Abstract
Background
Reduced synaptic connectivity in frontal cortex may contribute to schizophrenia symptoms. While altered mRNA and protein expression of various synaptic genes has been found, discrepancies between studies mean a generalisable synaptic pathology in schizophrenia has not been identified.
Methods
We determined if mRNAs encoding presynaptic proteins enriched in inhibitory [vesicular GABA transporter (VGAT) and complexin 1] and/or excitatory [vesicular glutamate transporter (VGluT1) and complexin 2] terminals are altered in the dorsolateral prefrontal cortex of subjects with schizophrenia (n=37 patients, n=37 controls). We also measured mRNA expression of markers associated with synaptic plasticity/neurite outgrowth [growth associated protein 43 (GAP43) and neuronal navigators 1 and 2 (NAV1 and NAV2)]; and mRNAs of other synaptic-associated proteins previously implicated in schizophrenia: dysbindin and vesicle-associated membrane protein (VAMP1) mRNAs using quantitative RT-PCR.
Results
No significant changes in complexin 1, VGAT, complexin 2, VGluT1, dysbindin, NAV2, or VAMP1 mRNA expression were found, however we observed reduced expression of mRNAs associated with plasticity/cytoskeletal modification (GAP43 and NAV1) in schizophrenia. Although dysbindin mRNA did not differ in schizophrenia compared to controls, dysbindin mRNA positively correlated with GAP-43 and NAV1 in schizophrenia, but not in controls, suggesting low levels of dysbindin may be linked to reduced plasticity in the disease state. No relationships between three dysbindin genetic polymorphisms previously associated with dysbindin mRNA levels were found.
Conclusions
A reduction in the plasticity of synaptic terminals supports the hypothesis that reduced modifiability of synaptic terminals may contribute to neuropathology and working memory deficits in schizophrenia.
Keywords: synapse, schizophrenia, complexin, GAP43, neuronal navigator, dorsolateral prefrontal cortex
Introduction
While the primary etiology of schizophrenia remains elusive, a dysregulation of synaptic connectivity of the frontal cortex may underlie the pathology and symptoms of the disease (1-4). Structural MRI studies suggest a reduced frontal grey matter volume in schizophrenics (5, 6) and increased packing density of cells (7, 8) implicates a reduction in volume of the neuropil, where synapses are found (9). However, at the molecular level there is a lack of consistent evidence of altered dorsolateral prefrontal cortex (DLPFC) expression of the synaptic membrane protein synaptophysin, considered one of the most valid markers of synapse density (10-12). To date, 8/10 studies report synaptophysin expression as unchanged in the DLPFC in patients with schizophrenia (13-20), while 3/10 report a reduction in synaptophysin in synaptosomal fraction or by immunohistochemistry in prefrontal cortex (18, 21, 22). This discrepancy suggests that synaptic reduction may not be widespread anatomically, may not be generalisable to all patients with schizophrenia, or that synaptic proteins may be reduced in a subset of terminals with a compensatory increase in others. Our previous study of five makers of presynaptic terminal proteins in patients with schizophrenia demonstrated that only vesicular associated membrane protein 1 (VAMP-1) was significantly reduced (16), however it is unknown if this fairly ubiquitous pre-synaptic terminal protein is reduced at the mRNA level in the DLPFC.
Considering that one of the most prominent pathologies in schizophrenia involves a deficit in GABAergic interneurons or changes in factors involved in GABA neurotransmission [ie reduction in GAD67, GAT1 and GABA receptor subunits (23-29)], this may suggest that inhibitory terminals are preferentially affected in schizophrenia. Indeed, complexin 1 protein, enriched in inhibitory terminals, may be reduced in schizophrenia (30), however mRNA is unchanged (31). Thus, the balance of inhibitory to excitatory synaptic terminals might be altered in the DLPFC in schizophrenia; however evidence suggests that there may also be a reduction in excitatory terminals with decreased spine density (32, 33) and reduced expression of vesicular glutamate transporter (VGluT1) and complexin 2 (enriched in excitatory terminals) in schizophrenia (31). This leaves the relative contribution of inhibitory and excitatory terminals to cortical synaptic change in schizophrenia, as well as the nature of any synaptic loss, unresolved. In this study, we examined the expression of multiple synaptic markers in one of the largest schizophrenia cohorts studied to date in order to test if putative synaptic changes may preferentially involve either inhibitory terminals [indexed by vesicular GABA transporter (VGAT) and complexin 1] or excitatory terminals (VGluT1 and complexin 2) to determine which are most altered.
