Abstract
Objective:
Numerous studies have examined the impact of alcohol on violence; however, only a small number have addressed differences elicited by different doses of alcohol. Such studies are limited by mixed findings, small sample sizes, inconsistent alcohol doses and control conditions, a bias toward studying only male participants, and the predominant use of only one particular measure to assess aggression. The present laboratory investigation was designed to elucidate and advance this literature by improving on these limitations.
Method:
Participants were 187 (95 men and 92 women) social drinkers. Following the consumption of one of six alcohol doses (i.e., 0.0 g/kg, 0.125 g/kg, 0.25 g/kg, 0.5 g/kg, 0.75 g/kg, and 1.0 g/kg), participants were tested on a laboratory task in which electric shocks were received from and administered to a fictitious opponent under the guise of a competitive reaction-time task. Aggression was operationalized as the intensity and duration of shocks administered to one's “opponent.”
Results:
Analyses revealed a highly significant positive linear trend between alcohol dose and aggression for both genders.
Conclusions:
Our data aid in clarifying a body of literature that has been afflicted with numerous limitations and will help guide the selection of alcohol doses for researchers conducting future laboratory-based aggression studies.
The fi rst draught serveth for health, the second for pleasure, the third for shame, and the fourth for madness. ∼ Anacharsis
The fact that alcohol intoxication facilitates violence is no longer in question. At present, scientists are working on identifying risk factors and underlying mechanisms of the alcohol-aggression relation. Other facets important to understanding the nature of intoxicated aggression are still understudied, however. One of these facets concerns the dose-response relation between the amount of alcohol consumed and the corresponding intensity of aggressive behavior. Available research on this topic falls into two main categories: (a) naturalistic/survey and (b) laboratory work. Both camps have revealed interesting data, but they tend to be mixed and incomplete, thus providing an important impetus for future research to help clarify the nature of the alcohol-aggression relation.
Naturalistic/survey research
A number of studies have investigated the relation between the quantity of alcohol consumed and the presence and intensity of violence. Some have used event-based designs in which alcohol drinking patterns were examined in the context of past incidents of violent behavior (e.g., Phillips et al., 2007), whereas others used live observations of barroom behavior (e.g., Graham et al., 2006a, 2006b). These reports suggest a close link between acute alcohol intoxication and aggression whereby larger quantities of alcohol are associated with increased aggression. Survey studies show that quantity of alcohol typically consumed on a drinking occasion is associated with aggression and hostility (Borders et al., 2007; Neal and Fromme, 2007; Wells et al., 2008). Such findings are consistent across a range of populations, including prison inmates (Phillips et al., 2007) and adolescents (Wells et al., 2006). Barroom observations reveal that degree of alcohol intoxication is strongly associated with more severe aggression (Graham et al., 2006a, 2006b), and similar findings are observed in general population studies (Wells and Graham, 2003; Wells et al., 2000). One such investigation found that aggressive incidents could be predicted by the number of drinks consumed 6 hours earlier (Wells and Graham, 2003). In summary, naturalistic/survey studies provide mounting support for a relation between the amount of alcohol consumed (i.e., higher doses) and violent behavior in real-world settings among a variety of populations.
The studies reviewed above provide invaluable information to the field; however, the precise measurement of key factors (e.g., blood alcohol content during the event, the exact dose consumed, the time over which beverages were consumed, the intensity of ensuing violence, as well as intervening environmental/contextual influences) are often not available in such investigations and thus expose a number of questions in need of attention. Fortunately, the data provided by these studies can be complemented by in vivo laboratory investigations that can exert the necessary experimental controls able to address the above limitations.
Laboratory research
Of the voluminous amount of experimental studies on alcohol and aggression (Bushman and Cooper, 1990; Cher-mack and Giancola, 1997), only a relatively small number have systematically tested different doses of alcohol on aggressive behavior. Most of these studies have assessed aggression with a task developed by Cherek (1981) known as the Point Subtraction Aggression Paradigm (PSAP) in which participants have the following set of response options: (a) pushing a button repeatedly to earn money, (b) pushing a button repeatedly to take money away from a fictitious opponent, or (c) administering a blast of loud noise to a fictitious opponent. (Cherek later removed this third option from his studies so that only the first two response options were available to participants; this may account for some of his inconsistent findings, reviewed below.) During the task, participants are intermittently “provoked” by having money removed from their total earnings by their fictitious opponent. Aggression is operationalized as either choosing to take money away from the opponent or choosing to deliver a loud blast of noise to this person.
These scientists conducted at least seven studies in which the PSAP was used to measure the effects of different doses of alcohol on aggression (Cherek et al., 1984, 1985, 1992; Dougherty et al., 1996, 1999; Kelly et al., 1988, 1989). The studies used very few participants with a repeated-measures design. Alcohol doses varied from study to study, with all but one investigation using three different doses and only three of the studies using a dose of greater than 0.5 g/kg. (See Table 1 for dose equivalence in standard drinks.)
Table 1.
