Abstract
Studies using transformed human cell lines suggest that most SIV strains use CCR5 as co-receptor. Our analysis of primary rhesus macaque CD4+ T-cell clones revealed marked differences in susceptibility to SIVmac239 infection. We investigated whether different levels of CCR5 expression account for clonal differences in SIVmac239 susceptibility. Macaque CD4+ T cells showed significant CCR5 downregulation 1-2 days following CD3 mAb stimulation, which gradually recovered at resting state, 7-10 days after activation. Exposure of clones to SIVmac239 during their CCR5low or CCR5high expression states revealed differences in SIV susceptibility independent of surface CCR5 levels. Furthermore, a CCR5 antagonist similarly reduced SIVmac239 infection of clones during their CCR5low or CCR5high expression states. Our data suggest a model where i) very low levels of CCR5 are sufficient for efficient SIV infection, ii) CCR5 levels above this threshold do not enhance infection, and iii) low level infection can occur in the absence of CCR5.
Keywords: AIDS, CCR5, CD4, SIV, rhesus macaques
Introduction
HIV-1 has dual co-receptor tropism, i.e., following interaction with the CD4 molecule, different isolates efficiently bind to CCR5 and/or CXCR4 for entry to different types of target cells (Glushakova et al., 1999; Granelli-Piperno et al., 1996; Scarlatti et al., 1997). HIV-1 isolates recovered early during acute infection are overwhelmingly R5-tropic while isolates recovered during late chronic phases can be X4-tropic, with a preserved ability to use CCR5 (Scarlatti et al., 1997). In addition to CCR5 and/or CXCR4, other chemokine and chemokine-like orphan receptors have been shown to be utilized by HIV as co-receptors for entry into different target cells with varying degrees of efficiency (Albright et al., 1999; Glushakova et al., 1999; Granelli-Piperno et al., 1996; Pohlmann, Krumbiegel, and Kirchhoff, 1999; Scarlatti et al., 1997; Xiao et al., 1998). In contrast to HIV, most SIV strains have been reported to be predominantly R5-tropic with very few variants shown to be X4-tropic (DeGottardi et al., 2008; Del Prete et al., 2009; Lauren et al., 2006; Means et al., 2001; Picker et al., 2004). The pathogenic SIVmac239 virus, a prototypic strain used for many studies, has been shown to be mainly R5-tropic (Edinger et al., 1998; Lauren et al., 2006; Pohlmann et al., 2000; Pohlmann et al., 1999; Sharron et al., 2000). However, cell-to-cell fusion assays and different entry assays using transformed cell lines found that chemokine receptor-like orphan receptors such as CXCR6 (STRL33/Bonzo) and GPR15 (BOB) might also be used, albeit less efficiently (Edinger et al., 1998; Lauren et al., 2006; Pohlmann et al., 2000; Pohlmann et al., 1999; Sharron et al., 2000).
A 32-base pair deletion (Δ32) in the human CCR5 gene resulting in loss of or significantly diminished expression of CCR5 (Blanpain et al., 2000; Connor et al., 1996; Liu et al., 1996; Mulherin et al., 2003; Paxton et al., 1996; Rana et al., 1997) has been associated with resistance to infection with R5-tropic HIV-1 strains (Hoffman et al., 1997; Huang et al., 1996; Oh et al., 2008; Zimmerman et al., 1997). Similar to the human Δ32 CCR5 gene mutation, a 24-base pair deletion (Δ24) in the CCR5 gene has been reported in some red-capped mangabeys (RCM), conferring resistance to R5-tropic SIV viruses (Chen et al., 1998). Therefore, Δ24 homozygote RCM derived PBMC are permissive only to the CCR2b-tropic SIVrcm strain (Chen et al., 1998). On the other hand, analyses of polymorphisms in the CCR5 gene from SIV-infected rhesus macaques did not find any associations between polymorphisms in CCR5 and control of virus replication (Weiler et al., 2006). Thus, the role of CCR5 expression levels in determining susceptibility of rhesus macaque CD4+ T cells to SIV infection is not clearly defined.
Studies comparing HIV/SIV infection of CCR5-expressing and non-expressing cells suggest that high levels of cell surface CCR5 are required for efficient target cell infection (DeGottardi et al., 2008; Glushakova et al., 1999; Granelli-Piperno et al., 1996; Lin et al., 2002; Means et al., 2001; Picker et al., 2004; Scarlatti et al., 1997). However, these studies typically assessed CCR5 expression at a single time point and assumed that the levels of surface expression remain constant over resting and activated cell states. Because HIV-1 infection and replication is more efficient in actively dividing cells (Vatakis et al., 2007; Vatakis et al., 2009), most in vitro protocols for SIV infection of rhesus macaque CD4+ T cells include an initial activation step, with mitogen or CD3 monoclonal antibody (mAb), 24-48 h prior to infection (Minang et al., 2009; Sacha and Watkins). We recently observed that primary rhesus macaque PBMC-derived CD4+ T-cell clones expressing similar levels of surface CD4, show clonal differences in susceptibility to infection with SIVmac239. We therefore asked whether differential levels of expression of CCR5 might account for the clonal differences in susceptibility to SIV. We found that clonal differences in susceptibility to infection of rhesus macaque CD4+ T cells by SIVmac239 is independent of levels of CCR5 surface expression.
Results
Dynamics of surface CCR5 expression by primary rhesus macaque CD4+ T-cell clones
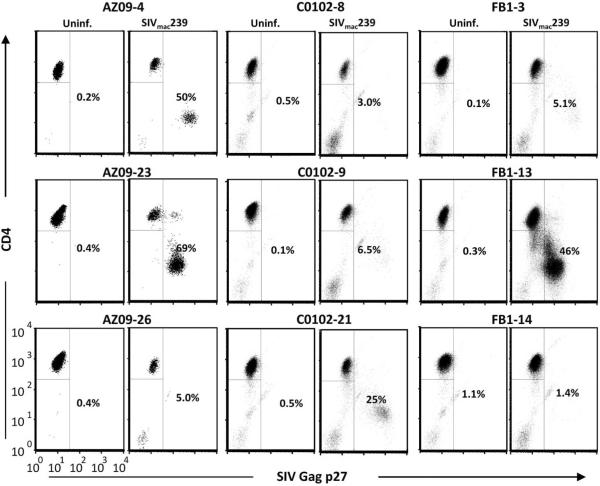
Infection of 9 CD4+ T-cell clones from 3 rhesus macaques 24hrs after plate-bound CD3 mAb stimulation revealed considerable clonal differences in their susceptibility to infection and kinetics of replication of SIVmac239 as measured by anti-p27 staining 5 days PI (Fig 1 and data not shown). Of the nine clones presented, three were highly infectable (H; SIV Gag p27+ cells ≥30%), five were poor hosts for SIV (i.e. low-to-resistant, L/R; SIV Gag p27+ cells <10%), and one had an intermediate number of infected cells (I; SIV Gag p27+ cells ≥10% but <30%). This relative difference in SIV susceptibility between clones was consistent in multiple infection experiments using additional clones from eight rhesus macaques (Supplemental Table I). The clones were stimulated on the same schedule and expressed high and comparable levels of surface CD4 at the time of infection (Supplemental Fig. 1; data not shown ), suggesting that these parameters or genetic differences between animals were not the cause of the observed variability. All clones were of effector memory phenotype (CD28−, CD95+) after in vitro culture (data not shown).
Figure 1.
Primary rhesus macaque CD4+ T cells show clonal differences in susceptibility to infection with SIVmac239. Nine CD4+ T-cell clones from three uninfected rhesus macaques were stimulated with plate-bound CD3 mAb. Twenty-four hours later, the cells were infected with cell-free SIVmac239 for 3 h, washed and plated in 24-well plates. The frequency of SIV Gag p27+ cells was determined by intracellular staining and flow cytometry on day 5 PI.