It is possible that overall synaptic density is unchanged, while synaptic plasticity is reduced in patients with schizophrenia as growth associated protein 43 (GAP43) mRNA has been reported to be reduced in the DLPFC (34) and other telencephalic areas (35-38) however, GAP43 protein has also been reported as unchanged or increased in schizophrenia (16, 17, 22, 36, 39, 40). Thus, we sought to measure the expression of markers associated with synaptic plasticity/neurite outgrowth [GAP43 and neuronal navigators 1 and 2 (NAV1 and NAV2)] to determine if synaptic plasticity may be altered in our cohort (41).
It is unclear to what extent putative synaptic pathology is directly related to the etiology of schizophrenia. While multiple studies suggest that schizophrenia susceptibility genes encode proteins with synaptic function, one of the most replicated, dysbindin, is localised to the synapse [reviewed by (42)]. We sought to replicate the reduction in dysbindin mRNA expression in the DLPFC (20, 43), and to determine if dysbindin mRNA levels may relate to dysbindin genotypes (20, 44) and/or may correlate with synaptic pathology as has been previously found in the hippocampus (43, 45). We chose this subset of mRNAs encoding presynaptic proteins based on the criteria that they were representative of either inhibitory or excitatory terminals and/or that they were previously reported to be altered in schizophrenia, with the aim to replicate findings in an additional postmortem cohort. NAV1 was chosen as a further candidate indicative of plasticity in order to corroborate or refute any GAP43 findings.
Methods and materials
Human post-mortem brain tissue
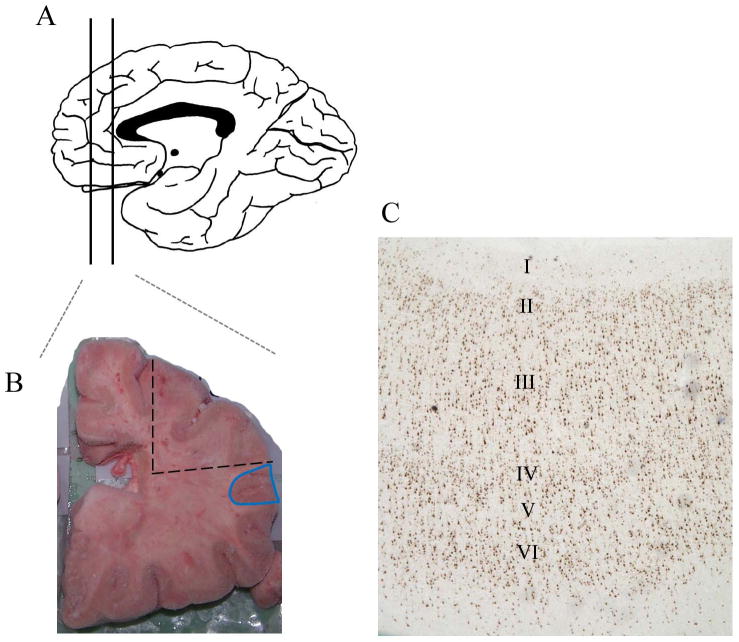
Tissue from the DLPFC of schizophrenia patients and matched controls was obtained from the New South Wales Tissue Resource Centre (Sydney, Australia; University of New South Wales Human Research Ethics Committee #HREC07261). This cohort consisted of 30 individuals diagnosed with schizophrenia and 7 individuals diagnosed with schizoaffective disorder with tissue from 37 controls where individuals were matched according to brain pH, age at death, RNA integrity number (RIN), and post-mortem interval (PMI) and groups did not differ on these factors (Table S1 in Supplement 1), as described previously (41). All schizophrenia and schizoaffective cases fulfilled the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) of the American Psychiatric Association. All subjects were prescribed antipsychotics at the time of death and the majority of patients (20/35 tested) had detectable levels of medication by toxicology. Control cases had no history of major psychiatric, neurological illnesses or drug abuse. Brains were hemisected and cut into coronal blocks approximately 1cm in thickness prior to freezing. From the slab just rostral to or at the genu of the corpus callosum, (Figure 1A) grey matter tissue was carefully trimmed from the underlying white matter with a dental drill (Cat # UP500-UG33, Brasseler, USA), typically along the inferior frontal sulcus [containing ventral middle frontal gyrus and dorsal inferior gyrus (blue region in Figure 1B)]. We verified that we were in a fairly consistent rostral-caudal level (from adjacent sections, dotted region in Figure 1B) by NeuN immunohistochemistry to confirm BA46 cytoarchitecture was present in the block for each case [criteria adapted from (46), Figure 1C]. Prior to RNA extraction, tissue was pulverised on dry ice. Total RNA was extracted using Trizol (Invitrogen) from 300mg of tissue and quality analysed by Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA, USA) (41).