Standard drink equivalents of laboratory alcohol doses: Number of standard drinks, by alcohol dose and body weight
| Dose | 100 lbs (45 kg) | 130 lbs.(59 kg) | 160 lbs.a (73 kg) | 190 lbs.b (86 kg) | 220 lbs (100 kg) | 250 lbs.(114 kg) |
| 0.125 g/kg | 0.4 | 0.5 | 0.7 | 0.8 | 0.9 | 1 |
| 0.25 g/kg | 0.8 | 1.1 | 1.3 | 1.5 | 1.8 | 2 |
| 0.5 g/kg | 1.6 | 2.1 | 2.6 | 3.1 | 3.6 | 4.1 |
| 0.75 g/kg | 2.4 | 3.2 | 3.9 | 4.6 | 5.4 | 6.1 |
| 1.0 g/kg | 3.2 | 4.2 | 5.2 | 6.1 | 7.1 | 8.1 |
Notes: Although the alcohol content of what is considered a “standard drink” varies considerably (Turner, 1990), for comparison purposes we use the National Institute on Alcohol Abuse and Alcoholism's (2000) definition of 14 g of pure alcohol as the measure of a standard drink. This is roughly equal to 12 oz. (355 ml) of beer, 5 oz. (148 ml) of wine, and 1.5 oz. (44 ml) of distilled spirits.
Average weight for women ages 20 years and older in the United States is 163 lbs. (74 kg; McDowell et al., 2005);
average weight for men ages 20 years and over in the United States is 190 lbs. (86 kg; McDowell et al., 2005).
Cherek et al.'s (1984) initial dose-response study used several very low doses of alcohol coupled with a placebo and found that alcohol increased aggression in three of four participants at alcohol doses of 0.1 g/kg, 0.21 g/kg, 0.42 g/kg, and 0.52 g/kg, with higher doses generally eliciting more aggression than lower doses. A follow-up study found that under high provocation, alcohol doses of both 0.23 g/kg and 0.46 g/kg increased aggression relative to a placebo, whereas a 0.12 g/kg dose did not (Cherek et al., 1985). This latter study unexpectedly found that the 0.23 g/kg dose increased aggression above that of the 0.46 g/kg dose, although the difference was not significant. Further studies by this group (Kelly et al., 1988, 1989) showed significant increases in aggression only at the highest doses administered (i.e., 0.5 g/kg and 0.75 g/kg). It is interesting to note that findings from another experiment were unexpected, with doses of 0.25 g/kg and 0.375 g/kg actually decreasing aggression in three of four participants (Cherek et al., 1992). Last, Dougherty and colleagues were the only researchers from this group of scientists to examine the alcohol-aggression relation in women. Their first study used three alcohol doses: 0.25 g/kg, 0.5 g/kg, and 1.0 g/kg, with only the highest dose resulting in increasing aggression relative to a placebo (Dougherty et al., 1996). Six of 10 participants evinced an increase between dose and aggression, whereas the remaining four participants counterintuitively exhibited the highest levels of aggression at lower doses. In their second study using both men and women, three doses of 0.35 g/kg were administered consecutively at 1-hour intervals (Dougherty et al., 1999). Results revealed a significant increase in aggression after the second drink to an equal extent in both men and women (cumulative dose = 0.7 g/kg) compared with a placebo group. After the third drink (cumulative dose = 1.05 g/kg), aggression levels were maintained in men but declined in women.
Apart from Cherek and colleagues' work, there has been scant research on dose-response effects in the alcohol-aggression relation. Taylor and Gammon (1975) compared the effects of two alcohol doses (0.34 g/kg and 1.0 g/kg) on 40 young men using the Taylor Aggression Paradigm (TAP; Taylor, 1967) in which mild electric shocks are substituted for point subtractions. Taylor and Gammon (1975) found that a high alcohol dose (1.0 g/kg) increased aggression significantly above a lower dose (0.34 g/kg). Following their first study, they recruited an additional 10 participants to constitute a sober control group. Despite being sober, these participants unexpectedly exhibited levels of aggression intermediate to the two alcohol dose groups. A follow-up study (Taylor et al., 1976) used the same two alcohol doses in addition to a placebo group (the latter of which was not used in the first study). Their findings did not reveal a significant difference between the placebo and low dose (0.34 g/kg) groups. Unfortunately, their follow-up study did not use a sober control group, making the nature of this lack of difference unclear. Aggression in the 1.0 g/kg dose group was still found to be significantly higher than in the two other groups (Taylor et al., 1976). Last, Bond and Lader (1986) compared a placebo with two alcohol doses (0.25 g/kg, 0.75 g/kg) in both men and women with a measure of aggression similar to the TAP. Results indicated that the high dose elicited more aggression than the low dose, which, in turn, elicited more aggression than the placebo.
Limitations
Unfortunately, a review of the above studies does not provide a clear picture of the dose effects of alcohol-related aggression. First, laboratory studies indicate that larger doses tend to increase aggression relative to placebo or sober controls. A number of significant notable exceptions, however, also demonstrate that higher doses elicit less aggression compared with low or moderate doses (i.e., Cherek et al., 1985, 1992) and that higher levels of aggression are observed in a knowingly sober control group compared with a low-dose group (Taylor and Gammon, 1975). Second, doses have been relatively inconsistent across studies (Table 2), with only 3 of 10 studies using an alcohol dose of 1.0 g/kg. Third, there is a lack of consistency in the use of control groups; some studies have used either a knowingly sober group or a placebo group but not both. Fourth, it is not clear whether there are consistent differences in the dose-response relation between men and women. Apart from three studies (Bond and Lader, 1986; Dougherty et al., 1996, 1999) in which a combined total of only 46 women took part, no other reports have explicitly assessed the dose-response relation in women. Fifth, as is evident in Table 2, many dose-response studies employed very small sample sizes. In fact, 4 of the 10 laboratory studies reviewed above used sample sizes of six participants or fewer. Given this, it is noteworthy that the study in which higher alcohol doses were found to decrease aggression (Cherek et al., 1992) used only four participants. A sixth reason justifying a new investigation into the dose effects of alcohol on aggression is that the majority of past studies were conducted in one laboratory by one group of researchers (i.e., Cherek and colleagues), using only one measure of aggression, the PSAP. Last, and most importantly, our review of the existing research in this area highlights the lack of understanding of how different alcohol doses affect aggression.