In light of reports suggesting that virus-specific effector memory CD4+ T cells may be more susceptible to HIV/SIV infection (Douek et al., 2002; Okoye et al., 2009), and because some of the CD4+ T-cell clones used in this study were generated from SIV DNA vaccinated macaques, we evaluated all the clones from vaccinated animals for reactivity to SIVmac239 Gag, Pol and Acc peptide pools (SIV Env was not included in the vaccine construct). Three CD4+ T-cell clones (C0102-17, -21 and -55) out of 26 tested clones were found to be SIV Gag reactive (Supplemental Fig. 2; IFN-γ responses by a representative SIV Gag-specific CD4+ T-cell clone). All three clones showed low or intermediate susceptibility to infection compared with the non-SIV reactive clones (Supplemental Table I).
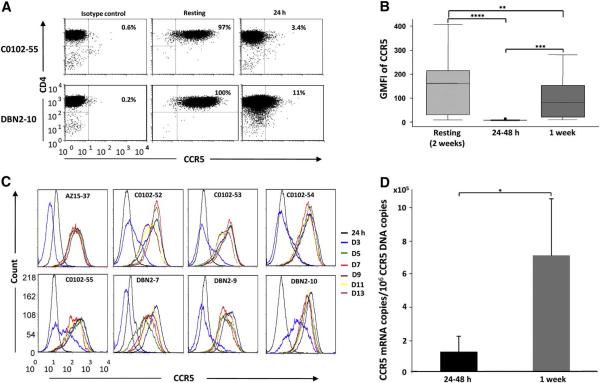
To test if CCR5 expression levels could account for the differences in SIV susceptibility, we measured surface expression of CCR5 on resting CD4+ T-cell clones and 24-48 h after activation. Using PE-conjugated mouse IgG1 k as isotype control mAb to establish background CCR5 staining, 88-100% and 2-15% of the clonal population of CD4+ T cells at resting and after activation, respectively, were positive for CCR5 expression (Figure 2A; data for 2 representative clones from 2 animals; and data not shown). When the GMFI of CCR5 expression was analyzed for 21 clones from 5 animals, resting CD4+ T-cell clones showed high but considerably variable levels of surface CCR5 (Fig. 2B). Surface CCR5 expression was significantly downregulated (up to 50-fold reduction for one clone) 24-48 h post stimulation, and partially recovered 1 week after, being significantly higher than the 24-48 h post stimulation levels (Fig. 2B; p<0.0005). Stimulation of CD4+ T-cell clones with phytohemagglutinin (PHA), soluble CD3 mAb or a cocktail of soluble CD3 and CD28 mAbs, yielded similar CCR5 downregulation (data not shown).
Figure 2.
CCR5 is differentially expressed at high levels in resting primary rhesus macaque CD4+ T cells but uniformly downregulated in response to T-cell receptor triggering. A) Percentage of CCR5 positive cells in CD4+ T-cell clones from two monkeys at resting and 24 h after CD3 stimulation compared to isotype control background staining. B) CCR5 expression by 21 CD4+ T-cell clones from 5 rhesus macaques analyzed at resting, 24-48 h and 1 week after CD3 mAb stimulation. Box plots show geometric mean fluorescent intensities (GMFI) of surface CCR5 expression determined by flow cytometry with the median, 95 percentile and maximum values indicated for each group. C) Surface CCR5 expression dynamics by 8 CD4+ T-cell clones from 3 rhesus macaques analyzed by flow cytometry every second day until day 13 following stimulation. D) CCR5 mRNA expression by 4 CD4+ T-cell clones from 2 rhesus macaques was determined by qPCR 24-48 h and 1 week after stimulation. Shown are means and standard deviations of CCR5 mRNA copies per 106 CCR5 DNA copies. The paired Students t test was used in analyses for B and D with significance set at a p value ≤ 0.05 where **** = p<0.00005, *** = p<0.0005, ** = p<0.005 and * = p<0.05.
To examine the kinetics of recovery in finer detail, surface CCR5 levels on 8 clones from 3 animals were measured 24 h after CD3 mAb stimulation, and every second day until day 13. Only a representative subset of the original 29 CD4+ T-cell clones were included in these experiments because of the technical difficulty of maintaining all clones in continuous culture for the total time of the study. Overall, CCR5 expression was downregulated 24 h post-stimulation with a gradual return over the next 7 days, reaching pre-stimulation levels between day 7-10 post stimulation (Fig. 2C). Similar results were seen when CCR5 expression was measured on freshly isolated PBMC from two monkeys, excluding the possibility that the dynamic CCR5 expression on CD4+ T-cell clones was a result of long-term in vitro culture (Supplemental Fig. 3). Studies in human cells found that surface CCR5 downregulation after activation is in part due to decreased CCR5 mRNA expression (Carroll et al., 1997). When levels of CCR5 mRNA expression in 4 clones from 2 animals were analyzed and normalized to number of cells (CCR5 DNA copies), we observed a 5-17 fold reduction in CCR5 mRNA copies for the cells analyzed 24-48 h compared to 1 week after stimulation (Fig. 2D; p=0.02). These data suggest that part of the activation-induced CCR5 downregulation on rhesus CD4+ T cells occurs at the transcriptional level, similar to the case in human T cells.
Levels of SIV Gag p27-expression or cell-associated viral DNA in SIV-infected macaque CD4+ T cells are not related to the levels of surface CCR5 at time of infection
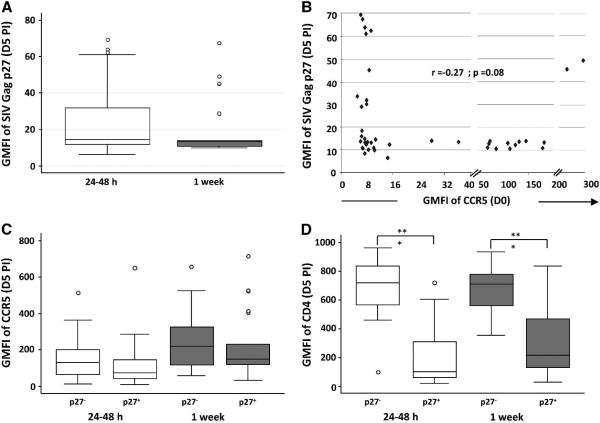
We next asked whether SIVmac239 infection and replication in macaque CD4+ T cells is affected by the levels of surface CCR5 at time of infection. We infected 21 CD4+ T-cell clones from 5 rhesus macaques 24-48 h (CCR5low) and 1 week (CCR5high) after CD3 mAb stimulation with cell-free SIVmac239, and assessed the levels of intracellular SIV Gag p27 expression on day 5 PI by flow cytometry. The levels of surface CD4 and CCR5 on the day of infection as well as at the time of harvest were also determined.
We observed a wide variation in the levels of intracellular SIV Gag p27 expression between the clones on day 5 PI. However, there was no significant difference in the median GMFI of SIV Gag p27 staining between matched cells infected at 24-48 h (CCR5low) and 1 week (CCR5high) post stimulation (Fig. 3A). Correlation analyses of the intensity of SIV Gag p27 staining on day 5 PI and the levels of surface CCR5 expression at the time of infection did not show a direct relationship between these parameters (Fig. 3B, r = −0.27). Productive infection did not significantly affect CCR5 expression: surface CCR5 levels were similar in the SIV Gag p27+ and SIV Gag p27− fractions of cells from the same clonal population on day 5 PI (Fig. 3C). As expected for SIV infection, surface CD4 levels were significantly downregulated in the SIV Gag p27+ cell fraction of cultures of SIVmac239-infected cells (Fig. 3D; p<0.0005).