Figure 1. Isolation of DLPFC tissue.
Coronal blocks of approximately 1cm thickness were cut from hemisected brains and frozen. The coronal block rostral to the corpus callosum, or containing the genu of the corpus callosum (A) was used to dissect grey matter tissue containing typically inferior frontal sulcus, ventral middle frontal gyrus and dorsal inferior gyrus (blue region in B). 14μm frozen coronal sections were cut from adjacent tissue (dotted region in B) and NeuN immunohistochemistry was used to determine BA46 cytoarchitecture (C).
Immunohistochemistry
Thawed 14μm tissue sections were fixed with 4% paraformaldehyde in phosphate buffered saline (10 min, 4°C) and immunohistochemistry was performed using anti-NeuN antibody (1:1000 in diluent, Millipore MAB377) as per the protocol described previously (47).
Quantitative Real Time PCR analysis
cDNA was synthesised using the SuperScript® First-Strand Synthesis kit and random hexamers (Invitrogen) in 3× 3μg total RNA per sample (pooled). Transcript levels were measured by quantitative real time-PCR (qPCR) using an ABI Prism 7900HT Fast Real time PCR system, 384-well format and TaqMan Gene Expression Assays (Applied Biosystems)(VAMP1, Hs00249911_m1; complexin 1, Hs00362510_m1; VGAT, Hs00369773_m1; complexin 2, Hs00932617_m1; VGluT1, Hs00220404_m1; GAP43, Hs00967138_m1; NAV1, Hs00368110_m1; NAV2, Hs00367864_m1; dysbindin, Hs01105865_m1). PCR cycling conditions were: 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. PCR data were obtained with the Sequence Detector Software (SDS version 2.0, Applied Biosystems). No template and no reverse transcriptase enzyme controls produced no signal. All measurements from each subject were performed in triplicate and relative quantities determined from a seven point standard curve. Measurement outliers were removed if the variance of triplicates was > 30% of the mean and the mean re-calculated based on two values. No subjects were excluded due to missing data. Transcript quantities were normalised by the geometric mean of four housekeeping genes: ubiquitin C (Hs00824723_m1), β-actin (Hs99999903_m1), glyceraldehyde-3-phosphate dehydrogenase (Hs99999905_m1), TATA box binding protein (Hs00427620_m1) determined to be unaltered in the DLPFC in schizophrenia (41).
SNP Genotyping
DNA was isolated from DLPFC tissue using a PUREGENE DNA purification kit (QIAGEN) from 20mg of tissue. SNP genotype was determined using TaqMan SNP genotyping assays [Applied Biosystems (48) as detailed in Table 1]. Heterozygotes were grouped with individuals homozygous for the rare allele to increase the group size for statistical analysis.
Table 1. Dysbindin SNP genotyping in schizophrenia and controls.
| valid n | ||||||||
|---|---|---|---|---|---|---|---|---|
| SNP identification number | SNP | minor allele frequency | SNP location | Chromosome 6 position* | TaqMan SNP assay | custom asay designed to* | 1/1 | 1/2 and 2/2 |
| rs2619537 | A/G | 0.12 | 5′ flanking region | 15664413 | AHWR12B (custom) | chr6:15662718-15663518 | 51 | 20 |
| rs2743864 | C/T | 0.09 | Intron 3 | 15640281 | C_114533_10 | n/a | 60 | 11 |
| rs1047631 | T/C | 0.15 | exon 10 (3′ UTR) | 15523101 | C_7460562_10 | n/a | 52 | 20 |
from the UCSC Genome Browser on Human Feb. 2009 (GRCh37/hg19) Assembly
Statistical analysis
For qPCR, outliers (+/- 2 SD from the mean) were removed from each diagnostic group (<6% of values). Data were normally distributed (all p>0.20) and all genes showed homogeneity of variance (p>0.29) with the exception of VGAT (p=0.008). Analysis of variance (ANOVA) was used to determine diagnostic group differences. Pearson's correlations were run to determine relationships between gene expression and demographics and, when appropriate, analysis of co-variance (ANCOVA) were run covarying for factors that correlated to gene expression in the whole cohort or controls only or schizophrenics only. All statistical analyses were performed using Statistica software (version 7.1).
Results
Expression of synaptic mRNAs in schizophrenia
The geometric mean of housekeepers used to normalise target gene expression did not differ between schizophrenia patients compared to controls (41). Normalised levels of many mRNAs encoding synaptic proteins correlated with pH, RIN and age (significantly decreasing with increased age) as expected (Table S2 in Supplement 1). Additionally, a few transcripts correlated significantly with PMI.
