈2 or more servings of fish/d) but not lower amounts of fish consumption were associated with an increased risk of diabetes. The magnitude of effect was larger in our cohort, with a 49% increased risk of T2D in the highest quintile of fish intake (median: 3.93 servings of fish/wk) than in the lowest quintile of fish intake (median: 0.46 servings of fish/wk). Because other nutrients in fish (other than EPA and DHA) could partially explain the positive association between fish consumption and T2D, we examined the possible mediation by EPA and DHA. The fish-diabetes relation was minimally attenuated after adjustment for EPA and was eliminated after adjustment for DHA. Such attenuation was consistent with a potential causal effect of DHA shown in fish. Furthermore, the fact that we observed a similar positive relation between dietary long-chain omega-3 and T2D risk in women who reported infrequent fish consumption further strengthened the hypothesis that DHA but no other nutrients in fish or contaminants in fish may be responsible for the observed findings. In our sample, 23% of long-chain omega-3 consumed was EPA whereas 77% was DHA, on average. This unbalanced distribution of EPA and DHA might provide an alternative explanation of why we observed only a minimal attenuation of the fish-diabetes association after adjustment for EPA.
We did not observe an association between plant-based ALA and incident diabetes. This result was consistent with the findings of the Nurses’ Health Study and Health Professionals Follow-Up Study, which also did not show a relation between ALA and incident diabetes (19). Likewise, the ARIC study (16) did not find a difference in plasma ALA concentrations between diabetes cases and noncases. It is possible that health benefits of ALA may differ from those of long-chain omega-3 fatty acids.
What biological mechanisms, if any, may support a causal relation between long-chain omega-3 fatty acids and incident diabetes? Compared with olive oil intake in patients with T2D, a 6-wk intervention with 4 g EPA or DHA led to a 1.40-mmol/L (P = 0.002) and 0.98-mmol/L (P = 0.002) increase in fasting glucose concentrations, respectively (15); In contrast, neither EPA nor DHA had any effect on fasting insulin concentrations, insulin sensitivity, or C-peptide or glycated hemoglobin (Hb A1c) concentrations (15). In a meta-analysis of 26 trials, fish-oil intake, compared with placebo intake, was associated with elevated fasting glucose concentrations; each increase of a gram of EPA per day led to a 0.38% (95% CI: 0.00%, 0.76%) increase in Hb A1c concentrations and the corresponding increase in Hb A1c concentrations with DHA was 0.6% (95% CI: 0.06, 1.15%) (31). In particular, every gram of DHA per day was associated with an increase in fasting glucose concentrations of 0.74 mmol/L (95% CI: 0.16, 1.32 mmol/L) (31). However, another meta-analysis of 18 trials did not find a significant effect of fish oil on fasting glucose or Hb A1c concentrations (32). In a 6-wk intervention trial in 59 overweight individuals, an intake of 4 g EPA/d was associated with a slight increase in fasting glucose concentrations (P = 0.062), whereas DHA intake had no effect on fasting glucose concentrations; in addition, EPA and DHA intakes were associated with an 18% (P = 0.035) and 27% (P = 0.001) increase in fasting insulin concentrations compared with those associated with a placebo intake (33). In another randomized trial, fish-oil (containing 1.8 g EDA/d and 3 g DHA/d) intervention was associated with increased fasting glucose concentrations and decreased insulin sensitivity in T2D subjects after 9 wk of intervention (14). In contrast, other randomized trials showed no effects of EPA and DHA on insulin sensitivity (34, 35). Although inconsistent across studies, there are possible biologic mechanisms to support a causal relation between higher intakes of long-chain omega-3 fatty acids and incident diabetes.
Our study has some limitations. First, we had only one dietary assessment for our analyses. Thus, we were unable to account for possible changes over time in omega-3 fatty acid intake in our cohort. However, similar relations were seen when analyses were restricted to the first 5 y of follow-up (the period within which dietary habits were less likely to change much), which suggested that our findings were unlikely to be explained by a possible bias introduced by a change in dietary habits over time. Second, subjects at risk of cardiovascular disease who may also be at increased risk of T2D may have been advised to consume more fish by their primary care physician. Such a scenario would have led to a bias of a positive association. The fact that we saw similar results in women who reported infrequent fish consumption, and individuals with prevalent coronary disease at baseline were excluded, minimizes the possibility of confounding by indication in our data. Third, we could not exclude exposure misclassification because of the self-reported nature of diet. However, because the collection of dietary information preceded the occurrence of diabetes, such misclassification was likely to be nondifferential and bias the observed results toward the null. Fourth, we could not exclude residual or unmeasured confounding because of observational nature of our study. We also lacked detailed data on fish-oil supplements. Fifth, we did not have data on fasting insulin and glucose concentrations to explore possible biological pathways by which dietary omega-3 may influence risk of diabetes. Given the high correlation between EPA and DHA (Pearson's r = 0.91) in our data, collinearity prevented us from completely examining the independent effects of EPA or DHA on diabetes (while adjusting for the other type of omega-3). Sixth, we did not have data on fasting glucose or hemoglobin A1c concentrations on all participants to fully examine relations between dietary omega-3 and those biomarkers. Lastly, participants were mostly white, female, health care professionals (95%), which thereby limited the generalizability of our findings. Although we showed a positive association between higher amounts of omega-3 and T2D in white women, we did not have adequate sample size to explore such a relation in other ethnic groups.
