Abstract
Background: Preterm delivery represents a substantial problem in perinatal medicine worldwide. Current knowledge on potential influences of probiotics in food on pregnancy complications caused by microbes is limited.
Objective: We hypothesized that intake of food with probiotics might reduce pregnancy complications caused by pathogenic microorganisms and, through this, reduce the risk of spontaneous preterm delivery.
Design: This study was performed in the Norwegian Mother and Child Cohort on the basis of answers to a food-frequency questionnaire. We studied intake of milk-based products containing probiotic lactobacilli and spontaneous preterm delivery by using a prospective cohort study design (n = 950 cases and 17,938 controls) for the pregnancy outcome of spontaneous preterm delivery (<37 gestational weeks). Analyses were adjusted for the covariates of parity, maternal educational level, and physical activity.
Results: Pregnancies that resulted in spontaneous preterm delivery were associated with any intake of milk-based probiotic products in an adjusted model [odds ratio (OR): 0.857; 95% CI: 0.741, 0.992]. By categorizing intake into none, low, and high intakes of the milk-based probiotic products, a significant association was observed for high intake (OR: 0.820; 95% CI: 0.681, 0.986).
Conclusion: Women who reported habitual intake of probiotic dairy products had a reduced risk of spontaneous preterm delivery.
INTRODUCTION
See corresponding editorial on page 3.
Preterm delivery (PTD) is one of the most common causes of perinatal mortality and morbidity worldwide. This condition accounts for most of the important adverse outcomes of pregnancy, including 70% of perinatal deaths and almost half of all subsequent postnatal neurologic complications, causing a substantial burden on society (1, 2). In Scandinavian countries, the rates of PTD are 6.4% in Norway, 6.3% in Denmark, and 5.6% in Sweden (3). Other European countries have comparable rates; however, in the United States the rate is as high as 12–13% (4–7).
PTD has a complex etiology, with the implication of several different potential causative pathways, such as decidual-chorioamniotic or systemic inflammation, decidual hemorrhage, activation of the maternal or fetal hypothalamic-pituitary-adrenal axis, or pathologic distention of the uterus (8). Clinically, PTD is divided into spontaneous and medically indicated PTD. Spontaneous PTD (sPTD) is further divided into preterm labor and preterm prelabor rupture of membranes. The remaining deliveries are medically indicated PTD that are induced for obstetrical reasons.
Infectious conditions are believed to account for ≈25–40% of PTD (9–13). In the lower genital tract (cervix, vagina, vulva), several types of bacterial infections can occur during pregnancy. In the vaginal tract, the main types of infections consist of bacterial vaginosis (BV), abnormal vaginal microbiota, and aerobic vaginitis, with different degrees of implication of these conditions in PTD. BV has been associated with PTD in a number of studies (14, 15). Women with BV, abnormal vaginal microbiota, or aerobic vaginitis during pregnancy were found to have a higher risk of PTD, especially severe forms (early) and additional miscarriage, when they had a partial BV as defined in Donders et al (16) together with the presence of Mycoplasma hominis (16). Bacterial infection of the intrauterine environment is more strongly implicated in cases of early PTD with a disproportionate high number of adverse outcomes (16).
The preterm parturition syndrome leading to PTD is partly explained as being caused by microbial invasion of the amniotic cavity and the activation of the innate immune system. Combined endotoxins from microbes and proinflammatory cytokines from the innate immune system increase the production of prostaglandins with concurrent augmentation of uterine contractility, causing increased risk of preterm labor; additional inflammatory mediators influence the degradation of extracellular matrix in the fetal membranes that contribute to processes leading toward preterm prelabor rupture of membranes (17, 18).
Maternal diet during pregnancy is of growing interest in relation to pregnancy outcomes and complications (19). Probiotics are shown to normalize infections of the lower genital tract (20) and have a modifying effect on lipopolysaccharide (LPS) inflammatory response (21, 22). We hypothesized that intake of food with probiotics might influence and reduce pregnancy complications and, through this, influence the rate of PTD. The rationale is based on a putative direct effect of probiotics on vaginal tract infections and on a reduction in the overall inflammatory state, which is in agreement with a systemic inflammation hypothesis based on the assumption that keeping inflammation at a subthreshold level avoids progesterone-induced labor.
