Abstract
In the past half century research efforts have defined a critical role for angiogenesis in tumor growth and metastasis. We previously reported that inhibition of a novel target, ENOX1, by a (Z)-2-benzylindol-3-ylmethylene) quinuclidin-3-ol, suppressed tumor angiogenesis. The present study was undertaken in order to establish structure-activity relationships for quinuclidine analogs. The angiogenesis inhibiting activity of a series of substituted (Z)-(±)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ols (1a–1k), (Z)-2-benzylindol-3-ylmethylene)quinuclidin-3-ones (2a–2h), (Z)-(±)-2-(1H/N-methyl-indol-3-ylmethylene)quinuclidin-3-ols (3a–3b), and substituted (Z)-(±)-2-(N-benzenesulfonylindol-3-yl-methylene)quinuclidin-3-ols and their derivatives (4a–4d) that incorporate a variety of substituents in both the indole and N-benzyl moieties was evaluated using Human Umbilical Vein Endothelial Cells (HUVECs) subjected to in vitro cell migration scratch assays, tubule formation in Matrigel, cell viability and proliferation assays. In total, 25 different analogs were evaluated. Based on in vitro cell migration scratch assays, eight analogs were identified as potent angiogenesis inhibitors at 10 µM, a concentration that was determined to be nontoxic by colony formation assay. In addition, this approach identified a potent antiangiogenic ENOX1 inhibitor, analog 4b.
Keywords: substituted (Z)-(±)-2-(N-benzylindol-3-ylmethylene) quinucli-din-3-ol/one analogs, indole, angiogenesis, Enox1, antiangiogenic activity, Human Umbilical Vein Endothelial Cells
Endothelial cells form the lining of all the blood vessels and play a critical role in tissue growth and wound healing. They achieve this by retaining the ability to divide and proliferate in an adult vascular system by the process of ‘angiogenesis’, where new blood vessels are formed from existing cells via development of small capillaries.1 Development of these capillaries, also known as neovascularization, plays a critical role in cell division, proliferation and movement.1,2
In the past half century research efforts have established a critical role for angiogenesis in tumor growth and metastasis.2 Without development of a capillary network by neovascularization, a tumor will not have sufficient nutrients to grow beyond a limited size.3 Thus, angiogenesis constitutes an important point in the control of cancer progression,3 and consequently, several major cancer therapy advances that target angiogenesis have been made in the recent years. These include development of antiangiogenesis drugs such as bevacizumab that binds to vascular endothelial growth factor (VEGF), a pro-angiogenic growth factor, and inhibits VEGF-VEGF receptor interactions. Sunitinib and Sorafenib, small molecule receptor tyrosine kinase inhibitors, represent two other examples.3–5 Other drugs that are currently in clinical trials include, AZD2171, a potent, indole-ether quinazoline that also targets all VEGF receptors in renal cell cancer.6 While these treatments have been shown to be effective in controlling initial cancer progression, their effects have been shown to be limited due to adaptation or previously existing resistance.6 This ability of tumor cells to adapt to current antiangiogenesis drugs underscores the need to develop novel drugs that not only target tumor vasculature but also complement cytotoxic therapy.6–7
We previously described the synthesis of novel compounds consisting of combinations of indole, benzyl, and quinuclidine moieties.8–10 In these studies, the effect of three specific compounds on cellular proliferation and tubule formation ability of endothelial cells was analyzed in detail.8 Both phenotypic screening and analysis of biochemical pathways affected by non toxic doses of the active compounds identified the ECTO-NOX (ENOX) family of cell surface enzymes as potential targets for these indole analogs.8 The ENOX family of enzymes exhibit cell surface protein disulfide-thiol interchange activity and oxidize NAD(P)H as an alternate substrate8. Enox enzymes play a critical role in cell proliferation and cancer.11 The family consists of constitutively expressed ENOX1 (previously called CNOX), which is ubiquitous in mammals and plants, a cancer specific ENOX2 (formally called tNOX) that is thought to be unregulated and responsive to inhibitors, and an age-related ENOX.12–13
Our studies demonstrated that inhibition of ENOX activity by these indole compounds inhibited endothelial cell proliferation, inhibited the ability to form tubules, and synergistically increased radiation-mediated xenograft tumor growth delay. These observations led us to further investigate and establish the relationship between structure and antiangiogenesis activity of a series of (Z)-(±)-2-(N-benzylindol-3-ylmethylene)quinuclidin-3-ols (Figure 1, 1a–1k), (Z)-2-benzylindol-3-ylmethylene)quinuclidin-3-ones (2a–2i), (Z)-(±)-2-(1H/N-methyl-indol-3-ylmethylene)quinuclidin-3-ol (3b), and (Z)-(±)-2-(N-benzenesulfonylindol-3-yl-methylene)quinuclidin-3-ols and their derivatives (Fig. 1, 4a–4d). In this study we describe the effect of 25 additional derivatives on cellular migration of HUVECs. The synthesis of these analogs has been previously described.10
Figure 1. Chemical Structures of Novel Quinuclidine Compounds.