Table 2.
Summary of dose-response studies of alcohol-related aggression
| Study | Alcohol doses (g/kg)a | Subjects (N) | Gender | Paradigm |
| Taylor and Gammon, 1975 | 0,b 0.34, 1.0 | 50 | Male | TAP |
| Taylor et al., 1976 | P,c 0.34, 1.0 | 30 | Male | TAP |
| Cherek et al., 1984 | P, 0.05,0.1,0.21, 0.42, 0.52 | 4 | Male | PSAP |
| Cherek et al., 1985 | P, 0.12, 0.23,0.46 | 11 | Male | PSAP |
| Bond and Lader, 1986 | P, 0.25, 0.75 | 45 | Male/female | TAP |
| Kelly et al., 1988 | P, 0.25, 0.5, 0.75 | 4 | Male | PSAP |
| Kelly et al., 1989 | P, 0 125, 0 25, 0 5 | 6 | Male | PSAP |
| Cherek et al., 1992 | P, 0.125,0.25, 0.375 | 4 | Male | PSAP |
| Dougherty et al., 1996 | P, 0.25,0.5, 1.0 | 10 | Female | PSAP |
| Dougherty et al., 1999 | P, 0.35d | 26 | Male/female | PSAP |
| Duke et al. (present investigation) | 0.0, 0.125,e 0.25, 0.5, 0.75, 1.0 | 187 | Male/female | TAP |
Notes: TAP = Taylor Aggression Paradigm; PSAP = Point Subtraction Aggression Paradigm.
Doses not originally given in g/kg were converted using conversion ratios consistent with other reviews (Turner, 1990);
Taylor and Gammon (1975) added a post hoc sober control group (n = 10);
P = placebo beverage;
Dougherty et al. (1999) used a cumulative dosing procedure in which 0.35 g/kg was administered three times at 1-hour intervals;
the 0.125 g/kg dose is considered an “active placebo” (Ross and Pihl, 1989) in the present investigation.
Dose-response models
Although it is beyond the scope of this article to describe all possible dose-response functions that alcohol can have on behavior, an understanding of different plausible models is essential before making experimentally derived inferences. In the case of alcohol, there is an inherent ceiling effect on how much aggression can be elicited while consuming high enough doses that will eventually lead to stupor, unconsciousness, coma, and even death. In other words, there is a point at which increased consumption of alcohol will obviously prohibit aggressive behavior. Whether there is a sudden precipitous drop in aggression as a result of physical incapacitation or whether this point of decreasing returns occurs gradually is unknown. Because ethical concerns limit maximal alcohol doses that can be administered to human participants in research settings, the effects of extremely high doses of alcohol on aggressive behavior will most likely remain uncertain.
Within the range of alcohol doses that are subject to ethical experimental inquiry, a number of dose-response functions are possible, including a strictly linear model in which more alcohol causes more aggression. Another possibility is a linear threshold model, whereby aggression increases only after a minimum threshold of alcohol exposure is met. The disruption model is an alternative in which aggression is present only after a certain alcohol threshold is achieved, but unlike the linear model, the effect does not increase in strength with further increases of the drug. A number of curvilinear dose-response relations are also possible. The inverted-U dose-response curve occurs when increasing alcohol doses lead to increased aggression until a nonspeci-fied peak is reached, after which further dosing leads to decreased aggression. As mentioned earlier, extremely high doses of alcohol will always lead to diminished capacity to aggress, and thus, this model is likely the most accurate of the entire spectrum of alcohol doses on aggression. Last, a J-shaped dose-response curve occurs if low doses of alcohol decrease aggression (because of relaxation) before observing an increase in aggression with higher doses.
Current study
The current study was designed to determine which of the above dose-response models is most representative of alcohol's influence on aggression. This was accomplished by addressing each of the above limitations as follows: (a) using the TAP as a well-established alternative to the PSAP; (b) assessing aggression in a different laboratory setting than that used in PSAP studies; (c) using six different alcohol doses that included a high dose (1.0 g/kg) as well as both sober and placebo control conditions; (d) including both men and women as subjects; and (e) using a significantly larger sample size than previous dose-response studies.
Method
Participants
Participants were 187 healthy male (n = 95) and female (n = 92) social drinkers between 21 and 34 years of age (M = 22.47, SD = 2.60) recruited from the greater Lexington, KY, area through newspaper advertisements and fliers. In terms of ethnicity, 173 identified themselves as White (88 men, 85 women), 10 as African American (7 men, 3 women), 1 as Hispanic (1 woman), 1 as Asian (1 woman), 1 as American Indian (1 woman), and 1 as “other” (1 woman). All participants were screened for problem drinking using the Short Michigan Alcoholism Screening Test (SMAST; Selzer et al., 1975). Anyone scoring an 8 or more on the SMAST was excluded. Participants were also screened and excluded for serious mental illnesses (e.g., psychosis, bipolar disorder, current mood disorders) and any medical condition in which receiving alcohol or mild electric shocks would be contra-indicated (e.g., liver cirrhosis, stomach cancer, heart condition, pacemaker, epilepsy). Anyone who tested positive on a urine drug test, breath alcohol concentration (BrAC) test, or pregnancy test was also excluded. Drug use (e.g., benzo-diazepines, barbiturates, cocaine, morphine, amphetamines, and marijuana) was assessed with the OnTrak Teststik drug testing kit (Roche Diagnostics, Indianapolis, IN). Pregnancy was tested using the Human Chorionic Gonadotropin One-Step Pregnancy Test (Mainline Technology, Ann Arbor, MI). Last, BrAC was determined using the Alco-Sensor IV breath analyzer (Intoximeters Inc., St.-Louis, MO).