In this study, we investigated the hypothesis that intake of milk products containing probiotics is associated with reduced risk of sPTD.
SUBJECTS AND METHODS
We performed a study in the Norwegian Mother and Child Cohort Study (MoBa) initiated by and maintained at the Norwegian Institute of Public Health. In brief, MoBa is a nationwide pregnancy cohort that from 1999 to 2008 has included >107,000 pregnancies. Pregnant women were invited to participate through a postal invitation after they signed up for routine ultrasound examination at their local hospital. The participation rate was 43% (23). The women were asked to provide biological samples and to answer questionnaires during pregnancy and after birth until age 7 y for the child. The first questionnaire (Q1), which was completed in gestational week 15, covered health, chemical, and physical factors in the environment; lifestyles; and socioeconomic and demographic factors. The second questionnaire (Q2) was a food-frequency questionnaire. The records of the participating women in the Medical Birth Registry of Norway (MBRN) were included in the data set (24). This study uses version 4 of the data files made available for research in 2008. The study was approved by the Regional Committee for Ethics in Medical Research and the Data Inspectorate in Norway.
Inclusion criteria and phenotype definition for sPTD were spontaneous onset of PTD in current pregnancy of either preterm labor or preterm prelabor rupture of membranes and onset of PTD from ≥22 to <37 gestational weeks in a singleton pregnancy with a maternal age of 20 to <35 y. Pregnancies were excluded on the basis of preexisting medical conditions such as diabetes, hypertension, thromboembolic disease, autoimmune diseases, inflammatory bowel disease, systemic lupus erythematosus, rheumatoid arthritis, and scleroderma. In addition, pregnancies were excluded on the basis of pregnancy complications due to preeclampsia, hypertension, diabetes, small for gestational age (according to intrauterine growth curves), abruption of the placenta, placenta previa, or deep venous thrombosis and serious fetal malformations and cervical cerclage. Immunocompromised patients were excluded. Controls were selected according to the following criteria: spontaneous onset of PTD, singleton pregnancy, a maternal age of 20–35 y, and a gestational age from ≥39 to <41 wk. The exclusion criteria were the same as for sPTD regarding preexisting medical conditions and pregnancy complications and the exclusion of immunocompromised patients.
Dietary information
A food-frequency questionnaire (FFQ; www.fhi.no/dav/22CA50E0D7.pdf) was completed in weeks 17–22 of gestation. The dietary data used in this study were collected from 2002 to 2007. The FFQ is a semiquantitative questionnaire designed to capture dietary habits and intake of dietary supplements during the first 4–5 mo of pregnancy, as has been described in detail elsewhere (25). The FFQ specifically asked for how often the women consumed probiotic milk or yogurt [Probiotic food item A: Biola milk (Tine, Oslo, Norway), Biola yogurt (Tine)] or probiotic milk [Probiotic food item B: Cultura milk (Tine)], with 11 alternative intake frequencies ranging from “never” to “8 or more times per day” (see Supplemental Figure 1 under ldquoSupplemental datardquo in the online issue). The lowest possible intake for each probiotic item was 6.6 g/d (once monthly), and the highest possible intake was 1600 g/d (≥8 times daily). FoodCalc (26) and the Norwegian Food Composition Table (27) were used to calculate food and nutrient intakes. A validation study showed that, relative to a dietary reference method and several biological markers, the FFQ produces a realistic estimate of habitual intake and is a valid tool for ranking pregnant women according to high and low intakes of energy, nutrients, and food (28).
The products classified as containing probiotics in this study are milk-based beverages containing probiotic lactobacilli. The probiotic milk-based food item A was Biola probiotic milk, including Biola yogurt, containing Lactobacillus acidophilus LA-5, Bifidobacterium lactis Bb12, and Lactobacillus rhamnosus (LGG); food item B consisted of Cultura probiotic milk-based beverage containing Lactobacillus acidophilus LA-5 and Bifidobacterium lactis Bb12.