Figure 3.
CCR5 expression levels by primary rhesus macaque CD4+ T cells at time of infection with SIVmac239 do not correlate with the levels of SIV Gag p27 expression. Rhesus macaque CD4+ T-cell clones were infected with cell-free SIVmac239 either 24-48 h or 1 week after -CD3 mAb stimulation. A) Geometric mean fluorescent intensity (GMFI) of SIV Gag p27 expression on day 5 PI. B) Correlation plot of GMFI of SIV Gag p27 staining on day 5 PI and surface CCR5 expression at time of infection. C) GMFI of surface CCR5 and D) CD4 expression by SIV Gag p27 negative and positive cell fractions on day 5 PI. All plots show data for 21 CD4+ T-cell clones from 5 rhesus macaques. Stained cell acquisition and analyses was by flow cytometry. Group comparisons were by the paired Students t test and correlation analyses by the Spear man rank order correlation with significance set at a p value ≤ 0.05 where *** = p<0.0005.
Because of the dynamic pattern of CCR5 expression during the 5 days incubation between infection and SIV Gag p27 analyses, we next investigated whether levels of cell-associated viral DNA measured early after infection could be related to the levels of surface CCR5 at the time of infection. To address this, we infected 4 CD4+ T-cell clones from 2 rhesus macaques with SIVmac239 24-48 h (CCR5low) and 1 week (CCR5high) after CD3 mAb stimulation. As indicated previously, a representative subset of the original 29 CD4+ T-cell clones that were intermediate-to-highly infectable were used in this set of experiments because of the technical difficulty of maintaining all the clones long term for the duration of the study. Cell-associated viral DNA was analyzed from aliquots of cell suspensions harvested 9, 24, 48 and 96 h PI by QPCR. Using our infection protocol, typically less than 1% of the cells are SIV Gag p27+ before 3 days post infection when analyzed by flow cytometry (Minang et al., 2008). Although the levels of cell-associated viral DNA varied between individual clones, the levels expressed by the same clones infected 24-48 h (CCR5low) and 1 week (CCR5high) post stimulation were similar at all tested time points PI (Fig. 4). Hence, the levels of surface CCR5 expression by macaque CD4+ T cells at the time of virus exposure had no measurable impact on the infection efficiency at early time points.
Figure 4.
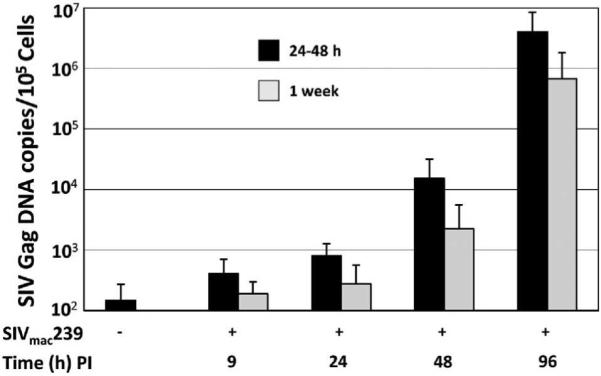
Copies of cell-associated viral DNA early (1-4 days PI) during viral replication are not related to levels of surface expression of CCR5 at time of infection. Four CD4+ T-cell clones from 2 rhesus macaques were infected 24-48 h and 1 week after CD3 mAb stimulation with cell-free DNase-treated SIVmac239, and levels of cell-associated viral DNA determined 9, 24, 48 and 96 h PI by QPCR.
Reduction of CCR5 by RNAi has no effect on SIVmac239 infection of macaque CD4+ T cells
It was possible that the observed recovery in CCR5 expression following CD3 mAb stimulation allowed for increased spread of the virus over time. To exclude this possibility, one of the CD4+ T-cell clones that showed intermediate susceptibility to SIV infection was transduced with a macaque CCR5 shRNA retroviral vector and two independent subclones were isolated by sorting for the GFP marker carried in the shRNA vector. The CCR5 shRNA transduced CD4+ T-cell subclones showed stable and markedly downregulated expression of CCR5 compared to the untransduced parental clone without affecting other surface markers such as CD4 (Fig. 5A and data not shown). CCR5 shRNA transduced CD4+ T-cell subclones and the untransduced parental clone were infected 1 week after CD3 mAb stimulation with SIVmac239. The frequency of SIV Gag p27-expressing cells was determined by intracellular staining and flow cytometry. Cultures of the CCR5 shRNA transduced (CCR5low) and parental untransduced (CCR5high) CD4+ T cells showed similar frequencies of SIV Gag p27+ cells 12 days after infection (Fig. 5B). Thus, CCR5 shRNA-mediated knockdown of CCR5 expression had no measurable impact on the level of SIVmac239 infection and replication efficiency as assessed by SIV Gag p27 expression.
Figure 5.
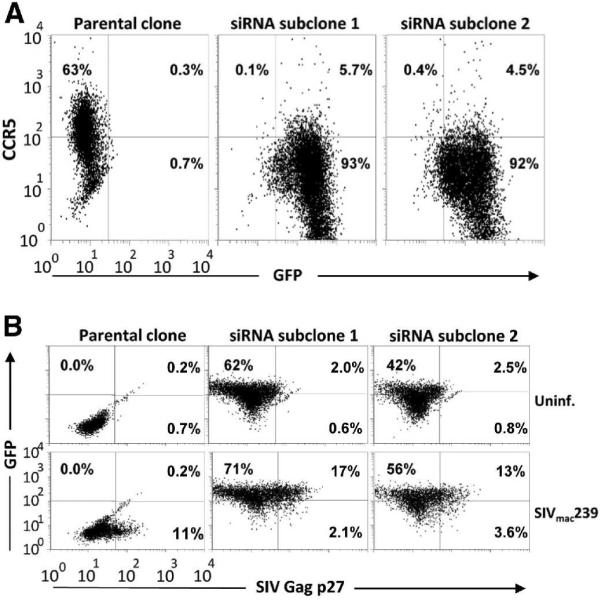
CCR5 RNAi-mediated inhibition of CCR5 expression has no impact on the susceptibility of macaque CD4+ T cells to SIVmac239. A) A rhesus macaque CD4+ T-cell clone (DBN2-9) was transduced with CCR5 shRNA using a retroviral vector construct that included the gene encoding green fluorescent protein (GFP) as a reporter. Surface CCR5 expression by the transduced and parental cells was determined by flow cytometry. B) CCR5 shRNA transduced and untransduced clones were infected with SIVmac239 1 week after CD3 mAb stimulation, and SIV Gag p27 expression was assessed on day 12 PI by flow cytometry.