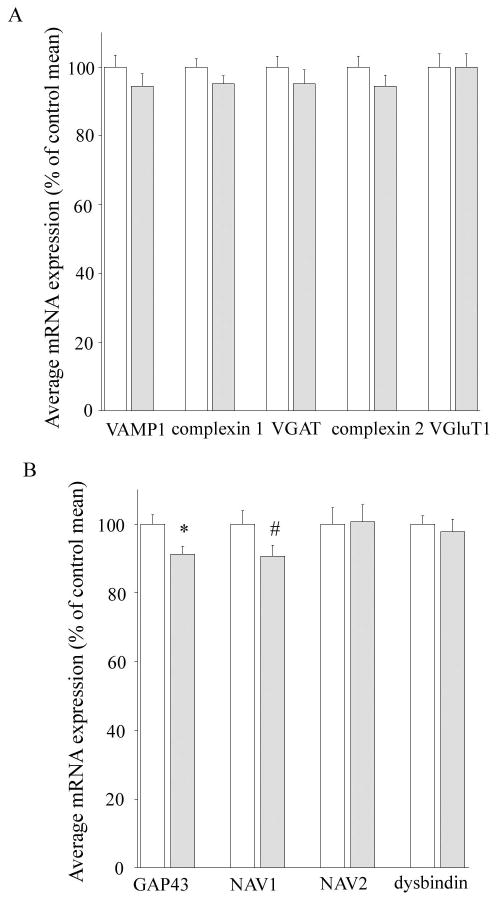
VAMP1 mRNA, detected in cells with VGAT or VGluT1 protein positive terminals (49) was unaltered in schizophrenia (co-varying for age, pH and PMI, F=1.43, df=66, p=0.25; Figure 2A; Table 2). Expression of genes encoding inhibitory terminal-enriched mRNAs (complexin 1 and VGAT) was not significantly altered in schizophrenia (F=1.90, df=68, p=0.17 and co-varying for age, pH, RIN, F=0.61, df=64, p=0.44, respectively; Figure 2A) although complexin 1 trends towards a significant reduction when a directional hypothesis was used (t=1.38, df=68, p=0.08). For mRNAs of excitatory-terminal enriched proteins, a slight reduction in complexin 2 mRNA expression was noted in schizophrenics but this failed to reach statistical significance (5.5%, covarying for pH and PMI, F=2.81, df=65, p=0.10). mRNA encoding the excitatory terminal marker VGluT1 was also not altered in schizophrenia (co-varying for age, pH, RIN, and PMI, F=0.02, df=66, p=0.89; Figure 2A).
Figure 2. Expression of synaptic mRNAs in schizophrenia.
(A) The general synaptic mRNA VAMP1 and mRNAs enriched in inhibitory terminals (complexin 1 and vesicular GABA transporter, VGAT) or excitatory terminals (complexin 2 and vesicular glutamate transporter, VGluT1) are not significantly reduced in schizophrenia. (B) Plasticity and axon guidance associated mRNAs (growth associated protein 43, GAP43; and neuronal navigators 1 and 2, NAV1 and NAV2) are reduced in the DLPFC in schizophrenia compared to controls. Dysbindin mRNA expression is not altered overall in the schizophrenia cohort. *p<0.05 by Student's t-test, # p<0.05 by ANCOVA co-varying for pH. White bars controls, grey bars schizophrenia cases.
Table 2. ANOVA/ANCOVA analyses of expression of mRNAs encoding synaptic proteins in controls and schizoprhenia subjects.