Statistical methods
Data were analyzed by using the binary logistic regression method in the statistical software PASW statistics 17 (SPSS Inc, Chicago, IL) with the covariates of maternal age, parity, previous spontaneous abortions early (<12 wk) or later (>12–24 wk) in pregnancy, marital status, body mass index before pregnancy, physical activity, smoking before pregnancy, smoking in pregnancy, alcohol intake, educational level, and combined partner income. Criteria for inclusion of confounders in the model were restricted to covariates associated with exposure at P < 0.05 (Table 1) and were associated with outcome in the model at P < 0.100. In addition to potential confounders, differences in total energy intake (kJ/d), added sugar (% of total energy intake), total carbohydrate (g/d), total fat (g/d), total protein (g/d), or dietary fiber (g/d) were assessed between cases and controls. The probiotic food types were tested for association for intake or no intake, then intake in g/d was grouped into categories of “none,” “low,” and “high” consumption for observable dose effect, and analyses were performed in the model to evaluate the potential interaction of sex and probiotic intake and parity and probiotic intake on sPTD. An observed P value at the 0.05 level was considered to be significant.
TABLE 1.
Intake of probiotics according to maternal characteristics in 18,888 pregnancies in the Norwegian Mother and Child Cohort Study from 2002 to 20071
| Number in category (n = 18,888) | Intake of probiotic milk item A2 | Intake of probiotic milk item B2 | Combined intake item A and item B3 | |
| n (%) | % of consumers | % of consumers | mL | |
| Maternal age | ||||
| 20–24 y | 2414 (12.8) | 16.9 | 16.8 | 18.9 ± 61.64 |
| 25–29 y | 7640 (40.4) | 23.7 | 18.7 | 27.3 ± 82.8 |
| 30–34 y | 8834 (46.8) | 25.0 | 18.4 | 28.7 ± 87.3 |
| P value | — | <0.0015 | 0.0075 | <0.0016 |
| Parity | ||||
| Primiparous | ||||
| 0 | 8408 (44.5) | 27.4 | 20.2 | 31.4 ± 90.0 |
| Multiparous | ||||
| 1 | 7571 (40.1) | 21.5 | 16.9 | 24.2 ± 29.0 |
| 2 | 2488 (13.2) | 17.0 | 17.0 | 21.3 ± 70.4 |
| ≥3 | 410 (2.2) | 16.8 | 15.9 | 17.7 ± 53.4 |
| P value | — | <0.0015 | <0.0015 | <0.0016 |
| Missing data | 11 (0.1) | 27.3 | 0 | 16.7 ± 34.2 |
| Previous spontaneous abortions <12 wk | ||||
| 0 | 15,851 (83.9) | 23.5 | 18.5 | 26.79 ± 82.2 |
| 1 | 2446 (13.0) | 23.2 | 17.2 | 26.87 ± 83.2 |
| ≥2 | 591 (3.1) | 22.7 | 18.3 | 28.85 ± 92.3 |
| P value | — | 0.8475 | 0.2845 | 0.7336 |
| Previous spontaneous abortions >12–21 (+6 d) wk | ||||
| 0 | 18446 (97.7) | 23.5 | 18.4 | 26.90 ± 82.6 |
| 1 | 428 (2.3) | 21.0 | 15.4 | 24.97 ± 85.1 |
| ≥2 | 14 (0.1) | 21.4 | 14.3 | 33.58 ± 77.6 |
| P value | — | 0.4835 | 0.2695 | 0.2246 |
| Fetus sex | ||||