Nevertheless, the large sample size, the lengthy (≥10 y) follow-up and nearly complete ascertainment of vital status in this cohort, the robustness of our results in sensitivity analyses, the standardized method of collection of data on various potential confounders, and the large number of incident-diabetes cases were major strengths of this study.
If confirmed in other populations, these findings can inform future recommendations on dietary approaches to prevent T2D. In consideration of the potential cardiovascular benefits of omega-3, future studies are warranted to determine a threshold below which cardiac benefits are preserved and risk of T2D remains neutral. Because of the lack of an association with ALA, plant-based omega-3 might appear attractive for people at increased risk of T2D (including individuals with a positive parental history of diabetes or with genetic polymorphism associated with an increased risk of diabetes).
In conclusion, our data are consistent with a modest and threshold relation between EPA and DHA intakes and incident T2D, whereas ALA intake does not appear to be associated with an increase risk. Because the observed risk of T2D was mostly seen with higher intakes of omega-3 or fish (≈≥2 servings omega-3 or fish/d), our data do not support the prohibition of long-chain omega-3 fatty acids when consumed in moderation. Additional studies that confirm these findings and that focus on biological mechanisms in nondiabetic individuals and possible differential effects of EPA compared with DHA on glucose metabolism and insulin sensitivity are warranted.
Acknowledgments
We thank the 39,876 dedicated and committed participants of the Women's Health Study and the entire Women's Health Study staff for their expert and unfailing assistance.
The authors’ responsibilities were as follows—LD and I-ML: had full access to all study data and were responsible for data integrity and accuracy of data analyses; LD: study concept and design, drafting of the manuscript, and statistical analyses; JEB: acquisition of data; JEB and I-ML: obtained funding; JMG and I-ML: study supervision; and all authors: analysis and interpretation of data and critical revision of the manuscript for important intellectual content. LD is currently the recipient of investigator-initiated grants from the National Institutes of Health. LD received investigator-initiated grants from the Huntington's Disease Society of America, the National Institutes of Health, and the Biomedical Research Institute, Brigham and Women's Hospital. JMG received investigator-initiated grants from the National Institutes of Health, the Veterans Administration, Amgen, and Veroscience and Pills and packaging for a research study from BASF, DSM, and Wyeth. JMG served as a consultant to Bayer and as a medical expert for Merck. JEB received investigator-initiated grants from the National Institutes of Health. I-ML received investigator-initiated grants from the National Institutes of Health and has served as a consultant to Virgin HealthMiles.