Pre-laboratory procedure
Persons were instructed not to consume any alcohol 24 hours before testing, to refrain from using recreational drugs from the time of the telephone interview, and to avoid eating 4 hours before testing. They were informed that they would be assigned to one of a number of alcohol dose groups ranging from 0.0 g/kg to 1.0 g/kg and that the highest dose condition would raise their BrAC to around 0.12%. Because of hormonal variations associated with menstruation that may affect aggressive responding, women were not tested between 1 week before menstruation and the beginning of menstruation. Participants were informed that they would receive $15 per hour as compensation for their time, which varied from 1 to 6 hours (including detoxification time), depending on the dose of alcohol consumed.
Experimental design and beverage administration
To more fully account for dose-related effects of alcohol on aggression, six different alcohol dose conditions were used in a 6 (dose) × 2 (gender) independent-groups design. Participants were randomly assigned into “sober” (16 women, 17 men), “active placebo” (0.125 g/kg; 16 women, 13 men), “low dose” (0.25 g/kg; 16 women, 16 men), “medium dose” (0.5 g/kg; 17 women, 16 men), “medium-high dose” (0.75 g/kg; 14 women, 18 men), or “high dose” (1.0 g/kg; 13 women, 15 men) beverage conditions. The “sober” condition was a veridical no-alcohol control group (i.e., given no alcohol/told no alcohol), whereas the “active placebo” condition controlled for the belief that alcohol had been consumed by administering a small amount of alcohol, insufficient to affect behavior yet detectable by olfactory and gustatory senses (Ross and Pihl, 1989). Rather than adding a few drops or milliliters of alcohol into a glass, we used a standardized 0.125 g/kg dose of alcohol for our active placebo group, in accordance with Ross and Pihl (1989), because it avoids confounding placebo manipulation variations between studies. Participants in the other alcohol conditions were informed that they were consuming alcohol but were not told how much (this was covered in the consent form). To achieve similar BrACs among both men and women, women were given doses containing 10% less alcohol to account for gender differences in body fat and gastric alcohol dehydrogenase activity.
All participants consumed their beverages individually. Other than the sober group that received only orange juice with no alcohol, those in all dose conditions received 95% alcohol mixed in a 1:5 ratio with Tropicana orange juice. Participants rinsed their mouths with water following beverage consumption to obtain accurate BrAC readings. Depending on the dose consumed, drinking times ranged from 1 to 20 minutes to accommodate the time necessary to consume the different beverage volumes. Beverage volume ranged from approximately 50 ml to approximately 750 ml (2–25 oz.), including alcohol. To achieve optimal BrACs during the TAP, postdrinking wait times following alcohol consumption and the beginning of the TAP varied between dose conditions from 5 minutes for the placebo dose to 40 minutes for the 1.0 g/kg dose.
Immediately before beginning the aggression task, participants provided subjective ratings of their level of intoxication. This was done using a specially constructed scale, the Subjective Intoxication Scale, that ranges from 0 to 11 (0 = not drunk at all, 8 = drunk as I have ever been, and 11 = more drunk than I have ever been). Regardless of beverage group assignment, all participants were informed that their opponent was intoxicated. This was done to ensure that the “drinking status” of the opponent would not confound any potential beverage group differences in aggression.
Aggression task
A modified version of the TAP (Taylor, 1967) was used to measure aggression. This task places participants in a situation in which electric shocks are received from, and administered to, a fictitious opponent during a supposed competitive reaction-time task. Aggression was operational-ized as the shock intensities and durations selected by the participants. They were seated at a table in a small room. On the table facing the participant were a computer screen and a keyboard. White adhesive labels marked “1” through “10” were attached to the number keys running across the top of the keyboard. The labels “low,” “medium,” and “high” were placed above keys “1,” “5,” and “10,” respectively, to indicate the subjective levels of shock corresponding to the number keys. The keyboard and monitor were connected to a computer located in an adjacent control room out of the participant's view. Aggression was operationalized as a combinatory index of mean shock intensity (1 through 10) and mean shock duration (in milliseconds) across all trials of the TAP. The score was calculated by transforming the first trial shock intensity and duration variables into z scores and then summing them, and so on. All summed z scores were then added to form the final aggression index. This was done to increase the reliability of both indices (i.e., shock intensity and duration) inasmuch as a meta-analytic study demonstrated that these variables are significantly related to one another and are considered to be part of a more general construct of aggression (Carlson et al., 1989). Although the TAP has been both criticized and defended as a measure of aggression, researchers may find it useful to add other behavioral measures of aggression to future studies.
Procedure
To disguise the fact that the TAP is a measure of aggression, participants were given a fictitious cover story. They were informed that the study was aimed at understanding the effects of alcohol on reaction-time in a competitive situation. Participants were told that they were going to compete against a person of the same gender in an adjacent room on a reaction-time task. In actuality, there was no opponent. Instructions for the TAP were given as participants began drinking their beverages. They were informed that shortly after the words “Get Ready” appeared on a computer screen, the words “Press the Spacebar” would appear at which time they had to press, and hold down, the spacebar. Following this, the words “Release the Spacebar” would appear at which time they had to lift their fingers off of the spacebar as quickly as possible. A “win” was signaled by the words, “You Won. You Get to Give a Shock,” and a “loss” was signaled by the words, “You Lost. You Get a Shock.” A winning trial allowed participants to deliver a shock to their fictitious opponent, and a losing trial resulted in receiving a shock from this individual. Participants viewed the shocks they selected and received on a “volt meter” and by the illumination of one of 10 “shock lights” (ranging from 1 [low] to 10 [high]) on the computer screen.