| Male | 9163 (48.5) | 23.9 | 18.5 | 27.5 ± 83.4 |
| Female | 9725 (51.5) | 23.0 | 18.2 | 26.3 ± 82.0 |
| P value | — | 0.1825 | 0.5995 | 0.1886 |
| BMI before pregnancy | ||||
| <18.5 kg/m2 | 630 (3.3) | 18.9 | 16.8 | 25.64 ± 80.7 |
| ≥18.5 to <25.0 kg/m2 | 12,593 (66.7) | 25.8 | 19.8 | 29.63 ± 86.5 |
| ≥25.0 to <30.0 kg/m2 | 3735 (19.8) | 20.2 | 15.7 | 22.21 ± 72.1 |
| ≥30.0 to <35.0 kg/m2 | 1064 (5.6) | 15.7 | 14.3 | 16.05 ± 63.8 |
| ≥35.0 kg/m2 | 370 (2.0) | 12.4 | 10.0 | 10.40 ± 40.6 |
| P value | — | <0.0015 | <0.0015 | <0.0016 |
| Missing data | 496 (2.6) | 19.8 | 17.3 | 28.70 ± 110.7 |
| Physical activity | ||||
| Nonexerciser | 2786 (14.8) | 15.8 | 12.3 | 16.21 ± 62.0 |
| Light | 3768 (19.9) | 20.9 | 17.4 | 21.39 ± 75.3 |
| Irregular | 5545 (29.9) | 24.5 | 18.5 | 26.77 ± 83.7 |
| Regular | 5173 (27.4) | 30.0 | 23.6 | 38.07 ± 96.2 |
| P value | — | <0.0015 | <0.0015 | <0.0016 |
| Missing data | 1616 (8.6) | 17.7 | 13.4 | 22.45 ± 76.8 |
| Marital status | ||||
| Married | 9073 (48.0) | 23.8 | 19.1 | 26.74 ± 80.9 |
| Cohabitants | 9202 (48.7) | 23.4 | 17.7 | 27.24 ± 85.3 |
| Single | 364 (1.9) | 18.1 | 15.4 | 20.22 ± 60.9 |
| P value | — | 0.0445 | 0.0235 | 0.0096 |
| Missing data | 52 (1.0) | 21.2 | 9.6 | 17.57 ± 49.1 |
| Smoking before pregnancy | ||||
| No smoking | 13,410 (71.0) | 24.9 | 19.0 | 28.92 ± 87.2 |
| Occasional | 1880 (10.0) | 28.6 | 21.0 | 29.89 ± 79.6 |
| Daily | 3433 (18.2) | 15.2 | 14.4 | 17.70 ± 64.4 |
| P value | — | <0.0015 | <0.0015 | <0.0016 |
| Missing data | 165 (0.9) | 20.6 | 17.6 | 16.07 ± 82.7 |
| Smoking during pregnancy | ||||
| No smoking | 17,347 (91.8) | 24.4 | 18.9 | 27.85 ± 84.6 |
| Occasional | 485 (2.6) | 17.7 | 11.8 | 20.11 ± 65.4 |
| n (%) | % of consumers | % of consumers | mL | |
| Daily | 1056 (5.6) | 9.8 | 11.8 | 13.78 ± 50.3 |
| P value | — | <0.0015 | <0.0015 | <0.0016 |
| Alcohol intake during pregnancy | ||||
| No | 16,787 (88.9) | 23.3 | 18.0 | 26.97 ± 84.3 |
| Yes | 2101 (11.1) | 25.2 | 21.1 | 26.00 ± 68.4 |
| P value | — | 0.0465 | <0.0015 | 0.0117 |
| Educational level | ||||
| <12 y | 3808 (20.2) | 14.9 | 14.0 | 1830 ± 63.7 |
| 12 y | 2329 (12.3) | 17.6 | 15.8 | 22.77 ± 84.0 |
| 13–16 y | 8196 (43.4) | 25.0 | 18.6 | 27.98 ± 83.8 |
| ≥17 y | 4175 (22.1) | 31.8 | 23.5 | 35.11 ± 93.4 |
| P value | — | <0.0015 | <0.0015 | <0.0016 |
| Missing data | 380 (2.0) | 18.9 | 15.5 | 23.28 ± 83.08 |
| Income for participant and partner8 | ||||
| Both have incomes <NOK 300 | 5777 (30.6) | 19.6 | 18.0 | 21.86 ± 71.9 |
| One has income <NOK 300 | 8246 (43.7) | 23.0 | 17.7 | 27.06 ± 80.4 |
| Both have incomes ≥NOK 300 | 3808 (20.2) | 31.6 | 20.5 | 35.31 ± 100.1 |
| P value | — | <0.0015 | <0.0015 | <0.0016 |
| Missing data | 1057 (5.6) | 18.2 | 17.5 | 22.22 ± 84.0 |
NOK, Norske kroner.