| control | schizophrenia | ANOVA/ANCOVA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | mean | SD | mean | SD | F | df | p | covariates | Cohen's d | |||
| CPLX1 | 6.09 | 0.89 | 5.80 | 0.84 | 1.90 | 68 | 0.17 | 0.33 | ||||
| VGAT | 5.03 | 0.94 | 4.79 | 1.23 | 0.61 | 64 | 0.44 | age | pH | RIN | 0.22 | |
| CPLX2 | 0.85 | 0.16 | 0.80 | 0.15 | 2.81 | 65 | 0.10 | pH | PMI | 0.30 | ||
| VGluT1 | 0.55 | 0.13 | 0.55 | 0.13 | 0.02 | 66 | 0.89 | age | pH | RIN | PMI | 0.00 |
| GAP43 | 5.39 | 0.87 | 4.92 | 0.72 | 5.84 | 66 | 0.02 | 0.57 | ||||
| NAV1 | 3.98 | 0.94 | 3.61 | 0.73 | 4.33 | 67 | 0.04 | pH | 0.43 | |||
| NAV2 | 6.19 | 1.75 | 6.23 | 1.77 | 0.01 | 62 | 0.91 | age | pH | RIN | PMI | -0.02 |
| VAMP1 | 5.03 | 1.05 | 4.74 | 1.09 | 1.43 | 66 | 0.25 | age | pH | PMI | 0.26 | |
| dysbindin | 5.08 | 0.73 | 4.97 | 1.11 | 0.41 | 68 | 0.53 | age | pH | 0.12 | ||
A statistically significant reduction with moderate effect size was found in the plasticity-associated mRNA, GAP43 mRNA in schizophrenia compared to controls (8.7% reduction, Cohen's d = 0.57; F=5.84, df=66, p=0.02; Figure 2B; Table 2), and in NAV1 mRNA co-varying for pH (9.8% reduction, Cohen's d = 0.43, co-varying for pH F=4.33, df=67, p=0.04; Figure 2B). No significant alterations were observed in NAV2 mRNA expression in the DLPFC of schizophrenia patients compared to controls (co-varying for age, pH, RIN, PMI, F=0.10, df=62, p=0.91; Figure 2B). No overall diagnostic differences were found for dysbindin mRNA (co-varying for age and pH, F=0.41, df=68, p=0.53; Figure 2B).
Dysbindin expression in schizophrenia – correlations with other mRNAs encoding synaptic markers
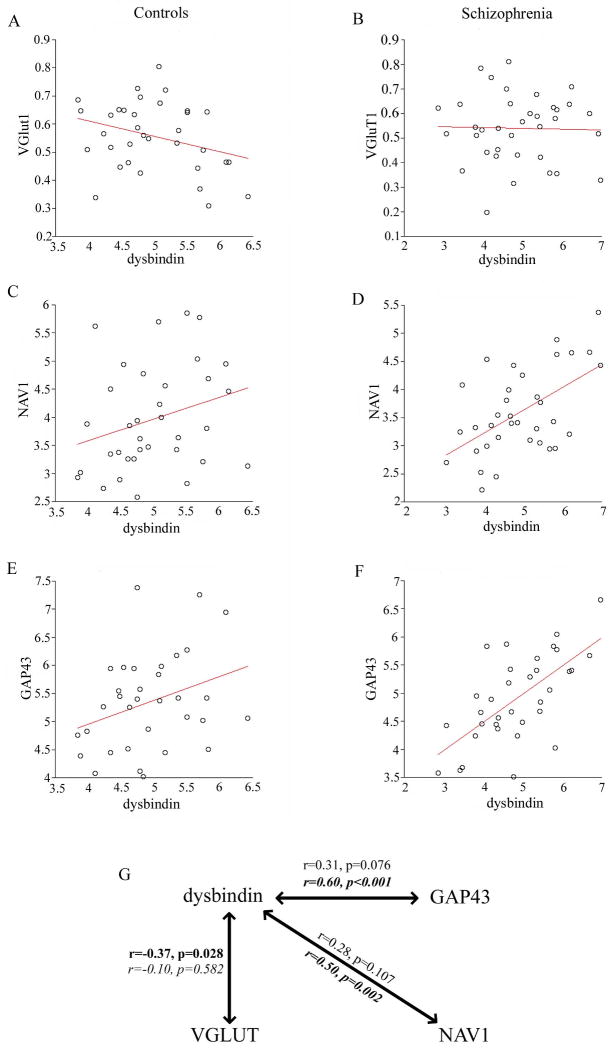
Given the proposed links of dysbindin to schizophrenia, we sought to examine the relationships with the plasticity related genes that we had determined to be altered in schizophrenia, GAP43 and NAV1 (Figure 3), and VGluT1 [previously reported to inversely correlate with dysbindin in schizophrenia (45)] by performing Pearson's correlations. Relationships between these mRNAs and dysbindin varied between control and schizophrenia groups (Figure 3). VGluT1 mRNA expression negatively correlated with dysbindin mRNA in controls but not schizophrenics (r=-0.37, p=0.03 in controls; r=-0.10, p=0.58 in schizophrenics; Table S3 in Supplement 1; Figure 3G). In contrast, GAP43 and NAV1 mRNAs strongly positively correlated with dysbindin mRNA in schizophrenia but was not significantly correlated in controls (r= 0.60, p=2.5 × 10-4 and r=0.50, p=0.002 in schizophrenics, r= 0.31, p=0.08 and r=0.28, p=0.11 in controls; Table S3 in Supplement 1; Figure 3G).
Figure 3. Dysbindin mRNA expression in schizophrenia.