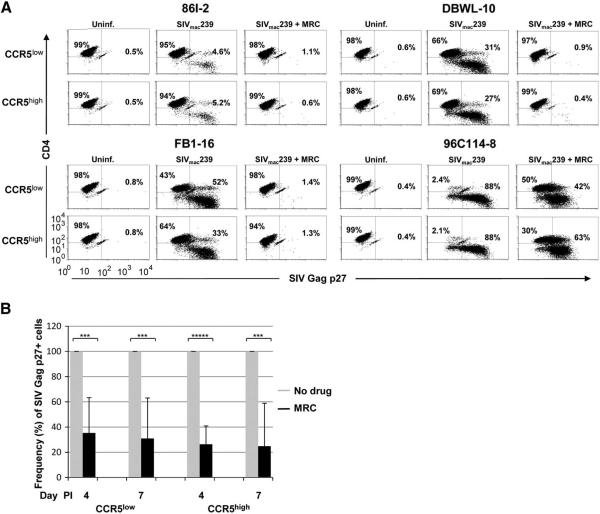
A CCR5 antagonist blocks SIVmac239 infection of macaque CD4+ T cells at CCR5low- and CCR5high-expressing states to comparable degrees
Because we found that RNAi-mediated CCR5 down-regulation does not impact SIVmac239 infection of macaque CD4+ T cells, we hypothesized that very low levels of CCR5 expression may be sufficient for SIVmac239 infection of macaque CD4+ T cells. To test this, 8 CD4+ T-cell clones with intermediate-to-high susceptibility to SIVmac239 infection from 5 rhesus macaques were pretreated with 10 μM of the CCR5 antagonist maraviroc at CCR5low (24-48 after stimulation) and CCR5high (resting) expression states before infection with SIVmac239. The frequency of SIV Gag p27-expressing cells was determined by intracellular staining and flow cytometry on day 4 and 7 PI. Pretreatment with maraviroc resulted in a marked reduction in the frequency of SIV Gag p27-expressing cells both in culture of cells infected at CCR5low- and CCR5high-expression states (Fig. 6A; data for 4 representative clones on day 7 PI). When the data for cultures with drug were normalized for all 8 tested clones, the differences were found to be significant at both time points (Fig. 6B). Noteworthy, the blocking effect of the drug for cells infected at CCR5low- and CCR5high-expression states was similar. Similar results were obtained when SIV Gag p28 levels in culture supernatants were determined by Enzyme-linked immunosorbent assay (data not shown). Our data suggests that a very low threshold level of surface CCR5 expression is necessary for efficient SIVmac239 infection of macaque CD4+ T cells, but that wide differences in CCR5 expression above this threshold do not affect susceptibility to infection.
Figure 6.
Pretreatment of macaque CD4+ T cells with the CCR5 antagonist maraviroc (MRC) before infection with SIVmac239 blocks infection of cells exposed to virus at CCR5low and CCR5high expression states to a comparable degree. A) CD4+ T-cell clones from four rhesus macaques were infected with SIVmac239 in the absence or presence of the 10 μM CCR5 antagonist, and the frequency of SIV Gag p27 positive cells determined on day 7 PI by flow cytometry. B) Frequency of SIV Gag p27 positive cells shown as mean and standard deviation on day 4 and 7 PI for eight CD4+ T-cell clones from five rhesus macaques infected in the absence (grey) or presence (black) of maraviroc at CCR5low or CCR5high expression states. The data were normalized for frequency of SIV Gag p27 positive cells in cultures of infected cells without drug.
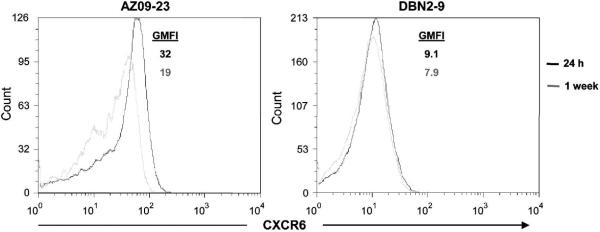
We next investigated whether other reported minor SIVmac239 co-receptors (Sharron et al., 2000; Unutmaz et al., 2000) are regulated similar to CCR5 in primary macaque CD4+ T cells. We analyzed expression of the chemokine-like orphan receptors CXCR6 (STRL33/Bonzo) and GPR15 (BOB) on 2 CD4+ T-cell clones (one that showed high and the other intermediate susceptibility to SIV infection) from 2 different animals 24-48 h and 1 week after CD3 mAb stimulation. CXCR6 expression ranged from stable-to-slightly-upregulated 24-48 h after stimulation (Fig. 7A), whereas GPR15 levels were generally low-to-undetectable and were unaffected by stimulation (data not shown). This suggests that surface expression of CCR5 and CXCR6 is differentially regulated, but the direct role of CXCR6 on SIV infection could not be tested due to lack of CXCR6-specific neutralizing mAb (Sharron et al., 2000).
Figure 7.
CXCR6 (Bonzo/STRL33) surface expression by primary rhesus macaque CD4+ T-cell clones are stable-to-slightly upregulated in response to T-cell receptor triggering. CXCR6 expression by 2 CD4+ T-cell clones from 2 rhesus macaques analyzed 24-48 h (black) and 1 week (grey) after CD3 mAb stimulation by flow cytometry.
Discussion
In this study we report substantial clonal differences in susceptibility of rhesus macaque CD4+ T-cells to SIV infection. We demonstrate that surface CCR5 levels vary considerably on different resting macaque CD4+ T-cell clones and that T-cell receptor (TCR) triggering results in dramatic downregulation of CCR5 expression. We also show that the level of surface CCR5 expression at time of infection is not related to susceptibility to SIVmac239. Finally, we show that CCR5 downregulation involves an RNA mechanism, similar to the prior results in human cells (Carroll et al., 1997). Together, our findings suggest that CCR5 density above a low threshold is not a factor determining susceptibility of macaque CD4+ T-cells to SIV infection.
Because most infection protocols for SIV suggest infection after stimulation of the target cells (Minang et al., 2009; Sacha and Watkins, 2010), our observation that surface CCR5 expression was markedly downregulated following CD3 mAb stimulation was intriguing. The dynamic changes in CCR5 expression have important implications for in vivo studies suggesting selective infection and depletion of CD4+CCR5+ T cells. Veazey et al., (Veazey et al., 2000) reported that CD4+CCR5+ T cells are selectively depleted in the gut during acute SIV infection of rhesus macaques. While the bulk of the CD4+ T cell loss is due to infection, it is possible that the lower frequencies of CD4+CCR5+ T cells in the gut compared with CD4+CCR5low/− cells after SIV infection is partly due to downregulation of CCR5, as CD4+ T cells become activated from the generalized inflammatory environment known to occur in this tissue during acute infection (Campillo-Gimenez et al., 2009; Meythaler et al., 2009). Therefore, the skewed levels of CD4+CCR5low/− T cells might be due to activation-induced CCR5 downregulation rather than a virus resistant CD4+CCR5low/− T-cell pool. Furthermore, IL-15 treatment during acute SIV infection of rhesus macaques was shown to result in a dramatic decrease in the frequency of CD4+CCR5+ T cells despite a demonstrated increase in the frequency of Ki-67+CD4+ (activated/proliferating) T cells (Mueller et al., 2008). However, it is possible that the Ki-67+CD4+ cells may have downregulated CCR5 expression consistent with our in vitro data with CD3 mAb stimulated CD4+ T cells. Pandrea et al., showed that uninfected natural SIV host species express lower levels of CCR5 compared to rhesus macaques (Pandrea et al., 2007). However, similar levels of acute viral load are seen in natural hosts and rhesus macaques (Lay et al., 2009; Mattapallil et al., 2005). This together with data from Mattapallil et al., is consistent with our model that low levels of CCR5 expression are sufficient for efficient infection (Mattapallil et al., 2005). Whereas total CD4+ T-cell loss has been reported in gut biopsies and PBMC from sooty mangabeys (natural hosts) acutely infected with SIV at levels comparable with acutely-infected rhesus macaques (Lay et al., 2009; Mattapallil et al., 2005), CD4+CCR5+ T-cell loss is more pronounced in rhesus macaques (Monceaux et al., 2007). This is consistent with the elevated immune activation observed in SIV-infected macaques. Because it is complex to control for time or duration of activation during in vivo studies, it is difficult to distinguish between cells that were consistently CCR5low/−, and those that were CCR5high but subsequently downregulated CCR5 upon activation. Our results suggest caution be applied to interpreting CCR5 data from in vivo studies.