Intake is defined as any intake >6.6 mL/d.
Intake of Biola (Tine, Oslo, Norway) or Cultura (Tine) according to mean consumption in group including nonconsumers.
Mean ± SD (all such values).
Pearson's chi-square asymptotic 2-sided test of intake frequencies in groups.
Kruskal-Wallis test.
Mann-Whitney U test.
Income × 1000 NOK.
RESULTS
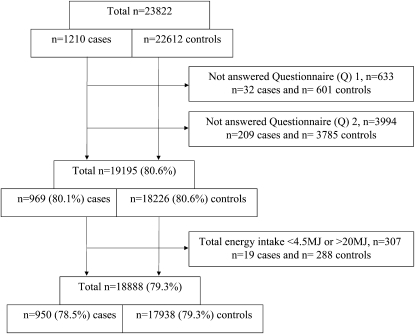
A total of 23,822 women were eligible for this study on the basis of information from the MBRN; of these, 1210 cases and 22,612 controls were identified, of whom 19,195 (80.6%) had answered both Q1 and Q2. A final total of 18,888 (79.3%) had a registered food intake >4.5 and <20 MJ/d and were included in the analyses (Figure 1). There were no observed differences in total energy intake (kJ/d), added sugar (% of total energy intake), total carbohydrate (g/d), total fat (g/d), total protein (g/d), or dietary fiber (g/d) between cases and controls (P > 0.05 for all).
FIGURE 1.
Flow diagram showing inclusion of study participants from the Norwegian Mother and Child Cohort Study.
The initial analysis was based on frequencies of any intake compared with no intake. The results showed a consistent association between intake of probiotic food items and sPTD (P = 0.044), which increased (P = 0.038; OR: 0.857; 95% CI: 0.741, 0.992) after adjustment for the following significant covariates: parity, maternal educational level, and physical activity (Table 1 and Table 2). The frequency analysis led to analyses using intake in milliliters. When grouped into no, low, and high intakes in milliliters, probiotic dairy products were significantly associated with high intake (P = 0.035; OR: 0.820; 95% CI: 0.681, 0.986) (Table 3), and all probiotic food item groups showed lower ORs with increasing dose, suggesting a weak dose-dependent effect with lower sPTD risk (data not shown). The content of probiotic bacteria in these beverages are 108 probiotic bacteria/mL, which corresponds to ≈2.85 × 109 – 2.0 × 1011 probiotic bacteria/d in the high-intake group. There was no significant interaction observed for parity and probiotic intake. No significant interaction was observed for fetus sex and probiotic intake.
TABLE 2.
Risk of spontaneous preterm delivery (sPTD) according to any or no consumption of probiotic food items1
| sPTD (n = 950) | Controls (n = 17,938) | P value2 | P value3 | OR (95% CI) | |
| n (%) | n (%) | ||||
| Intake of probiotic milk items A or B | 0.044 | 0.038 | 0.857 (0.741, 0.992) | ||
| No | 667 (70.2) | 12,029 (67.1) | |||
| Yes4 | 283 (29.8) | 5909 (32.9) |
OR, odds ratio (for adjusted model).
Unadjusted.
Adjusted for parity, physical activity, and maternal education.
Range: 6.6–2000 mL/d; median: 28 mL/d.
TABLE 3.
Risk of spontaneous preterm delivery (sPTD) according to consumption (in mL) of probiotic food items1
| sPTD (n = 950) | Controls (n = 17,938) | P value2 | P value3 | OR (95% CI) | |
| n (%) | n (%) | ||||
| Probiotic milk items A or B [median (min – max)] | 0.105 | 0.088 | — | ||
| No intake | 667 (70.2) | 12,029 (67.1) | — | Ref | — |
| Low intake: 13 mL/d (6.6–26 mL/d) | 133 (14.0) | 2653 (14.8) | — | 0.302 | 0.903 (0.745, 1.096) |
| High intake: 85 mL/d (28–2000 mL/d) | 150 (15.8) | 3256 (18.2) | — | 0.035 | 0.820 (0.681, 0.986) |
OR, odds ratio (for adjusted model); Ref, reference.