Structures of potent antiangiogenic agents with various substituents incorporated into the indole and N-benzyl moieties.
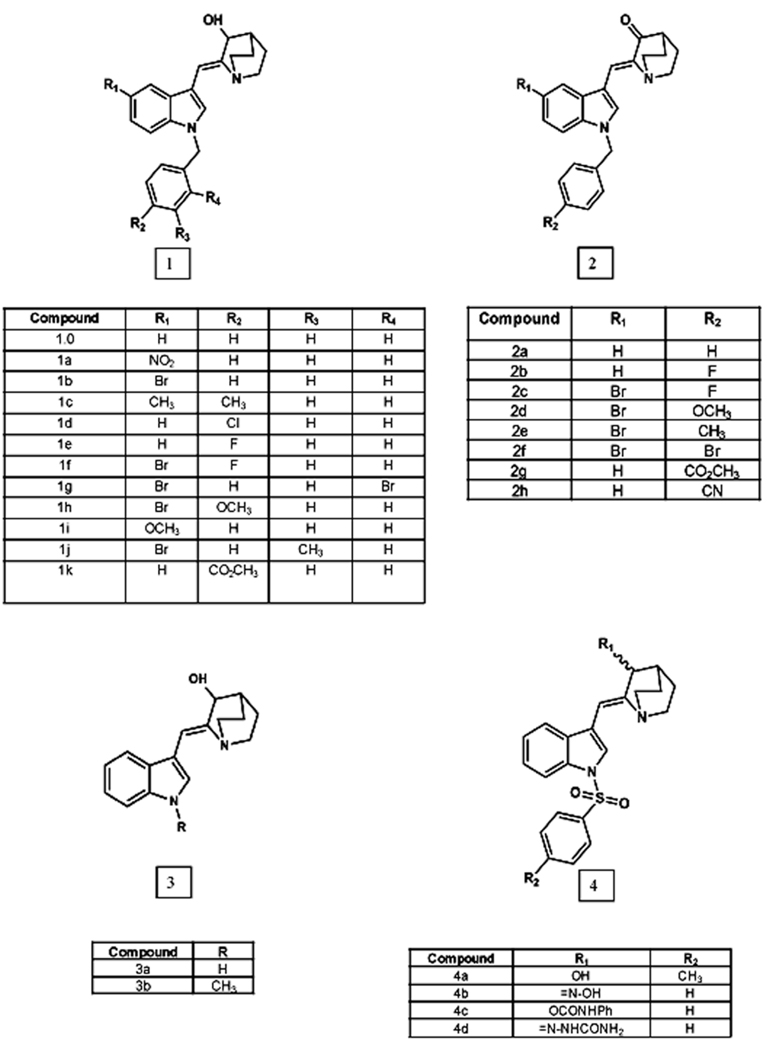
Initially, 25 novel analogs (Figure 1) were analyzed for their ability to inhibit the migration of HUVECs in an in vitro cell migration scratch assay at a fixed concentration (25 µM). The scratch assay was performed as described in Geng et al.8 HUVECs were seeded in 24-well culture plates. When confluent, a scratch was created using a 10 µl sterile pipette tip. Immediately following scratching, cells were washed twice with PBS to remove cell debris. Analogs were added at a final concentration of 25 µM in 1 ml final volume of HUVEC medium. Cells were incubated at 37°C for 20 hours. Microscopic images were taken at 0 hour (immediately after drug addition) and at 20 hours and the samples analyzed for cellular migration in the presence or absence of the test compound. Among analogs 1a–1k, introduction of either halogen, methyl or methoxy substituents into the benzyl group did not yield a more potent compound than the unsubstituted analog (1.0) (Figure 2). Introduction of electron withdrawing nitro group on the indole nucleus (1a) increased the potency of the parent compound (1.0). Interestingly, none of the (Z)-2-benzylindol-3-ylmethylene)quinuclidin-3-ones analogs (2a–2i) inhibit migration of HUVECs, suggesting that the OH group of the azabicyclo moiety is essential for inhibition of cell migration. Analogs that lacked aromatic substitution on the indole-NH were found to be inactive in migration assays (i.e. compounds 3a and 3b, Figure 1).8 Compound 3a had been tested in our previous study8, and was used as negative control. Methyl substitution on the indole-NH (3b) did not improve the efficacy of compound 3a. This observation suggests that benzyl substitution on the indole-NH is an important structural component that governs potency. Of the remaining analogs, 8 compounds showed ≥ 50% decrease in HUVEC cell migration (Figure 2). In our earlier study (Z)-(±)-2-(1-benzenesulfonylindol-3-ylmethylene)quinuclidin-3-ol was shown to exhibit potent antiangiogenic properties.8 Since we have identified the OH group in quinuclidine moiety as an essential structural element, we modified this group to study the effect on cell migration. Among these modifications replacement of the OH group with an oxime group (4b) afforded a very potent compound (Figure 2). Substitutions with semicarbazide and phenyl carbamate moieties yielded moderately active compounds. A total of eight of the 25 tested analogs (i.e. 1a–1c, 1e, 1f, and 4b–4d) were found to be potent inhibitors of cellular migration in HUVECs (Figure 2) at 25 µM concentration. The ability to inhibit cell migration by these 8 potent analogs was further tested at even lower concentrations (10 µM and 5µM). All eight analogs were found to be effective cell migration inhibitors at 10 µM concentration (data not shown).
Figure 2. Scratch Assay.
The ability of HUVECs to migrate is inhibited by indole analogs. Eight of the 25 tested analogs were found to inhibit cellular migration of HUVECs at 25 µM concentration. Confluent endothelial cells in 24 well plates were scratched with a sterile 10 µl pipette tip. The cells were then allowed to grow in fresh medium with or without 25 µM of analog for 20 hours.
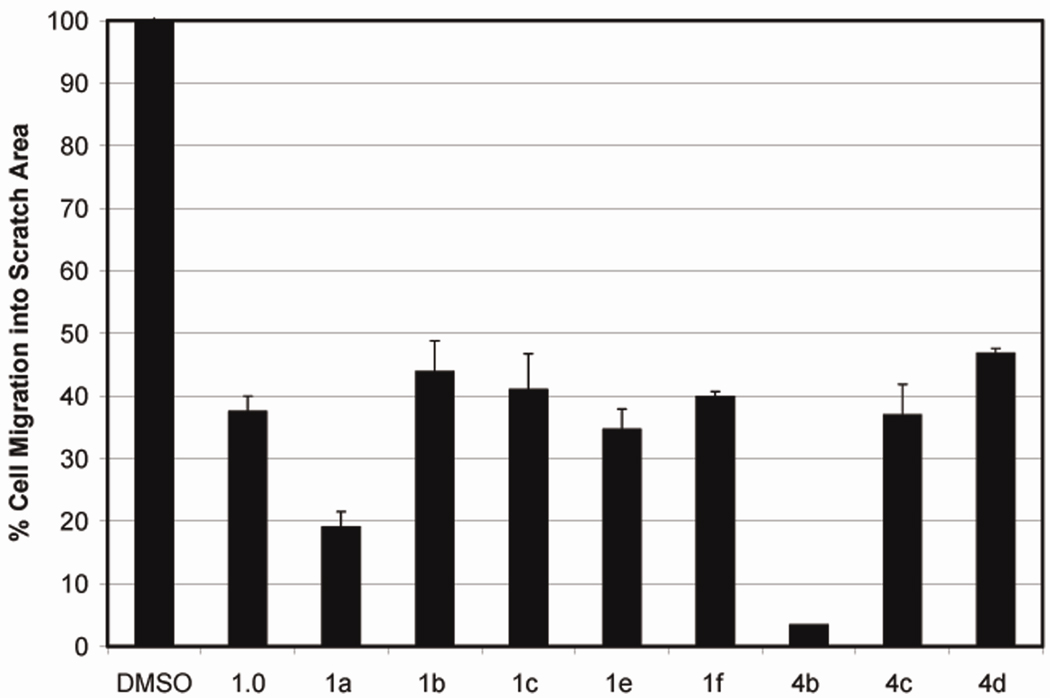
Based on results obtained from analysis of the scratch assay we chose the above eight analogs and tested their potency to inhibit the ability of HUVECs to form tubule-like structures. The assay was conducted in 24-well plates coated with Matrigel (BD Biosciences). HUVECs were plated onto the Matrigel in the presence or absence of the analogs (10 µM). Microscopic images were taken 7 hours after incubation, and the number of tubules formed was quantified by TotalLab software (Figure 3). Consistent with our scratch assay, the tubule-like structure forming ability of HUVECs was suppressed by 83% in presence of analog 4b.