Correlation plots depicting the relationship between dysbindin and VGluT1 and plasticity-related genes altered in schizophrenia (NAV1, GAP43) in control (A, C, E) and schizophrenia cohorts (B, D, F). (G) Correlations between dysbindin and VGluT1, NAV1, and GAP43 are dysregulated in schizophrenia (italic) compared to controls (regular text). Bold text represents statistically significant correlations.
SNPs in dysbindin gene did not alter gene expression
.None of the three dysbindin SNPs tested appeared to influence dysbindin mRNA expression and there was no effect of diagnosis or no genotype by diagnosis interaction effect by ANCOVA co-varying for age and pH (Table 3).
Table 3. Effect of dysbindin genotype on mRNA expression.
| 2 way ANCOVA (covarying for age and pH) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| effect of diagnosis | effect of genotype | interacteraction effect | |||||||
| F | df | p | F | df | p | F | df | p | |
| rs2619537 | 0.00 | 63 | 0.95 | 0.10 | 63 | 0.76 | 1.23 | 63 | 0.27 |
| rs2743864 | 0.07 | 64 | 0.80 | 0.15 | 64 | 0.70 | 0.53 | 64 | 0.47 |
| rs1047631 | 0.10 | 63 | 0.75 | 0.36 | 63 | 0.55 | 0.01 | 63 | 0.94 |
Affect of clinical characteristics on gene expression of synaptic proteins in schizophrenia
Correlations of synaptic marker gene expression with disease demographics and medications showed significant negative correlation with both excitatory and inhibitory neurotransmitter transporter (VGluT1 and VGAT) mRNA expression and duration of illness (r=-0.49, p=0.002, and r=-0.39, p=0.02, respectively; Table S4 in Supplement 1) that lost significance when partial correlation with age was performed. Similarly, the trend for correlation of VGluT1 and VGAT with lifetime chlorpromazine exposure is lost when partial correlations for age are performed (r=-0.04, p=0.82 and r=-0.15, p=0.41, respectively). There is a significant positive correlation of NAV2 mRNA expression with age of onset (r=0.35, p=0.05). We found that manner of death had no significant effect on gene expression (results not shown). Similarly, alcohol consumption also had no effect on gene expression (results not shown).
Discussion
Numerous reviews claim altered synaptic abundance to be one of the core cortical pathologies in schizophrenia (2, 9, 50-53); however this claim is based on indirect and variable evidence. Microarray analysis revealed that genes involved in presynaptic secretory function were some of the most changed genes in patients with schizophrenia (4), implicating schizophrenia as a disease of the synapse (1, 3, 4). However, the reduction in mRNAs of individual synaptic proteins varied from person to person; and thus, no clear and generalisable pattern of synaptic reduction was identified. In the present study, we report that there is little overall group change in the mRNA expression of transcripts encoding synaptic genes, including complexin 1, VGAT, complexin 2, VGluT1 and VAMP1 in the DLPFC in our cohort (Figure 2), demonstrating that changes in synaptic mRNAs may be subtle and variable, and this does not support that synaptic reduction is widespread and ubiquitous as often portrayed. We do, however, report reductions in expression of two mRNAs encoding proteins associated with plasticity of the synapse and cytoskeletal stabilisation (GAP43 and NAV1, Figure 2) which may implicate an overall dysregulation of synaptic plasticity in the frontal cortex of people with schizophrenia in the context of apparently normal synaptic content or density.
Overall, we do not see any overt alterations in expression of the more specific markers of inhibitory or excitatory terminals (complexins or vesicular neurotransmitter transporters) in the DLPFC of patients with schizophrenia (Figure 2A). It may be that any “synaptic marker” can play a distinct role in a particular subset of terminals and could therefore be altered within specific terminals and not others. Complexins participate in synaptic vesicle fusion and help stabilise the SNARE complex in a fusogenic state (54). Early studies determined that complexins are predominantly co-localised to either inhibitory (complexin I) or excitatory (complexin II) terminals (55-57) and may, therefore, represent a good molecular measurement of overall inhibitory vs excitatory synapses. Indeed, reduced complexin I in the superior temporal cortex (31) and PFC (30) suggests an overall reduction in inhibitory terminals in schizophrenia. However, these reductions in complexin 1 are not consistently found in all brain regions in schizophrenia (31, 56, 58, 59). The variable nature of this reduction is consistent with our finding of a trend towards reduced complexin 1 in schizophrenia and suggests that complexin 1 mRNA may be reduced in certain subsets of patients. Reports are similarly varied for complexin II mRNA and protein expression in schizophrenic subjects (30, 31, 56, 59), suggesting that alterations in complexins may vary by brain region, between cohorts and among individuals. Additionally, findings may be influenced by disease heterogeneity, sample size, molecular technique used (eg in situ hybridization, quantitative PCR, and western blotting), age, and medication status of patients. We have reviewed recent studies examining synaptic proteins in the DLPFC in schizophrenia [since (16)] in Table 4, showing that most synaptic proteins are unchanged in the schizophrenic DLPFC and that an overall discrepancy between findings at protein and/or mRNA level exists. In a further recent review, Brose demonstrated that most studies of complexin 1 and 2 report either a reduction or no change in mRNA/protein of complexins in multiple brain regions (54).