Levine et al., showed that TCR triggering using CD3 mAb stimulation together with CD28 co-stimulation rendered CD4+ T cells from HIV-1 infected and uninfected donors highly resistant to infection by macrophage tropic (R5) HIV-1 (Levine et al., 1996). It was later demonstrated that their resistance to HIV-1 infection correlated with the absence of detectable CCR5 mRNA expression (Carroll et al., 1997). Our data extends this finding of activation-induced transcriptional control of CCR5 to the rhesus model. However, unlike HIV-1 infection of human PBMC or T-cell lines shown to correlate with cell surface CCR5 density in vitro (Lin et al., 2002; Salkowitz et al., 2003), we show that reduced levels of CCR5 expression by macaque CD4+ T cells after activation has no measurable impact on the efficiency of SIVmac239 infection. In line with this, Mattapallil et al. showed that the level of viral infection, as measured by SIV-Gag DNA, was essentially the same in sorted CD4+ CCR5+ and CD4+ CCR5− cells obtained from different tissues of rhesus macaques acutely infected with SIVmac239 (Mattapallil et al., 2005). We did not find any difference in susceptibility to infection between macaque CD4+ T cells that were CCR5low (following activation or RNAi transduction) and those that were CCR5high (resting) at the time of infection, and pretreatment of cells with saturating amounts of a CCR5 antagonist significantly reduced infection of both cell populations. Noteworthy, pretreatment of CD4+ T-cell clones that showed the highest susceptibility to SIVmac239 infection (typically ≥80% SIV Gag p27+ cells by day 5 PI) was considerably less effective compared with blocking of more resistant clones. These data suggest that the highly susceptible clones express alternative receptor(s) in addition to CCR5, and more resistant clones express this putative receptor(s) at lower densities. In line with this, Riddick et al. recently showed that a mutated CCR5 gene encoding a truncated molecule that is not expressed on the cell surface does not protect sooty mangabeys from infection with the “R5-tropic” SIVsmm virus in vivo (Riddick et al., 2010). This, together with our data, support previous studies (Forte et al., 2003) suggesting that other surface molecules may also serve as co-receptors for SIV. SIV Env has been shown to mediate target cell entry in cell-to-cell fusion assays via interaction with chemokine receptor-like orphan receptors such as CXCR6 (STRL33/Bonzo) and GPR15 (BOB) (Edinger et al., 1998; Lauren et al., 2006; Pohlmann et al., 1999). In contrast to CCR5 regulation, we observed stable-to-slightly elevated surface expression of CXCR6 by primary macaque CD4+ T cells following CD3 mAb stimulation, but its role in our model could not be directly tested due to lack of suitable reagents. More studies are therefore needed to more clearly define the possible role of CXCR6 and other as of yet unknown receptors in mediating SIV entry into primary macaque CD4+ cells.
Our data fit a model where a very low threshold level of surface CCR5 expression is required and sufficient for efficient SIVmac239 infection of macaque CD4+ T cells, while low levels of infection can occur through an alternate entry pathway also in the absence of CCR5. Because CD4+ T cells that are CCR5low following CD3 mAb stimulation or RNAi transduction express surface CCR5 at levels above this hypothetical threshold, they are still highly susceptible to SIV. Thus, attempts to prevent SIVmac239 infection by RNAi or other methods that markedly reduce but fail to completely abrogate CCR5 expression may not be effective, and direct silencing at the gene level using reagents such as zinc-finger nucleases targeted to CCR5 may be more successful (Perez et al., 2008).
Materials and Methods
Generation of primary rhesus macaque CD4+ T-cell clones
CD4+ T-cell clones were generated from PBMC isolated from three naïve (96C114, DBGR and DBWL2) and five SIV DNA vaccinated (AZ09, AZ15, C0102, DBN2 and FB1) (Minang et al., 2010) Indian Rhesus macaques, Macaca mulatta. Both male and female animals were included in the study and ranged in age between 4-14 years. The CD4+ T-cell clones were maintained using bi-weekly stimulation with antihuman-CD3 monoclonal antibodies (mAb) (30 ng/mL; clone SP34-2; BD Biosciences, San Diego, CA, USA) and irradiated human PBMC and a human Epstein-Barr virus transformed B-cell line (TM B-LCL; kindly provided by Drs. S.R. Riddell and P. D. Greenberg, FHCRC, Seattle, WA) as feeder cells as described (Minang et al., 2008). Feeder cells were irradiated in a Mark I Cs137 γ-irradiator (Shepherd & Associates, San Fernando, CA) at 6000 and 12500 rad for PBMC and TM B-LCL, respectively.
All CD4+ T-cell clones from the vaccinated animals were screened for reactivity to SIV by assessing IFN-γ responses to vaccine antigens using autologous PBMC pulsed with SIV Gag, Pol and Accessory (Acc) peptide pools as described (Minang et al., 2010). Animal care was according to the guidelines of the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, and the Health and Human Services guidelines “Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996, National Academy Press, Washington, D.C.), under an Institutional Animal Care and Use Committee approved protocol.
Retroviral vectors expressing rhesus macaque CCR5 shRNA
MicroRNA-30-adapted CCR5 small hairpin RNA (miR-shRNA) were designed by using the Cold Spring Harbor Laboratory web RNAi design tool at http://katahdin.cshl.org/siRNA/RNAi.cgi?type=shRNA for Macaca mulatta mRNA CCR5 target sequence (GenBank accession number U73739). miR-shRNA oligonucleotide cassettes were inserted into the retroviral vector MSCV-LTRmiR30-PIG (LMP) (Open Biosystems, Huntsville, AL) (Dickins et al., 2005) according to the manufacturer’s recommendations. The resulting miR-shRNA-expressing constructs (LMP-miR-shRNA) were verified by sequencing.
To generate retroviral vector stocks, GP2-293 packaging cells (Clontech, Mountain View, CA) were co-transfected with LMP-miR-shRNA plasmid DNAs and vesicular stomatitis virus envelope G glycoprotein (VSV-G)-expressing construct using TransIt®-293 transfection reagent (Mirus Bio, Madison, WI). Forty-eight hours post transfection, supernatants were harvested and retroviral vector particles concentrated by centrifugation at 50,000 × g as described (Kutner, Zhang, and Reiser, 2009) and resuspended in PBS to produce vector stocks.
Retroviral transduction of rhesus macaque CD4+ T cells
Activated proliferating macaque CD4+ T cells were transduced with LMP-miR-shRNA retroviral vectors using RetroNectin transduction technique (Hanenberg et al., 1996) according to the manufacturer’s recommendations. Briefly, a 12-well cell culture plate was coated with RetroNectin® reagent (TaKaRA Bio USA, Madison, WI) and washed with PBS. To bind retroviral particles to the retronectin, retroviral vector stocks were added and the plate was centrifuged at 2,000 × g (32°C) for 2 h. The wells were washed with PBS. The CD4+ T cells were resuspended in cell culture medium (RPMI 1640 supplemented with 10% fetal bovine serum, penicillin/streptomycin [100 units/mL] and IL-2 [100 IU/mL]) and plated at 5 × 105 cells per well. The plate was centrifuged at 500 × g (32°C) for 2 h and the cells cultured for an additional 48 h at 37°C. Transductants were identified and sorted by flow cytometry using a BD FACS Aria (BD Biosciences) based on GFP fluorescence. The cells were stained with flourochrome-conjugated anti-CCR5 mAb and CCR5 surface expression analyzed by flow cytometry (see section on flow cytometric “quantitation of SIV Gag p27 expression”).
Virus stocks
Cell-free virus stocks used in this study were derived from the molecular clone spxfl SIVmac239 (gift of Dr. Ronald Desrosiers, New England Primate Research Center) (Genbank accession no. M33262.1) (Kestler et al., 1990). Virus stocks were produced as previously described (Minang et al., 2009).