Unadjusted.
Adjusted for parity, physical activity, and maternal education.
DISCUSSION
In this study we observed a protective effect of intake of probiotic milk products with sPTD, with a suggested weak dose-dependent effect. Our finding is of importance to perinatal care and has the potential to improve current pregnancy health care. Intake of milk products that contain probiotics might influence and reduce pregnancy complications, possibly through an effect of probiotics on vaginal tract infections and a reduction in overall inflammatory state in keeping with a systemic inflammation hypothesis.
There are several strengths of this study, which included the prospective design with collection of dietary data and the FFQ completed during 17–22 wk of gestation, before pregnancy delivery to avoid confounding by retrospectively answered questionnaires. The strict and extensive sample inclusion and exclusion criteria make this a very homogenous set of cases and controls. Furthermore, the sample size is large and represents women from all over Norway with diverse dietary habits and a wide range of intake frequencies of probiotic products. A true picture of dietary intake is difficult to obtain with the use of FFQs. Although this method provides an acceptable representation of dietary habits, its use represents a weakness in the study along with the absence of confirmation of results through examination of vaginal microbiota and known but unmeasured confounders such as use of fertility medications, interval between pregnancies, psychological problems including depression, anxiety, stress, weight gain during pregnancy, traumatic events during pregnancy, and genetic factors.
We investigated intake of milk products containing probiotics as an environmental exposure that is potentially important in the moderation of infection and in influencing the outcome of sPTD. The investigated probiotic dairy products contain probiotic bacteria such as lactobacilli that normalize levels of, among others, the 4 main BV microorganisms—Gardnerella vaginalis, Mobiluncus, Bacteroides, and M. hominis—and additionally Prevotella bivia and Peptostreptococcus anaerobicus (29). Thus, probiotic lactobacilli strains have been documented to have an antagonistic effect on most bacteria involved in BV, and the probiotic effect is assumed to contribute to a normalization of the bacterial microbiota through reestablishment of lactobacilli (20).
In our study, a significant association was observed between high intake of probiotic dairy products and sPTD (Table 3). The use of probiotics in female urogenital health care and especially BV has been advocated in previous studies (30, 31). In a study on oral intake of probiotic lactobacilli that investigated the effect on vaginal microbiota, a significant reduction in asymptomatic BV was observed after treatment with oral capsules containing Lactobacillus rhamnosus and Lactobacillus fermentum, resulting in restoration of the vaginal microbiota to normal, lactobacilli-dominant status (20). In addition, infections of M. hominis that are associated with PTD (32) are sensitive to probiotic bacteria such as those found in the milk-based probiotic dairy products included in this study.
The observed results of a protective effect of probiotic intake and an association between high intake of probiotic dairy products and sPTD (Table 3) are especially interesting in light of a recent study by Yeganegi et al (21), in which supernatant fluid of L. rhamnosus GR-1 was found to influence the LPS response in placental trophoblast cells, with an increased modifying effect for male cells. Yeganegi et al (21) suggested that the observed sex difference in the studied placental trophoblast inflammatory response might explain some of the skewness observed toward increased ratio of male fetuses in PTD and that lactobacilli might have a therapeutic effect on PTD. Because we observed a protective effect of intake of probiotic dairy products and an increase in risk after adjustment for potential confounders, our results are partly in agreement with Yeganegi et al's hypothesis. However, we did not observe a more prominent contribution regarding the probiotic food item containing the additional probiotic bacteria L. rhamnosus (LGG) (data not shown), and no significant interaction was observed for fetus sex and probiotic intake or parity and probiotic intake.