Figure 3. Tubule Formation in Matrigel.
The ability of HUVECs to form capillary-like structures is inhibited by analog 4b. Endothelial cells were added to 24-well plates coated with Matrigel with or without 10 µM of analog 4b. Plates were incubated for 7 hours to allow formation of capillary-like structures. A. Effect of various analogs on tubule formation ability of HUVECs. B. The ability of HUVECs to form tubules in presence of analog 4b (10 µM) was reduced by 83%.
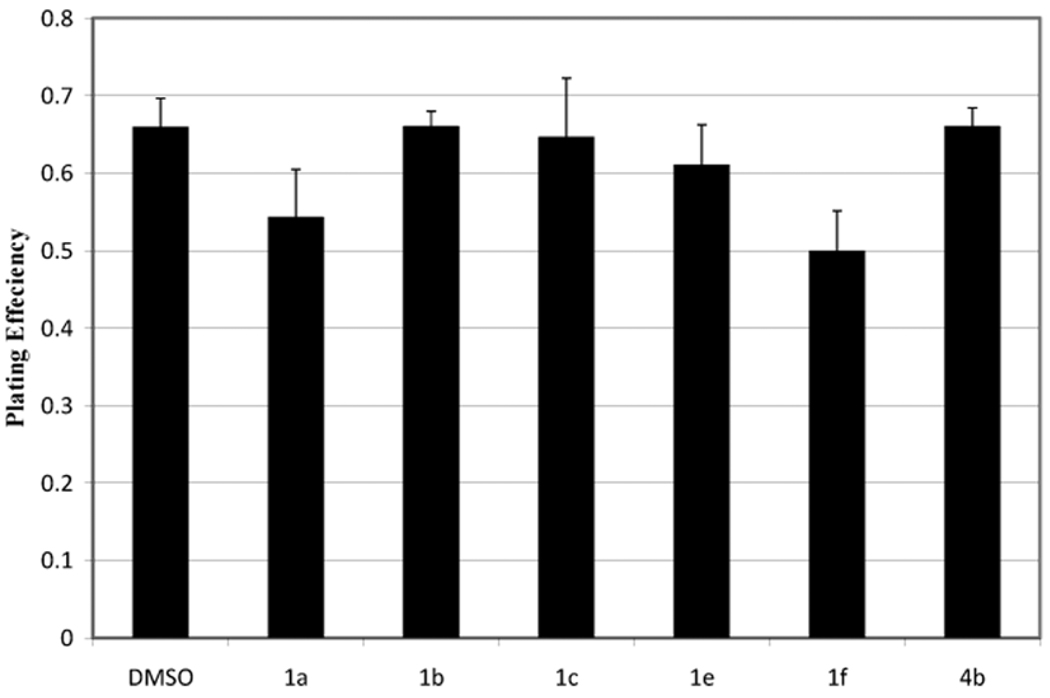
A clonal plating efficiency assay10 was used to assess the toxicity of the six most potent inhibitors (1a–1c, 1e, 1f and 4b) identified from the two functional assays. These inhibitor analogs did not exhibit toxicity, defined as a plating efficiency of less than 75% relative to the DMSO control, when tested at concentrations used in the tubule formation assay.