REFERENCES
- 1.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–90 [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Kamineni A, Carnethon M, Djousse L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med 2009;169:798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–7 [DOI] [PubMed] [Google Scholar]
- 4.Djousse L, Hunt CS, Tang W, Eckfeldt JH, Province MA, Ellison CR. Dietary linolenic acid and fasting glucose and insulin: The National Heart, Lung, and Blood Institute Family Heart Study. Obesity (Silver Spring) 2006;14:295–300 [DOI] [PubMed] [Google Scholar]
- 5.Kochar J, Djousse L, Gaziano JM. Breakfast cereals and risk of type-2 diabetes in the Physicians’ Health Study I. Obesity (Silver Spring) 2007;15:3039–44 [DOI] [PubMed] [Google Scholar]
- 6.Djousse L, Arnett DK, Carr JJ, et al. Dietary linolenic acid is inversely associated with calcified atherosclerotic plaque in the coronary arteries: the NHLBI Family Heart Study. Circulation 2005;111:2921–6 [DOI] [PubMed] [Google Scholar]
- 7.Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n−3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002;105:1897–903 [DOI] [PubMed] [Google Scholar]
- 8.de Lorgeril M, Renaud S, Mamelle N, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994;343:1454–9 [DOI] [PubMed] [Google Scholar]
- 9.Albert CM, Campos H, Stampfer MJ, et al. Blood levels of long-chain n−3 fatty acids and the risk of sudden death. N Engl J Med 2002;346:1113–8 [DOI] [PubMed] [Google Scholar]
- 10.Jenkins DJ, Josse AR, Dorian P, et al. Heterogeneity in randomized controlled trials of long chain (fish) omega-3 fatty acids in restenosis, secondary prevention and ventricular arrhythmias. J Am Coll Nutr 2008;27:367–78 [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–8 [DOI] [PubMed] [Google Scholar]
- 12.Kasim SE, Stern B, Khilnani S, McLin P, Baciorowski S, Jen KL. Effects of omega-3 fish oils on lipid metabolism, glycemic control, and blood pressure in type II diabetic patients. J Clin Endocrinol Metab 1988;67:1–5 [DOI] [PubMed] [Google Scholar]
- 13.Friday KE, Childs MT, Tsunehara CH, Fujimoto WY, Bierman EL, Ensinck JW. Elevated plasma glucose and lowered triglyceride levels from omega-3 fatty acid supplementation in type II diabetes. Diabetes Care 1989;12:276–81 [DOI] [PubMed] [Google Scholar]
- 14.Mostad IL, Bjerve KS, Bjorgaas MR, Lydersen S, Grill V. Effects of n−3 fatty acids in subjects with type 2 diabetes: reduction of insulin sensitivity and time-dependent alteration from carbohydrate to fat oxidation. Am J Clin Nutr 2006;84:540–50 [DOI] [PubMed] [Google Scholar]
- 15.Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr 2002;76:1007–15 [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003;78:91–8 [DOI] [PubMed] [Google Scholar]
- 17.Laaksonen DE, Lakka TA, Lakka HM, et al. Serum fatty acid composition predicts development of impaired fasting glycaemia and diabetes in middle-aged men. Diabet Med 2002;19:456–64 [DOI] [PubMed] [Google Scholar]
- 18.Meyer KA, Kushi LH, Jacobs DR, Jr, Folsom AR. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care 2001;24:1528–35 [DOI] [PubMed] [Google Scholar]
- 19.Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, Hu FB. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr 2009;90:613–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA 2005;294:56–65 [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005;352:1293–304 [DOI] [PubMed] [Google Scholar]
- 22.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA 2005;294:47–55 [DOI] [PubMed] [Google Scholar]
- 23.Ding EL, Song Y, Manson JE, Pradhan AD, Buring JE, Liu S. Accuracy of administrative coding for type 2 diabetes in children, adolescents, and young adults. Diabetes Care 2007;30:e98. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Lee IM, Song Y, et al. Vitamin E and risk of type 2 diabetes in the women's health study randomized controlled trial. Diabetes 2006;55:2856–62 [DOI] [PubMed] [Google Scholar]
- 25.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 26.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26 [DOI] [PubMed] [Google Scholar]
- 27.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8S [DOI] [PubMed] [Google Scholar]
- 28.Lee I-M, Paffenbarger RS., Jr Design of present-day epidemiologic studies of physical activity and health : Lee I-M. Epidemiologic methods in physical activity studies. New York, NY: Oxford University Press, 2009 [Google Scholar]
- 29.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9 [DOI] [PubMed] [Google Scholar]
- 30.Hodge AM, English DR, O'Dea K, et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007;86:189–97 [DOI] [PubMed] [Google Scholar]
- 31.Friedberg CE, Janssen MJ, Heine RJ, Grobbee DE. Fish oil and glycemic control in diabetes. A meta-analysis. Diabetes Care 1998;21:494–500 [DOI] [PubMed] [Google Scholar]
- 32.Montori VM, Farmer A, Wollan PC, Dinneen SF. Fish oil supplementation in type 2 diabetes: a quantitative systematic review. Diabetes Care 2000;23:1407–15 [DOI] [PubMed] [Google Scholar]
- 33.Mori TA, Burke V, Puddey IB, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr 2000;71:1085–94 [DOI] [PubMed] [Google Scholar]
- 34.Kabir M, Skurnik G, Naour N, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr 2007;86:1670–9 [DOI] [PubMed] [Google Scholar]
- 35.Griffin MD, Sanders TA, Davies IG, et al. Effects of altering the ratio of dietary n−6 to n−3 fatty acids on insulin sensitivity, lipoprotein size, and postprandial lipemia in men and postmenopausal women aged 45-70 y: the OPTILIP Study. Am J Clin Nutr 2006;84:1290–8 [DOI] [PubMed] [Google Scholar]