Before beginning the TAP, participants' pain thresholds and tolerances were assessed to determine the intensity parameters for the shocks they would receive. This was accomplished via the administration of short duration shocks that increased in intensity in a stepwise manner from the lowest available shock setting, which was imperceptible, until the shocks reached a subjectively reported “painful” level. All shocks were administered through two finger electrodes attached to the index and middle fingers of the nondominant hand using Velcro straps. Participants were instructed to inform the experimenter when the shocks were “first detectable” and then when they reached a “painful” level. Later, during the actual testing, participants received shocks that ranged from “1” to “10.” These shocks were respectively set at 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, and 100% of the highest tolerated shock intensity.
The entire TAP procedure consisted of 34 trials. Participants were told that they had a choice of 10 different shock intensities to administer at the end of each winning trial for a duration of their choosing. Following a losing trial, they received 1 of 10 shock intensities that lasted 1 second. Shock intensities (including winning and losing trials) were administered in a random pattern. Immediately following the TAP, BrACs were measured, and participants were again asked to rate their subjective state of intoxication. In addition, they were asked whether the alcohol they drank caused them any impairment, on a scale ranging from 0 to 10, with 0 = no impairment, 5 = moderate impairment, and 10 = strong impairment. Participants were then asked a yes/no question regarding whether they believed that they had consumed alcohol.
Results
Manipulation checks
Taylor Aggression Paradigm checks.
To assess the success of the TAP deception, participants were asked about their opponent, the reaction-time task, and their own performance on the task. Participants were asked whether their opponent “played fair,” whether they tried their best, and whether they believed the task was a good measure of reaction time. They responded in such a way as to convince the researchers that the TAP deception was successful.
Subjective intoxication/placebo checks.
All individuals in the placebo condition indicated that they believed they had consumed alcohol. Subjective intoxication ratings are presented in Table 3 for all doses except the sober condition in which no alcohol was administered. No gender differences were found.
Table 3.
Subjective Intoxication Scale scores means (standard deviations)
| Dose | Pre-TAP intoxication M(SD) | Post-TAP intoxication M(SD) |
| 0.125 g/kg | 1.34(1.14) | 1.56(1.13) |
| 0.25 g/kg | 1.59(1.21) | 2.53(1.46) |
| 0.5 g/kg | 3.45 (1.82) | 3.33 (1.94) |
| 0.75 g/kg | 3.17(1.02) | 3.27(1.16) |
| 1.0 g/kg | 3.86(1.21) | 4.04 (2.45) |
Notes: This scale ranged from 0 to 11, on which 0 = not drunk at all, 8 = drunk as I have ever been, and 11 = more drunk than I have ever been. TAP = Taylor Aggression Paradigm.
Breath alcohol concentration levels.
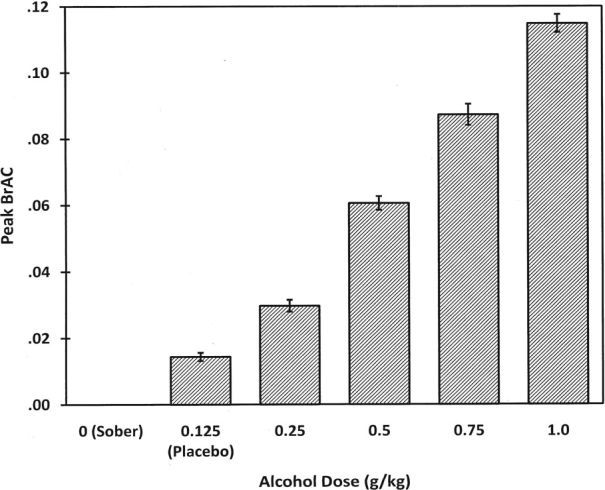
As noted above, all participants were assessed to ensure a BrAC of 0.0% before administering alcohol. BrACs were measured immediately before the beginning the TAP and immediately after its completion. Pre- and post-TAP BrACs are presented in Table 4 for all doses except the sober condition, in which no alcohol was administered. Mean peak BrACs are presented in Figure 1. No gender differences were found.
Table 4.
Breath alcohol concentration means (standard deviations)
| Dose | Pre-TAP M(SD) | Post-TAP M(SD) | 4 min.post-TAP M(SD) | 10 min.post-TAP M(SD) |
| 0.125 g/kg | .014 (.008) | .010 (.006) | .009 (.006) | .010 (.006) |
| 0.25 g/kg | .023 (.011) | .028 (.010) | .027 (.008) | .026 (.007) |
| 0.5 g/kg | .051 (.016) | .056 (.010) | .055 (.009) | .053 (.009) |
| 0.75 g/kg | .074 (.021) | .085 (.018) | .083 (.016) | .076 (.016) |
| 1.0 g/kg | .104 (.013) | .112 (.014) | .110 (.015) | .103 (.013) |
Note: TAP = Taylor Aggression Paradigm; min. = minute.
Figure 1.
Mean peak breath alcohol concentrations (BrAC) for the six alcohol dose conditions. Error bars indicate ±1 standard error.
Aggression data
Shock intensity and shock duration had high internal reliabilities (Cronbach α's = .94 and .98, respectively) and were significantly correlated (r = .57, p < .001), thus confirming Carlson et al.'s (1989) finding that their combined influence represents aspects of an underlying aggression trait. Thus, as discussed earlier, these measures were standardized and summed to create a more reliable measure of aggression.