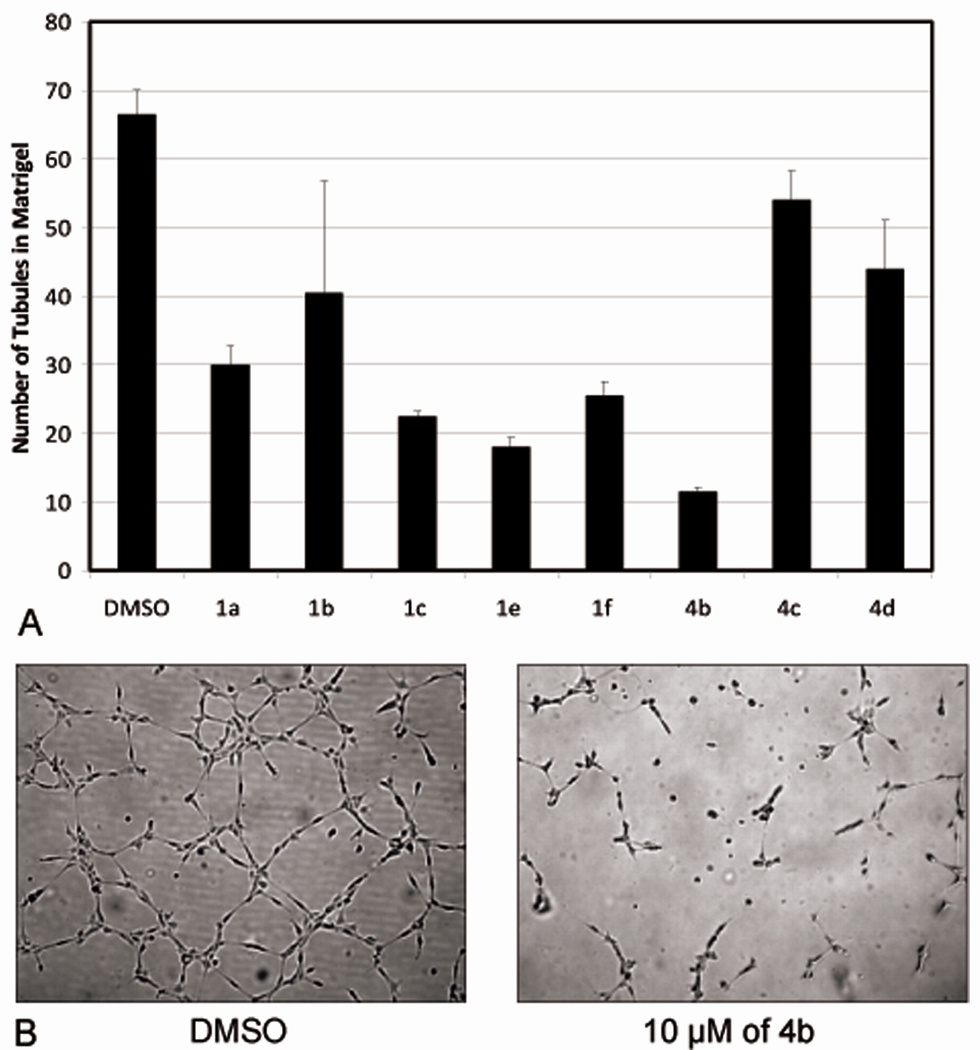
To determine if these analogs affected ECTO-NOX activity, we analyzed the reduction of 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1)8 in HUVECs in the presence of various concentrations of above six inhibitors.. HUVECs in 100 µl of culture medium were seeded in 96-well plates and after 24 hours of growth the cells were treated with analog (0.5, 1, 5, and 10 µM) or solvent (DMSO) in duplicate for 20 hours. This was followed by a WST-1 assay, as per the manufacturer’s (Calbiochem) recommendations. The optical density of each well was measured with a spectrophotometric microplate reader (Bio-Rad) at 450 nm. Interestingly none of the compounds inhibited WST-1 reduction, except for analog 4b at 10 µM concentration (Figure 5).
Figure 5. Cleavage of the Tetrazolium Salt WST-1.
(4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate): six potent analogs identified by scratch assay were evaluated for their ability to directly inhibit ECTO-NOX proteins by WST-1 assay at the indicated concentrations. Analog 4b exhibited significant ENOX inhibition at 10µM.
The goal of this study was to analyze the angiogenesis-inhibiting activity of novel indole-based analogs. From a pool of 25 different indole analogs, we identified 8 analogs that had angiogenesis inhibition properties based on phenotypical assays: i.e., cell migration and tubule formation in Matrigel. Compound 4b was the most effective inhibitor and demonstrated an ability to inhibit ENOX activity, as determined by the WST-1 assay, as well as inhibition of phosphorylation of p70S6K1 at T389 (data not shown).8 The kinase p70S6K1 has been previously shown to play a critical role in cell enlargement, proliferation and tubule formation.14–16
Indolone based compounds such as 3-substituted-2-oxoindole analogs, have been reported to possess antiangiogenic properties16 independent of their serine/threonine kinase inhibition properties. Indolone analog SU5416, which inhibits VEGF2 activity, has been reported to possess antiangiogenic properties.17 All these compounds lack a substitutiuent on indole-NH. The striking difference between known antiangiogenic indole compounds and the compounds reported in this study is the presence of benzyl or benzene-sulfonyl substituent on the indole-NH. Lack of such a substituent renders the latter analogs inactive. Structural modifications with electron donating groups of either the indole moiety or the phenyl ring of the benzyl group on the indole-NH are not tolerated by these compounds with regard to their inhibition of cell migration and tubule formation. In conclusion, the present study has identified a potent ENOX inhibitor, 4b that exhibits tyrosine kinase receptor-independent antiangiogenic properties.