The lactic acid bacteria included here constitute different lactobacilli strains, which adds to the findings by Yeganegi et al (20). To this end, we can consider a possible mechanism by which intake of diverse strains of probiotics may contribute to a reduction in overall inflammatory state in keeping with a systemic inflammation hypothesis, further keeping inflammation at a subthreshold level to avoid progesterone-induced labor. Because the effects of specific probiotics in relation to sPTD are strain specific, it is important that studies are conducted at a strain-specific level.
Because the implicated pregnancy conditions in PTD are presumably atypical and subclinical variants of BV, and the biological dynamics of probiotic food intake and effect of maintaining these dynamics under control are unknown, the amount and concentration of probiotic intake needed for an effect is an important aspect of this discussion. Our results imply that high intake (in mL) of probiotic food items, which in this study corresponded to a mean intake of 138.4 mL/d, had an effect (Table 3) with a range of ≈2.85 × 109 – 2.0 × 1011 probiotic bacteria/d in the high-intake group. This is more than the >109 probiotic bacteria/capsule that was applied in a daily dose in the previously mentioned clinical trial by Reid et al (20).
Our results thus fit a general hypothesis of subsets of sPTD being caused partly by an increased infection or inflammation state representing an increased level of systemic inflammation. Dietary probiotics fit according to this hypothesis if they are assumed to function as a “rescue mechanism” by lowering overall inflammation in combination with providing a healthy vaginal microbiological environment.
In this era of multidrug-resistant bacteria, it is especially important to be open to more alternative and innovative methods for the prevention and treatment of resource-demanding conditions such as preterm hospitalization, which is a major cost in the United States (2) and the rest of the world.
In conclusion, this study shows that intake of probiotics through milk products might be associated with reduced risk of sPTD. Further investigation is warranted with the use of randomized controlled trials for evaluation as to whether to view presence of foods containing probiotics as protective and their absence as a risk factor for sPTD and to improve understanding of health complications during pregnancy to further facilitate effective health promotion strategies. Successive reduction in PTD may be achieved by targeting dietary health issues and evaluating intake of probiotics, with consideration of nutritional interventions early in pregnancy or prepregnancy.
Supplementary Material
Acknowledgments
We thank the Norwegian Institute of Public Health and Sahlgrenska University Hospital in Sweden for their inspiring and supportive research environments and for making this collaboration possible.
The authors’ responsibilities were as follows—RM and BJ: contributed to the conception and design of the study, interpretation of results, and writing of the manuscript; RM, ALB, MH, and HMM: contributed to statistical analysis, writing of the manuscript, and interpretation of results; SM, VS, and ALB: were involved in selection of cases and controls; and HKG: provided statistical expertise and contributed to the interpretation of results. All authors participated in the evaluation of the data and approved the final manuscript. The authors declared that they had no conflicts of interest.
REFERENCES
- 1.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med 1985;312:82–90 [DOI] [PubMed] [Google Scholar]
- 2.Russell RB, Green NS, Steiner CA, et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics 2007;120:e1–9 [DOI] [PubMed] [Google Scholar]
- 3.Morken NH, Vogel I, Kallen K, et al. Reference population for international comparisons and time trend surveillance of preterm delivery proportions in three countries. BMC Womens Health 2008;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med 2006;19:773–82 [DOI] [PubMed] [Google Scholar]
- 6.Ananth CV, Joseph KS, Oyelese Y, Demissie K, Vintzileos AM. Trends in preterm birth and perinatal mortality among singletons: United States, 1989 through 2000. Obstet Gynecol 2005;105:1084–91 [DOI] [PubMed] [Google Scholar]
- 7.Davidoff MJ, Dias T, Damus K, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol 2006;30:8–15 [DOI] [PubMed] [Google Scholar]