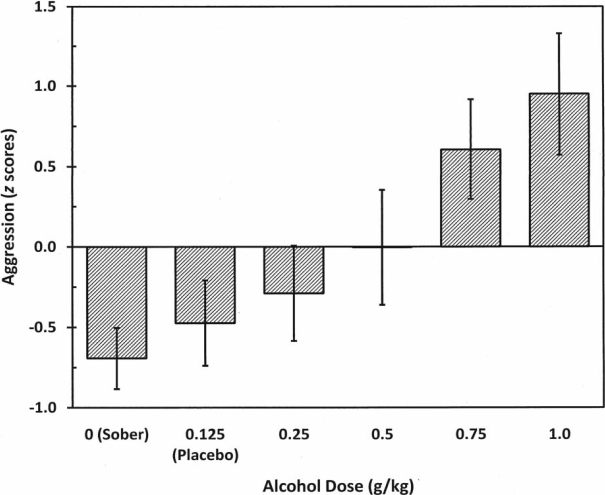
A 6 (dose) × 2 (gender) between-groups design analysis of variance revealed a significant main effect for dose, F(5, 175) = 4.47, p < .001 (Table 5), η2 = .094, and gender, F(1, 175) = 36.16, p < .001, η2 = .152 (men: M = 0.56, SD = 1.51; women: M = -0.58, SD = 1.04). A trend analysis using polynomial contrasts evinced a highly significant linear trend for alcohol dose on aggression F(1, 181) = 21.55, p < .001, η2 = .106, but did not find any significant curvilinear effects (quadratic: p = .844; cubic: p = .914). In addition, we found no Dose × Gender interaction. See Figure 2 for a graphic representation of mean aggression scores and associated standard errors for each dose condition. Least significant difference post hoc analyses were conducted to determine which dose conditions differed significantly (p < .05) from one another. Differences were detected between the 1.0 g/kg group and all other dose groups with the exception of the 0.75 g/kg dose. The 0.75 g/kg group differed from only the 0.25 g/kg, placebo, and sober groups. The 0.5 g/kg group differed from only the 1.0 g/kg group, whereas the 0.25 g/kg group differed from both the 0.75 g/kg and the 1.0 g/kg groups. Consistent with meta-analytic studies, the sober and placebo doses did not differ from one another, nor did they differ from the 0.25 g/kg or the 0.5 g/kg dose conditions. Nevertheless and most importantly, despite any significant or nonsignificant dose group differences, what is of paramount importance is the highly significant linear trend analysis, noted above, that delineates a strong and pronounced effect between increased alcohol dose and aggressive behavior, thus clearly supporting the linear effect model.
Table 5.
Mean (standard deviation) shock intensity, duration, and combined intensity/duration z scores for each dose condition
| Dose | Mean shock intensity M(SD) | Mean shock duration (seconds) M(SD) | Combined shock intensity and duration (summed z scores) M(SD) |
| 0.0 g/kg | 4.65 (1.69) | 0.64 (0.63) | −0.57 (0.82) |
| 0.125 g/kg | 4.80(1.90) | 0.87(1.11) | −0.34(1.10) |
| 0.25 g/kg | 4.77 (2.01) | 1.18(1.61) | −0.22 (1.35) |
| 0.5 g/kg | 5.28 (2.69) | 1.24(1.51) | 0.00(1.61) |
| 0.75 g/kg | 5.73 (1.54) | 1.85(1.73) | 0.47 (1.44) |
| 1.0 g/kg | 6.12(1.99) | 2.10(2.10) | 0.77 (1.66) |
Figure 2.
Aggression (combined shock intensity and duration for all Taylor Aggression Paradigm trials standardized and then summed) as a function of alcohol dose. Error bars indicate ±1 standard error.
Discussion
As can be seen in Figure 2, our results demonstrate that higher alcohol doses produce a significant linear increase in aggression. This pattern was evidenced for both men and women using an aggression paradigm (i.e., the TAP) that has never been used to assess the effects of alcohol dose with the number and range of doses used herein. Moreover, we were able to show that the relation between alcohol dose and aggression is best defined by a linear-shaped function up to a high dose of 1.0 g/kg, as opposed to a curvilinear or threshold-shaped function. This suggests that, in general, the more alcohol an individual consumes, the more likely it is that the individual will exhibit aggressive behavior.
An important result that emerged from this experiment is that, although a significant trend was detected, there were no statistical differences in aggression between any of the lower doses (i.e., 0.0 g/kg, 0.125 g/kg, 0.25 g/kg, and 0.5 g/kg). Compared with the lower doses just mentioned, significant increases in aggression were observed only in the 0.75 g/kg and the 1.0 g/kg doses but particularly in the 1.0 g/kg dose group, which produced the greatest amount of aggression. These data confirm the findings of more naturalistic studies indicating that violent behavior tends to occur at higher BrAC levels.
Moreover, we were able to clarify the mixed results of past studies by designing an investigation that specifically addressed a number of previously limiting issues. The first issue addressed in the present study was the inconsistency of experimental alcohol dose conditions. As is evinced in Table 2, past studies have used either a sober or a placebo control group. The present study was the first to explicitly include both a sober and a placebo control condition, thus allowing us to use two different baselines from which to compare the effects of different alcohol doses. Although meta-analytic studies indicate that placebo groups do not differ from sober groups with respect to aggression, we wanted to be comprehensive to account for this possibility. As is also evidenced in Table 2, previous studies differed considerably on both the number of alcohol doses administered (between three and six) as well as the highest dose administered (0.46 g/kg to 1.0 g/kg). The present investigation used six alcohol conditions (Table 2) with a maximum dose of 1.0 g/kg, making it only the second alcohol-aggression study to use six alcohol doses (Cherek et al., 1984, used six doses in one previous study with a maximum dose of 0.52 g/kg). Having more alcohol doses that span a wider range of alcohol amounts allows for stronger inferences regarding how alcohol affects aggression at different doses.