Figure 4. Colony Formation Assay.
Colony formation assays were used to assess the potency of 6 drug analogs.9 HUVECs growing exponentially in T25 flasks (n = 4) were exposed to DMSO/analog for 6 hours at 37 °C. Analog was then washed off, and cells were incubated in fresh medium for 14 days. Viable cells formed colonies that were stained with crystal violet and counted. The plating efficiency was defined as the ratio of the number of colonies counted divided by the initial number of cells exposed to vehicle control (DMSO) for 6 h. Vehicle treatment (6 h) was not toxic to cells, as survival was not significantly different from plating efficiency measured in the absence of vehicle (p>0.05 Student’s t-test) and was arbitrarily set at 1.0. All analogs were tested at 10 µM. The percentage of surviving cells following exposure to analog alone was 75% or greater.
Acknowledgement
This research was supported by in part by R01CA140409, P50CA095103 and T32CA093240.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bruce Alberts, et al. Molecular Biology of the Cell. 3rd edition. Garland Publisihing, Inc.; 1994. [Google Scholar]
- 2.Kufe DW, Pollock RE, Weichselbaum RR, Bast RC Jr, Gansler TS, Holland JF, Frei Emil III, editors. Cancer Medicine. Hamilton (Canada): BC Decker Inc.; 2003. [Google Scholar]
- 3.Folkman J. Semin Oncol. 2002;29:15. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 4.FDA Approval Summary for Bevacizumab - National Cancer Institute. 2008 November 10; http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab.
- 5.Mena AC, Pulido EG, Guillén-Ponce C. Anticancer Drugs. 2010;21:3. doi: 10.1097/01.cad.0000361534.44052.c5. [DOI] [PubMed] [Google Scholar]
- 6.Jeanny BA, William LD. Update Cancer Ther. 2009;3:182. [Google Scholar]
- 7.Waldner MJ, Neurath MF. Mol Aspects Med. 2010;31:171. doi: 10.1016/j.mam.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Geng L, Rachakonda G, Morré DJ, Morré DM, Crooks PA, Sonar VN, Roti JL, Rogers BE, Greco S, Ye F, Salleng KJ, Sasi S, Freeman ML, Sekhar KR. FASEB J. 2009;23:2986. doi: 10.1096/fj.09-130005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekhar KR, Sonar VN, Muthusamy V, Sasi S, Laszlo A, Sawani J, Horikoshi N, Higashikubo R, Bristow RG, Borrelli MJ, Crooks PA, Lepock JR, Roti Roti JL, Freeman ML. Cancer Res. 2007;67:695. doi: 10.1158/0008-5472.CAN-06-3212. [DOI] [PubMed] [Google Scholar]
- 10.Reddy YT, Sekhar KR, Sasi N, Reddy PN, Freeman ML, Crooks PA. Bioorg Med Chem Lett. 2010;20:600. doi: 10.1016/j.bmcl.2009.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morré DJ, Morré DM. Free Radic Res. 2003;37:795. doi: 10.1080/1071576031000083107. [DOI] [PubMed] [Google Scholar]
- 12.Tang X, Tian Z, Chueh PJ, Chen S, Morré DM, Morré DJ. Biochemistry. 2007;46:12337. doi: 10.1021/bi700973k. [DOI] [PubMed] [Google Scholar]
- 13.Morré DJ. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. p. 121. [Google Scholar]
- 14.Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, Thomas G. Science. 1999;285:2126. doi: 10.1126/science.285.5436.2126. [DOI] [PubMed] [Google Scholar]
- 15.Dufner A, Thomas G. Exp. Cell. Res. 1999;253:100–109. doi: 10.1006/excr.1999.4683. [DOI] [PubMed] [Google Scholar]
- 16.Abadi AH, Abou-Seri SM, Abdel-Rahman DE, Klein C, Lozach O, Meijer L. Eur. J. Med. Chem. 2006;41:296. doi: 10.1016/j.ejmech.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Angelov L, Salhia B, Roncari L, McMahon G, Guha A. Cancer Res. 1999;59:5536. [PubMed] [Google Scholar]