Table 4. Studies of presynaptic proteins and mRNAs in schizophrenia PFC since 2003.
| Study | reference | Gene | Brain region | Molecule | Assay | Finding | Number of subjects (con, scz) | Mean age (con, scz) |
|---|---|---|---|---|---|---|---|---|
| Sawada et al, 2002 | 30 | complexin 1 | BA9/10 | protein | ELISA | reduced | 11, 13 | 46.3, 47.1 |
| Eastwood and Harrison, 2005 | 31 | complexin 1 | BA9/46 | mRNA | in situ hybridisation | no change | 9, 9 (subset of whole cohort) | 60.4, 55 (whole cohort) |
| Sawada et al, 2002 | 30 | complexin 2 | BA9/10 | protein | ELISA | no change | 11, 13 | 46.3, 47.1 |
| Eastwood and Harrison, 2005 | 31 | complexin 2 | BA9/46 | mRNA | in situ hybridisation | reduced | 8, 10 (subset of whole cohort) | 60.4, 55 (whole cohort) |
| Eastwood and Harrison, 2005 | 31 | VGluT1 | BA9/46 | mRNA | in situ hybridisation | reduced | 10, 10 (subset of whole cohort) | 60.4, 55 (whole cohort) |
| Halim et al, 2003 | 16 | GAP43 | PFC | protein | western blot | no change | 23, 18 | 49.1, 50.8 |
| Dean et al, 2007 | 63 | NAV2 | BA46 | mRNA | restriction fragment differential display | reduced | 10, 10 | 47.6, 45.8 |
| Halim et al, 2003 | 16 | VAMP | PFC | protein | western blot | reduced | 23, 18 | 49.1, 50.8 |
| Scarr et al, 2006 | 19 | VAMP | BA9 | protein | western blot | no change | 20, 20 | 55.7, 55.9 |
| Gray et al, 2010 | 15 | VAMP | BA10/46 | protein | western blot | no change | 20, 20 | 55.7, 55.9 |
| Tang et al, 2009 | 72 | dysbindin | DLPFC | protein | western blot | reduced (dysbindin-1C) | 28, 28 | 83.7, 81.7 |
| Tang et al, 2009 | 72 | dysbindin | DLPFC | mRNA | real-time PCR | increased (dysbindin-1A, -1B) | 28, 28 | 83.7, 81.7 |
| Weickert et al, 2004 | 20 | dysbindin | DLPFC | mRNA | in situ hybridisation | reduced | 15, 14 | 49.5, 50.5 |
| Halim et al, 2003 | 16 | SNAP25 | PFC | protein | western blot | no change | 23, 18 | 49.1, 50.8 |
| Scarr et al, 2006 | 19 | SNAP25 | BA9 | protein | western blot | no change | 20, 20 | 55.7, 55.9 |
| Scarr et al, 2006 | 19 | SNAP25 | BA9 | mRNA | real-time PCR | no change | 18, 18 (subset of whole cohort) | 55.7, 55.9 (whole cohort) |
| Gray et al, 2010 | 15 | SNAP25 | BA10/46 | protein | western blot | no change | 20, 20 | 55.7, 55.9 |
| Halim et al, 2003 | 16 | synaptophysin | PFC | protein | western blot | no change | 23, 18 | 49.1, 50.8 |
| Weickert et al, 2004 | 20 | synaptophysin | DLPFC | mRNA | in situ hybridisation | no change | 15, 14 | 49.5, 50.5 |
| Scarr et al, 2006 | 19 | synaptophysin | BA9 | protein | western blot | no change | 20, 20 | 55.7, 55.9 |
| Gray et al, 2010 | 15 | synaptophysin | BA10/46 | protein | western blot | no change | 20, 20 | 55.7, 55.9 |
| Halim et al, 2003 | 16 | syntaxin | PFC | protein | western blot | no change | 23, 18 | 49.1, 50.8 |
| Scarr et al, 2006 | 19 | syntaxin | BA9 | protein | western blot | no change | 20, 20 | 55.7, 55.9 |
| Gray et al, 2010 | 15 | syntaxin | BA10/46 | protein | western blot | no change | 20, 20 | 55.7, 55.9 |
More recent reports suggest that complexins 1 and 2 display some functional redundancy in complexin 1 and complexin 2 deficient mice suggesting that they are not necessarily localised to specific inhibitory or excitatory terminals (60). Consequently, we also examined the mRNA expression of the vesicular GABA and glutamate transporters (VGAT and VGluT1, respectively) as more specific inhibitory/excitatory transmission markers. A reduction in VGluT1 mRNA and/or protein in the PFC and hippocampus (31, 61), but also an increase in VGluT1 protein in the hippocampus (45) in people with schizophrenia have been reported. In our cohort, however we failed to detect a significant diagnostic difference in expression of either VGAT or VGluT1 mRNAs, and we also do not find any significant alterations in the expression of several other synaptic or cytoskeletal genes previously associated with schizophrenia: VAMP1 [part of the SNARE complex involved in vesicle fusion; previously reported to show overall reduced protein levels in the DLPFC (16)], and NAV2 [associated with actin