- 8.Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatr Perinat Epidemiol 2001;15(suppl 2):78–89 [DOI] [PubMed] [Google Scholar]
- 9.McGregor JA, French JI, Richter R, et al. Antenatal microbiologic and maternal risk factors associated with prematurity. Am J Obstet Gynecol 1990;163:1465–73 [DOI] [PubMed] [Google Scholar]
- 10.Menon R, Fortunato SJ. Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract Res Clin Obstet Gynaecol 2007;21:467–78 [DOI] [PubMed] [Google Scholar]
- 11.Leitich H, Kiss H. Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol 2007;21:375–90 [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol 2002;7:259–74 [DOI] [PubMed] [Google Scholar]
- 13.Anderson BL, Simhan HN, Simons K, Wiesenfeld HC. Additional antibiotic use and preterm birth among bacteriuric and nonbacteriuric pregnant women. Int J Gynaecol Obstet 2008;102:141–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meis PJ, Goldenberg RL, Mercer B, et al. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 1995;173:1231–5 [DOI] [PubMed] [Google Scholar]
- 15.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 1995;333:1737–42 [DOI] [PubMed] [Google Scholar]
- 16.Donders GG, Van Calsteren K, Bellen G, et al. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG 2009;116:1315–24 [DOI] [PubMed] [Google Scholar]
- 17.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 2006;113(suppl 3):17–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000;342:1500–7 [DOI] [PubMed] [Google Scholar]
- 19.Shapira N. Prenatal nutrition: a critical window of opportunity for mother and child. Womens Health (Lond Engl) 2008;4:639–56 [DOI] [PubMed] [Google Scholar]
- 20.Reid G, Burton J, Hammond JA, Bruce AW. Nucleic acid-based diagnosis of bacterial vaginosis and improved management using probiotic lactobacilli. J Med Food 2004;7:223–8 [DOI] [PubMed] [Google Scholar]
- 21.Yeganegi M, Watson CS, Martins A, et al. Effect of Lactobacillus rhamnosus GR-1 supernatant fluid and fetal sex on lipopolysaccharide-induced cytokine and prostaglandin-regulating enzymes in human placental trophoblast cells: implications for treatment of bacterial vaginosis and prevention of preterm labor. Am J Obstet Gynecol 2009;200:532e1–8 [DOI] [PubMed] [Google Scholar]
- 22.Bloise E, Torricelli M, Novembri R, et al. Heat-killed lactobacillus rhamnosus GG modulates urocortin and cytokine release in primary trophoblast cells. Placenta 2010;31;10:867–72 [DOI] [PubMed] [Google Scholar]
- 23.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 2006;35:1146–50 [DOI] [PubMed] [Google Scholar]
- 24.Irgens LM. The Medical Birth Registry of Norway: epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand 2000;79:435–9 [PubMed] [Google Scholar]
- 25.Meltzer HM, Brantsaeter AL, Ydersbond TA, Alexander J, Haugen M. Methodological challenges when monitoring the diet of pregnant women in a large study: experiences from the Norwegian Mother and Child Cohort Study (MoBa). Matern Child Nutr 2008;4:14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauritsen J. FoodCalc. Available from: http://www.ibt.ku.dk/jesper/foodcalc (cited February 2006)
- 27.Norwegian Food Safety Authority, Directorate for Health and the Department of Nutrition at the University of Oslo The Norwegian Food Composition Table 2006 (MVT-06). Available from: http://www.norwegianfoodcomp.no/ (cited 15 October 2010)
- 28.Brantsaeter AL, Haugen M, Alexander J, Meltzer HM. Validity of a new food frequency questionnaire for pregnant women in the Norwegian Mother and Child Cohort Study (MoBa). Matern Child Nutr 2008;4:28–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strus M, Malinowska M, Heczko PB. In vitro antagonistic effect of Lactobacillus on organisms associated with bacterial vaginosis. J Reprod Med 2002;47:41–6 [PubMed] [Google Scholar]
- 30.Reid G, Burton J, Devillard E. The rationale for probiotics in female urogenital healthcare. MedGenMed 2004;6:49. [PMC free article] [PubMed] [Google Scholar]
- 31.Cribby S, Taylor M, Reid G. Vaginal microbiota and the use of probiotics. Interdiscip Perspect Infect Dis 2008;2008:256490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massaro G, Scaravilli G, Simeone S, et al. Interleukin-6 and Mycoplasma hominis as markers of preterm birth and related brain damage: Our experience. J Matern Fetal Neonatal Med 2009;22:1–5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.