SIV infection of primary rhesus macaque CD4+ T cells
Polyclonal or clonal populations of PBMC-derived primary rhesus macaque CD4+ T cells were infected 24-48 h or 1 week after activation with CD3 mAb. Cells were infected by incubating with aliquots of virus stock for 2-3 h using the Viromag magnetofection reagents and procedures according to manufacturer’s recommendation (OzBiosciences, Marseille, France). Virus concentration and cell numbers were as described (Minang et al., 2009). Virus-exposed CD4+ T cells were washed twice with PBS to remove residual non-incorporated viral material and put in culture at a final volume of 2 mL/well in 24-well tissue culture plates. For CCR5 blocking experiments, CD4+ T-cell clones were pretreated with 10 μM CCR5 antagonist maraviroc (AIDS Research and Reference Program, Division of AIDS, NIAID) prior to virus exposure and the drug added to the cell cultures every 2-3 days until harvest and analyses. Initial titration experiments revealed that drug concentrations above 10 μM did not convey any increased blocking activity (data not shown).
Quantitation of SIV Gag p27 expression
The frequency of productively infected cells as a percentage of target cell population was determined by staining for intracellular SIV Gag expression using a FITC-conjugated SIV Gag p27 mAb (Clone 55-2F12; NIH AIDS Research and Reference Reagent Program), in concert with staining for other surface markers at defined time points post infection (PI). All mAbs were obtained from BD Biosciences (San Jose, CA, USA) unless otherwise indicated. The following mAbs conjugated to the indicated fluorochromes were used for surface staining: CD4-FITC or -PerCP-Cy5.5 (clone L200), CCR5-PE or -APC (clone 3A9), CXCR6 (STRL33/Bonzo)-APC (clone 56811; R&D Systems), GPR15 (BOB) (Clone 367902; R&D Systems) in combination with rat anti-mouse IgG2a+b PE (clone X57). The surface and intracellular staining procedure and subsequent analyses by flow cytometry was as previously described (Minang et al., 2009).
Analysis of cell-associated viral DNA
To assess the levels of cell-associated viral DNA, cell samples were harvested 9, 24, 48 and 96 h after infection of the CD4+ T cells and cell-associated viral DNA copies quantified by gag gene-specific real time PCR as previously described (Cline et al., 2005).
Statistical Analysis
Differences between geometric mean fluorescent intensities (GMFI) of surface CCR5 expression 24-48 h compared to 1 week post CD3 mAb stimulation, and those of surface CD4 and CCR5 expression by SIV Gag p27+ compared to SIV Gag p27− cells on day 5 PI were analyzed using the paired Students t test. Comparison of CCR5 mRNA copies by different clones at resting and after CD3 mAb stimulation were also performed using the paired Students t test. A Spearman rank order correlation analysis was performed to assess any correlation between the GMFI of surface CCR5 expression at time of infection and SIV Gag p27 expression on day 5 PI. A p value ≤ 0.05 was considered statistically significant. All tests were performed using the software STATA 10.0 (StataCorp LP, College Station, TX, USA).
Supplementary Material
Supplemental Figure 1 Resting CD4+ T-cell clones generated from different rhesus macaques show high and comparable levels of surface CD4 but variable CCR5 levels. 21 resting CD4+ T-cell clones from five rhesus macaques were stained with flourochrome-conjugated mAbs against CD4 and CCR5 and surface expression of these molecules determined by flow cytometry.
Supplemental Figure 2 SIV Gag-specific primary macaque CD4+ T cells produce IFN-γ following stimulation with SIV Gag peptide-pulsed autologous cells. An SIV Gag-specific CD4+ T-cell clone generated from an uninfected SIV DNA vaccinated rhesus macaque was stimulated with SIV Gag peptide-pulsed autologous virus non-specific CD4+ T cells for 4 h and IFN-γ production assessed by intracellular cytokine staining and flow cytometry. Shown is the percentage of intracellular IFN-γ-expressing cells within the clonal population.
Supplemental Figure 3 Freshly isolated rhesus macaque PBMC-derived CD4+ T cells show dynamic CCR5 expression after ex vivo CD3 mAb stimulation. The CD4-enriched fraction of freshly ficolled PBMC from two rhesus macaques, B001 and 86I, were stimulated with CD3 mAb, and CCR5 expression determined 24 h later by flow cytometry. The CD3 mAb stimulated CD4-enriched PBMC were cultured for an additional 8 days with IL-2 addition (50 IU/mL) every 2-3 days and CCR5 expression determined on day 2, 4, 6 and 8. A PE-conjugated mouse IgG isotype control mAb was included and showed similar staining pattern as indicated for the unstimulated cells on day 8.
Acknowledgements
The authors thank Jeffrey D. Lifson for valuable suggestions, Rebecca Shoemaker for help with the cell-associated viral DNA (QPCR) analyzes and Andy Wu for help with sample preparation. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: IL-2 from Hoffman-La Roche Inc., NJ; SIVmac p27 hybridoma (55-2f12) from Dr Niels Pedersen; SIVmac 239 Gag, Pol, Acc (15-mer) Peptides - Complete Sets; Maraviroc (Cat #11580). This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract number HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albright AV, Shieh JT, Itoh T, Lee B, Pleasure D, O’Connor MJ, Doms RW, Gonzalez-Scarano F. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73(1):205–13. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lee B, Tackoen M, Puffer B, Boom A, Libert F, Sharron M, Wittamer V, Vassart G, Doms RW, Parmentier M. Multiple nonfunctional alleles of CCR5 are frequent in various human populations. Blood. 2000;96(5):1638–45. [PubMed] [Google Scholar]
- Campillo-Gimenez L, Laforge M, Fay M, Brussel A, Cumont MC, Monceaux V, Diop O, Levy Y, Hurtrel B, Zaunders J, Corbeil J, Elbim C, Estaquier J. Non pathogenesis of SIV infection is associated with reduced inflammation and recruitment of plasmacytoid dendritic cells to lymph nodes, not to lack of an interferon type I response, during the acute phase. J Virol. 2009 doi: 10.1128/JVI.01496-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RG, Riley JL, Levine BL, Feng Y, Kaushal S, Ritchey DW, Bernstein W, Weislow OS, Brown CR, Berger EA, June CH, St Louis DC. Differential regulation of HIV-1 fusion cofactor expression by CD28 costimulation of CD4+ T cells. Science. 1997;276(5310):273–6. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- Chen Z, Kwon D, Jin Z, Monard S, Telfer P, Jones MS, Lu CY, Aguilar RF, Ho DD, Marx PA. Natural infection of a homozygous delta24 CCR5 red-capped mangabey with an R2b-tropic simian immunodeficiency virus. J Exp Med. 1998;188(11):2057–65. doi: 10.1084/jem.188.11.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline AN, Bess JW, Piatak M, Jr., Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34(5-6):303–12. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- Connor RI, Paxton WA, Sheridan KE, Koup RA. Macrophages and CD4+ T lymphocytes from two multiply exposed, uninfected individuals resist infection with primary non-syncytium-inducing isolates of human immunodeficiency virus type 1. J Virol. 1996;70(12):8758–64. doi: 10.1128/jvi.70.12.8758-8764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGottardi MQ, Lew SK, Piatak M, Jr., Jia B, Feng Y, Lee SJ, Brenchley JM, Douek DC, Kodama T, Lifson JD, Evans DT. Comparison of plasma viremia and antibody responses in macaques inoculated with envelope variants of single-cycle simian immunodeficiency virus differing in infectivity and cellular tropism. J Virol. 2008;82(1):321–34. doi: 10.1128/JVI.01094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete GQ, Haggarty B, Leslie GJ, Jordan AP, Romano J, Wang N, Wang J, Holmes MC, Montefiori DC, Hoxie JA. Derivation and characterization of a simian immunodeficiency virus SIVmac239 variant with tropism for CXCR4. J Virol. 2009;83(19):9911–22. doi: 10.1128/JVI.00533-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickins RA, Hemann MT, Zilfou JT, Simpson DR, Ibarra I, Hannon GJ, Lowe SW. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat Genet. 2005;37(11):1289–95. doi: 10.1038/ng1651. [DOI] [PubMed] [Google Scholar]
- Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417(6884):95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Hoffman TL, Sharron M, Lee B, O’Dowd B, Doms RW. Use of GPR1, GPR15, and STRL33 as coreceptors by diverse human immunodeficiency virus type 1 and simian immunodeficiency virus envelope proteins. Virology. 1998;249(2):367–78. doi: 10.1006/viro.1998.9306. [DOI] [PubMed] [Google Scholar]
- Forte S, Harmon ME, Pineda MJ, Overbaugh J. Early- and intermediate-stage variants of simian immunodeficiency virus replicate efficiently in cells lacking CCR5. J Virol. 2003;77(17):9723–7. doi: 10.1128/JVI.77.17.9723-9727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glushakova S, Yi Y, Grivel JC, Singh A, Schols D, De Clercq E, Collman RG, Margolis L. Preferential coreceptor utilization and cytopathicity by dual-tropic HIV-1 in human lymphoid tissue ex vivo. J Clin Invest. 1999;104(5):R7–R11. doi: 10.1172/JCI7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O’Doherty U, Paxton W, Koup R, Mojsov S, Bhardwaj N, Clark-Lewis I, Baggiolini M, Steinman RM. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184(6):2433–8. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanenberg H, Xiao XL, Dilloo D, Hashino K, Kato I, Williams DA. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat Med. 1996;2(8):876–82. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- Hoffman TL, MacGregor RR, Burger H, Mick R, Doms RW, Collman RG. CCR5 genotypes in sexually active couples discordant for human immunodeficiency virus type 1 infection status. J Infect Dis. 1997;176(4):1093–6. doi: 10.1086/516519. [DOI] [PubMed] [Google Scholar]
- Huang Y, Paxton WA, Wolinsky SM, Neumann AU, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau NR, Phair J, Ho DD, Koup RA. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2(11):1240–3. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, et al. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248(4959):1109–12. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc. 2009;4(4):495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- Lauren A, Vodros D, Thorstensson R, Fenyo EM. Comparative studies on mucosal and intravenous transmission of simian immunodeficiency virus (SIVsm): evolution of coreceptor use varies with pathogenic outcome. J Gen Virol. 2006;87(Pt 3):581–94. doi: 10.1099/vir.0.81408-0. [DOI] [PubMed] [Google Scholar]
- Lay MD, Petravic J, Gordon SN, Engram J, Silvestri G, Davenport MP. Is the gut the major source of virus in early simian immunodeficiency virus infection? J Virol. 2009;83(15):7517–23. doi: 10.1128/JVI.00552-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BL, Mosca JD, Riley JL, Carroll RG, Vahey MT, Jagodzinski LL, Wagner KF, Mayers DL, Burke DS, Weislow OS, St Louis DC, June CH. Antiviral effect and ex vivo CD4+ T cell proliferation in HIV-positive patients as a result of CD28 costimulation. Science. 1996;272(5270):1939–43. doi: 10.1126/science.272.5270.1939. [DOI] [PubMed] [Google Scholar]
- Lin YL, Mettling C, Portales P, Reynes J, Clot J, Corbeau P. Cell surface CCR5 density determines the postentry efficiency of R5 HIV-1 infection. Proc Natl Acad Sci U S A. 2002;99(24):15590–5. doi: 10.1073/pnas.242134499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86(3):367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434(7037):1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- Means RE, Matthews T, Hoxie JA, Malim MH, Kodama T, Desrosiers RC. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J Virol. 2001;75(8):3903–15. doi: 10.1128/JVI.75.8.3903-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meythaler M, Martinot A, Wang Z, Pryputniewicz S, Kasheta M, Ling B, Marx PA, O’Neil S, Kaur A. Differential CD4+ T-lymphocyte apoptosis and bystander T-cell activation in rhesus macaques and sooty mangabeys during acute simian immunodeficiency virus infection. J Virol. 2009;83(2):572–83. doi: 10.1128/JVI.01715-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minang JT, Barsov EV, Yuan F, Trivett MT, Piatak M, Jr., Lifson JD, Ott DE, Ohlen C. Efficient inhibition of SIV replication in rhesus CD4+ T-cell clones by autologous immortalized SIV-specific CD8+ T-cell clones. Virology. 2008;372(2):430–41. doi: 10.1016/j.virol.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Minang JT, Trivett MT, Bolton DL, Trubey CM, Estes JD, Li Y, Smedley J, Pung R, Rosati M, Jalah R, Pavlakis GN, Felber BK, Piatak M, Jr., Roederer M, Lifson JD, Ott DE, Ohlen C. Distribution, persistence, and efficacy of adoptively transferred central and effector memory-derived autologous Simian Immunodeficiency Virus-specific CD8(+) T cell clones in rhesus macaques during acute infection. J Immunol. 2010;184(1):315–26. doi: 10.4049/jimmunol.0902410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minang JT, Trivett MT, Coren LV, Barsov EV, Piatak M, Jr., Ott DE, Ohlen C. Nef-mediated MHC class I down-regulation unmasks clonal differences in virus suppression by SIV-specific CD8(+) T cells independent of IFN-gamma and CD107a responses. Virology. 2009;391(1):130–9. doi: 10.1016/j.virol.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monceaux V, Viollet L, Petit F, Cumont MC, Kaufmann GR, Aubertin AM, Hurtrel B, Silvestri G, Estaquier J. CD4+ CCR5+ T-cell dynamics during simian immunodeficiency virus infection of Chinese rhesus macaques. J Virol. 2007;81(24):13865–75. doi: 10.1128/JVI.00452-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller YM, Do DH, Altork SR, Artlett CM, Gracely EJ, Katsetos CD, Legido A, Villinger F, Altman JD, Brown CR, Lewis MG, Katsikis PD. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J Immunol. 2008;180(1):350–60. doi: 10.4049/jimmunol.180.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulherin SA, O’Brien TR, Ioannidis JP, Goedert JJ, Buchbinder SP, Coutinho RA, Jamieson BD, Meyer L, Michael NL, Pantaleo G, Rizzardi GP, Schuitemaker H, Sheppard HW, Theodorou ID, Vlahov D, Rosenberg PS. Effects of CCR5-Delta32 and CCR2-64I alleles on HIV-1 disease progression: the protection varies with duration of infection. Aids. 2003;17(3):377–87. doi: 10.1097/01.aids.0000050783.28043.3e. [DOI] [PubMed] [Google Scholar]