Cherek and colleagues have contributed substantially to understanding dose-dependent effects of alcohol on aggression, and their work with the PSAP is laudable. Studies from this group of scientists constitute the majority of the experimental literature on this subject (7 of 10 studies reviewed herein); however, the extent to which their findings generalize across other laboratory settings and other measures of aggression is a crucial question. Our results have, in general, supported their findings while simultaneously providing clarification on past inconsistencies such as their 1992 study (Cherek et al.), which found that higher doses of alcohol unexpectedly decreased aggression. One possible reason for such mixed findings is the use of small sample sizes. For example, Cherek et al. (1992) used only four participants; more importantly, the median number of participants for all 10 experimental studies reviewed in this article was only eight. The current investigation provides a substantial improvement in sample size over past studies by examining dose-dependent effects of alcohol on aggression in 187 participants. Another limitation addressed by the present investigation concerns the tendency of most previous studies to use only male participants. Only three prior studies examined the alcohol dose-response effects on aggression in women (Bond and Lader, 1986; Dougherty et al., 1996, 1999).
Unfortunately, there is no clear threshold speaking to when alcohol begins to elicit aggression. Despite our findings that a statistically significant increase in aggression did not occur until a dose of 0.75 g/kg or higher, our trend analyses revealed that the dose-response model that best fits the data is a linear model, indicating that the lower doses did indeed have a meaningful effect on aggression. As is apparent in Figure 2, there were progressively increasing mean levels of aggression for every increase in alcohol dose, findings that are certainly consistent with a linear model.
As explained earlier, the positive linear relation between alcohol dose and aggression is only one of several theoretically possible associations. Another dose-response relation could have been that alcohol produced aggression only above a particular “dose threshold,” after which aggression would either remain stable or continue to increase with higher doses. Some researchers have suggested, conversely, that lower doses of alcohol may actually decrease aggression by producing a state of affective tranquility (Taylor and Gammon, 1975). Our results argue against such hypotheses. It is important to note, however, that our conclusions pertain only to blood alcohol concentrations within a certain range. Given the ethical limitations of how much alcohol one can administer in experimental settings, we cannot definitively determine what dose or BrAC constitutes the point at which aggression will begin to decline. Animal studies pose one possible source of information concerning this issue. For example, one study with mice demonstrated that the aggression-eliciting effects of alcohol begin to attenuate somewhere between doses of 1.0 g/kg and 3.0 g/kg of alcohol (Miczek et al., 1998). Unfortunately, animal studies have yielded inconsistent results concerning this question, and it is unclear how such findings would generalize to humans with different alcohol tolerance levels. However, our findings, in addition to commonsense reasoning, suggest that the alcohol-aggression relation is likely best characterized by an inverted U-shaped curve. Specifically, elevations in alcohol doses will increase aggression to an indeterminate point, depending on a number of individual difference factors, but then, after a particular blood alcohol content is reached, deleterious aggression-attenuating effects such as fatigue, nausea, stupor, coma, and even death will set in.
Conclusion
Numerous experimental studies have investigated the impact of alcohol on aggression. Only a few of these, however, have examined the effects of different doses of alcohol on aggression, and a clear picture of this dose-response relation has not yet been forthcoming. We believe that our findings help to clarify this literature by demonstrating a positive linear relation between alcohol and aggression in both men and women up to a dose of 1.0 g/kg. Our findings also have clear public health implications: Violence and excessive drinking go hand in hand. Aggression is just one more reason why drinking more is a bad idea. Understanding the dose-response relation between alcohol and aggression is a critical step on the road to understanding exactly how alcohol influences aggression.
Footnotes
This research was supported by grant R01 -AA-11691 from the National Institute on Alcohol Abuse and Alcoholism and the National Center for Research Resources awarded to Peter R. Giancola
References
- Bond A, Lader M. The relationship between induced behavioral aggression and mood after the consumption of two doses of alcohol. British Journal of Addiction. 1986;81:65–75. doi: 10.1111/j.1360-0443.1986.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Borders A, Barnwell SS, Earleywine M. Alcohol-aggression expectancies and dispositional rumination moderate the effect of alcohol consumption on alcohol-related aggression and hostility. Aggressive Behavior. 2007;33:327–338. doi: 10.1002/ab.20187. [DOI] [PubMed] [Google Scholar]
- Bushman BJ, Cooper HM. Effects of alcohol on human aggression: An integrative research review. Psychological Bulletin. 1990;107:341–354. doi: 10.1037/0033-2909.107.3.341. [DOI] [PubMed] [Google Scholar]
- Carlson M, Marcus-Newhall A, Miller N. Evidence for a general construct of aggression. Personality and Social Psychology Bulletin. 1989;15:377–389. [Google Scholar]