- Oh DY, Jessen H, Kucherer C, Neumann K, Oh N, Poggensee G, Bartmeyer B, Jessen A, Pruss A, Schumann RR, Hamouda O. CCR5Delta32 genotypes in a German HIV-1 seroconverter cohort and report of HIV-1 infection in a CCR5Delta32 homozygous individual. PLoS One. 2008;3(7):e2747. doi: 10.1371/journal.pone.0002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye A, Park H, Rohankhedkar M, Coyne-Johnson L, Lum R, Walker JM, Planer SL, Legasse AW, Sylwester AW, Piatak M, Jr., Lifson JD, Sodora DL, Villinger F, Axthelm MK, Schmitz JE, Picker LJ. Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J Exp Med. 2009;206(7):1575–88. doi: 10.1084/jem.20090356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, Sumpter B, Roques P, Marx PA, Hirsch VM, Kaur A, Lackner AA, Veazey RS, Silvestri G. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109(3):1069–76. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton WA, Martin SR, Tse D, O’Brien TR, Skurnick J, VanDevanter NL, Padian N, Braun JF, Kotler DP, Wolinsky SM, Koup RA. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat Med. 1996;2(4):412–7. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, Wang N, Lee G, Bartsevich VV, Lee YL, Guschin DY, Rupniewski I, Waite AJ, Carpenito C, Carroll RG, Orange JS, Urnov FD, Rebar EJ, Ando D, Gregory PD, Riley JL, Holmes MC, June CH. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26(7):808–16. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Jr., Wang C, Allison DB, Maino VC, Lifson JD, Kodama T, Axthelm MK. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200(10):1299–314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann S, Krumbiegel M, Kirchhoff F. Coreceptor usage of BOB/GPR15 and Bonzo/STRL33 by primary isolates of human immunodeficiency virus type 1. J Gen Virol. 1999;80(Pt 5):1241–51. doi: 10.1099/0022-1317-80-5-1241. [DOI] [PubMed] [Google Scholar]
- Pohlmann S, Lee B, Meister S, Krumbiegel M, Leslie G, Doms RW, Kirchhoff F. Simian immunodeficiency virus utilizes human and sooty mangabey but not rhesus macaque STRL33 for efficient entry. J Virol. 2000;74(11):5075–82. doi: 10.1128/jvi.74.11.5075-5082.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann S, Stolte N, Munch J, Ten Haaft P, Heeney JL, Stahl-Hennig C, Kirchhoff F. Co-receptor usage of BOB/GPR15 in addition to CCR5 has no significant effect on replication of simian immunodeficiency virus in vivo. J Infect Dis. 1999;180(5):1494–502. doi: 10.1086/315097. [DOI] [PubMed] [Google Scholar]
- Rana S, Besson G, Cook DG, Rucker J, Smyth RJ, Yi Y, Turner JD, Guo HH, Du JG, Peiper SC, Lavi E, Samson M, Libert F, Liesnard C, Vassart G, Doms RW, Parmentier M, Collman RG. Role of CCR5 in infection of primary macrophages and lymphocytes by macrophage-tropic strains of human immunodeficiency virus: resistance to patient-derived and prototype isolates resulting from the delta ccr5 mutation. J Virol. 1997;71(4):3219–27. doi: 10.1128/jvi.71.4.3219-3227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick NE, Hermann EA, Lamorris ML, Elliot ST, Wey WC, Cervasi B, Taafe J, Engram JC, Li B, Else JG, Li Y, Hahn BH, Derdeyn CA, Sodora DL, Apetrei C, Paiardini M, Silvestri G, Collman RG. A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use In Vivo. PLoS Pathog. 2010;6(8) doi: 10.1371/journal.ppat.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacha JB, Watkins DI. Synchronous infection of SIV and HIV in vitro for virology, immunology and vaccine-related studies. Nat Protoc. 5(2):239–46. doi: 10.1038/nprot.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkowitz JR, Bruse SE, Meyerson H, Valdez H, Mosier DE, Harding CV, Zimmerman PA, Lederman MM. CCR5 promoter polymorphism determines macrophage CCR5 density and magnitude of HIV-1 propagation in vitro. Clin Immunol. 2003;108(3):234–40. doi: 10.1016/s1521-6616(03)00147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlatti G, Tresoldi E, Bjorndal A, Fredriksson R, Colognesi C, Deng HK, Malnati MS, Plebani A, Siccardi AG, Littman DR, Fenyo EM, Lusso P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3(11):1259–65. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- Sharron M, Pohlmann S, Price K, Lolis E, Tsang M, Kirchhoff F, Doms RW, Lee B. Expression and coreceptor activity of STRL33/Bonzo on primary peripheral blood lymphocytes. Blood. 2000;96(1):41–9. [PubMed] [Google Scholar]
- Unutmaz D, Xiang W, Sunshine MJ, Campbell J, Butcher E, Littman DR. The primate lentiviral receptor Bonzo/STRL33 is coordinately regulated with CCR5 and its expression pattern is conserved between human and mouse. J Immunol. 2000;165(6):3284–92. doi: 10.4049/jimmunol.165.6.3284. [DOI] [PubMed] [Google Scholar]
- Vatakis DN, Bristol G, Wilkinson TA, Chow SA, Zack JA. Immediate activation fails to rescue efficient human immunodeficiency virus replication in quiescent CD4+ T cells. J Virol. 2007;81(7):3574–82. doi: 10.1128/JVI.02569-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatakis DN, Nixon CC, Bristol G, Zack JA. Differentially stimulated CD4+ T cells display altered human immunodeficiency virus infection kinetics: implications for the efficacy of antiviral agents. J Virol. 2009;83(7):3374–8. doi: 10.1128/JVI.02161-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, Mansfield KG, Tham IC, Carville AC, Shvetz DE, Forand AE, Lackner AA. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol. 2000;74(23):11001–7. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler A, May GE, Qi Y, Wilson N, Watkins DI. Polymorphisms in eight host genes associated with control of HIV replication do not mediate elite control of viral replication in SIV-infected Indian rhesus macaques. Immunogenetics. 2006;58(12):1003–9. doi: 10.1007/s00251-006-0166-6. [DOI] [PubMed] [Google Scholar]
- Xiao L, Rudolph DL, Owen SM, Spira TJ, Lal RB. Adaptation to promiscuous usage of CC and CXC-chemokine coreceptors in vivo correlates with HIV-1 disease progression. Aids. 1998;12(13):F137–43. doi: 10.1097/00002030-199813000-00001. [DOI] [PubMed] [Google Scholar]
- Zimmerman PA, Buckler-White A, Alkhatib G, Spalding T, Kubofcik J, Combadiere C, Weissman D, Cohen O, Rubbert A, Lam G, Vaccarezza M, Kennedy PE, Kumaraswami V, Giorgi JV, Detels R, Hunter J, Chopek M, Berger EA, Fauci AS, Nutman TB, Murphy PM. Inherited resistance to HIV-1 conferred by an inactivating mutation in CC chemokine receptor 5: studies in populations with contrasting clinical phenotypes, defined racial background, and quantified risk. Mol Med. 1997;3(1):23–36. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Resting CD4+ T-cell clones generated from different rhesus macaques show high and comparable levels of surface CD4 but variable CCR5 levels. 21 resting CD4+ T-cell clones from five rhesus macaques were stained with flourochrome-conjugated mAbs against CD4 and CCR5 and surface expression of these molecules determined by flow cytometry.
Supplemental Figure 2 SIV Gag-specific primary macaque CD4+ T cells produce IFN-γ following stimulation with SIV Gag peptide-pulsed autologous cells. An SIV Gag-specific CD4+ T-cell clone generated from an uninfected SIV DNA vaccinated rhesus macaque was stimulated with SIV Gag peptide-pulsed autologous virus non-specific CD4+ T cells for 4 h and IFN-γ production assessed by intracellular cytokine staining and flow cytometry. Shown is the percentage of intracellular IFN-γ-expressing cells within the clonal population.
Supplemental Figure 3 Freshly isolated rhesus macaque PBMC-derived CD4+ T cells show dynamic CCR5 expression after ex vivo CD3 mAb stimulation. The CD4-enriched fraction of freshly ficolled PBMC from two rhesus macaques, B001 and 86I, were stimulated with CD3 mAb, and CCR5 expression determined 24 h later by flow cytometry. The CD3 mAb stimulated CD4-enriched PBMC were cultured for an additional 8 days with IL-2 addition (50 IU/mL) every 2-3 days and CCR5 expression determined on day 2, 4, 6 and 8. A PE-conjugated mouse IgG isotype control mAb was included and showed similar staining pattern as indicated for the unstimulated cells on day 8.