- Cherek DR. Effects of smoking different doses of nicotine on human aggressive behavior. Psychopharmacology. 1981;75:339–345. doi: 10.1007/BF00435849. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Spiga R, Egli M. Effects of response requirement and alcohol on human aggressive responding. Journal of the Experimental Analysis of Behavior. 1992;58:577–587. doi: 10.1901/jeab.1992.58-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherek DR, Steinberg JL, Manno BR. Effects of alcohol on human aggressive behavior. Journal of Studies on Alcohol. 1985;46:321–328. doi: 10.15288/jsa.1985.46.321. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Steinberg JL, Vines RV. Low doses of alcohol affect human aggressive responses. Biological Psychiatry. 1984;19:263–267. [PubMed] [Google Scholar]
- Chermack ST, Giancola PR. The relationship between alcohol and aggression: An integrated biopsychosocial approach. Clinical Psychology Review. 1997;17:621–649. doi: 10.1016/s0272-7358(97)00038-x. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Bennett RH, Moeller FG. The effects of a cumulative alcohol dosing procedure on laboratory aggression in women and men. Journal of Studies on Alcohol. 1999;60:322–329. doi: 10.15288/jsa.1999.60.322. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Cherek DR, Bennett RH. The effects of alcohol on the aggressive responding of women. Journal of Studies on Alcohol. 1996;57:178–186. doi: 10.15288/jsa.1996.57.178. [DOI] [PubMed] [Google Scholar]
- Graham K, Bernards S, Osgood DW, Wells S. Bad nights or bad bars? Multi-level analysis of environmental predictors of aggression in late-night large capacity bars and clubs. Addiction. 2006a;101:1569–1580. doi: 10.1111/j.1360-0443.2006.01608.x. [DOI] [PubMed] [Google Scholar]
- Graham K, Osgood DW, Wells S, Stockwell T. To what extent is intoxication associated with aggression in bars? A multilevel analysis. Journal of Studies on Alcohol. 2006b;67:382–390. doi: 10.15288/jsa.2006.67.382. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Cherek DR, Steinberg JL. Concurrent reinforcement and alcohol: Interactive effects on human aggressive behavior. Journal of Studies on Alcohol. 1989;50:399–405. doi: 10.15288/jsa.1989.50.399. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Cherek DR, Steinberg JL, Robinson D. Effects of provocation and alcohol on human aggressive behavior. Drug and Alcohol Dependence. 1988;21:105–112. doi: 10.1016/0376-8716(88)90055-5. [DOI] [PubMed] [Google Scholar]
- McDowell MA, Fryar CD, Hirsch R, Ogden CL. Anthro-pometric reference data for children and adults: U.S. population, 1999-2002. Advance Data From Vital and Health Statistics, No. 361, DHHS Publication No. (PHS) 2005-1250 05-0345. Hyattsville, MD: National Center for Health Statistics; 2005. [Google Scholar]
- Miczek KA, Barros HM, Sakoda L, Weerts EM. Alcohol and heightened aggression in individual mice. Alcoholism: Clinical and Experimental Research. 1998;22:1698–1705. [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Tenth special report to the U.S. Congress on alcohol and health: Highlights from current research (NIH Publication No. 00-1583) Bethesda, MD: Author; 2000. [Google Scholar]
- Neal DJ, Fromme K. Event-level covariation of alcohol intoxication and behavioral risks during the first year of college. Journal of Consulting and Clinical Psychology. 2007;75:294–306. doi: 10.1037/0022-006X.75.2.294. [DOI] [PubMed] [Google Scholar]
- Phillips S, Matusko J, Tomasovic E. Reconsidering the relationship between alcohol and lethal violence. Journal of Interpersonal Violence. 2007;22:66–84. doi: 10.1177/0886260506294997. [DOI] [PubMed] [Google Scholar]
- Ross DF, Pihl RO. Modification of the balanced-placebo design for use at high blood-alcohol levels. Addictive Behaviors. 1989;14:91–97. doi: 10.1016/0306-4603(89)90021-x. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A Self-Administered Short Michigan Alcoholism Screening Test (SMAST) Journal of Studies on Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Taylor SP. Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. Journal of Personality. 1967;35:297–310. doi: 10.1111/j.1467-6494.1967.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Taylor SP, Gammon CB. Effects of type and dose of alcohol on human physical aggression. Journal of Personality and Social Psychology. 1975;32:169–175. doi: 10.1037/h0076812. [DOI] [PubMed] [Google Scholar]
- Taylor SP, Vardaris RM, Rawtich AB, Gammon CB, Cranston JW, Lubetkin AI. The effects of alcohol and delta-9-tetra-hydrocannabinol on human physical aggression. Aggressive Behavior. 1976;2:153–161. [Google Scholar]
- Turner C. How much alcohol is in a ‘standard drink’? An analysis of 125 studies. British Journal of Addiction. 1990;85:1171–1175. doi: 10.1111/j.1360-0443.1990.tb03442.x. [DOI] [PubMed] [Google Scholar]
- Wells S, Graham K. Aggression involving alcohol: Relationship to drinking patterns and social context. Addiction. 2003;98:33–42. doi: 10.1046/j.1360-0443.2003.00253.x. [DOI] [PubMed] [Google Scholar]
- Wells S, Graham K, Speechley M, Koval J. Do predisposing and family background characteristics modify or confound the relationship between drinking frequency and alcohol-related aggression? A study of late adolescent and young adult drinkers. Addictive Behaviors. 2006;31:661–675. doi: 10.1016/j.addbeh.2005.05.046. [DOI] [PubMed] [Google Scholar]
- Wells S, Graham K, West P. Alcohol-related aggression in the general population. Journal of Studies on Alcohol. 2000;61:626–632. doi: 10.15288/jsa.2000.61.626. [DOI] [PubMed] [Google Scholar]
- Wells S, Mihic L, Tremblay PF, Graham K, Demers A. Where, with whom, and how much alcohol is consumed on drinking events involving aggression? Event-level associations in a Canadian national survey of university students. Alcoholism: Clinical and Experimental Research. 2008;32:522–533. doi: 10.1111/j.1530-0277.2007.00596.x. [DOI] [PubMed] [Google Scholar]