cytoskeleton remodelling (62) and reported to be downregulated in schizophrenia (63)] (Figure 2). This suggests that within our cohort there is no overall change in many of the mRNAs encoding presynaptic proteins which have previously been found to be reduced in patients with schizophrenia. This may be viewed as consistent with the evidence from most studies that fail to detect an alteration in synaptophysin widely considered a general marker of overall synapse number [reviewed by (16)], however synaptophysin may be enriched in glutamatergic vesicles (64). An alternative interpretation of our data might suggest that we failed to detect alterations in any one of these genes due to the heterogeneous nature of schizophrenia. This heterogeneity could implicate alterations in any one or more genes in a functional pathway that could then disrupt synaptic communication and lead to alterations in synaptic communication. A similar mechanism has been proposed to account for the numerous genes in the neurotransmission machinery of the synapse that have been implicated in the neuropathology of schizophrenia (3, 4). Indeed this may also be seen for VGAT and complexin 2 mRNA expression where a few individuals have particularly low expression of these genes, while average expression in the schizophrenia cohort as a whole is not significantly altered (Figure 2A).
Our finding of reduced GAP43 mRNA expression in schizophrenia (Figure 2B) is consistent with our previously reported reduction in GAP43 mRNA expression in layers III, V, and VI in the DLPFC of schizophrenia patients (34), although another study showed no alteration in GAP43 mRNA in the DLPFC in people with schizophrenia (36). While Perrone-Bizzozero and colleagues (22) reported an increase in GAP43 protein in the visual and frontal cortices, our previous work and that of others did not show any alteration in GAP43 protein in the DLPFC of patients with schizophrenia (16, 17). However, as GAP43 mRNA is robustly expressed in deeper cortical layers and GAP-43 protein is shipped via fast-axonal transport to the presynaptic terminals (65), we predict reductions in GAP-43 protein levels may be more readily detected in the thalamus or in the caudate to which the infragranular cortical neurons project. The potential roles of GAP43 in neuronal pathfinding and branching in development and neurotransmitter release, long-term potentiation and learning in the adult brain [reviewed by (65)] demonstrate a variety of mechanisms through which GAP43 deficiency might lead to altered plasticity of the brain and schizophrenia symptoms. Although there is the possibility of type 1 error, we also report a reduced mRNA expression encoding another cytoskeletal modulator (NAV1 see below) in the DLPFC of people with schizophrenia in this cohort (Figure 2B); however, to further strengthen the case for reduced synaptic plasticity in schizophrenia, these findings need to be replicated in another cohort.
The reduction we find in NAV1 mRNA in schizophrenia (Figure 2B) supports dysregulation of cytoskeleton reorganisation in schizophrenia. NAV1 localises to the growth cone and branch points in development similar to that of GAP43, and has a role in bundling microtubules and in pathfinding, axogenesis and synaptic maturation (66). It therefore appears that an overall reduction in synaptic plasticity may be a generalised finding in the schizophrenia cortex, as has been suggested (34, 35, 59). NAV1 is homologous to NAV2 with deletion of several exons from a gene duplication, but with a similar proposed function (67). NAV2 is also associated with neurite outgrowth and acts to remodel the cytoskeleton through association with growing microtubules (68-70) and it has been suggested that this protein contributes to actin cytoskeleton remodelling through its CH domain linking ABI-1 homologue and the ARP2/3 complex (62). We did not detect a change in NAV2 in our cohort, however NAV2 mRNA was found to be reduced in the DLPFC of people with schizophrenia (63). While the Navigators have been implicated in migration and neurite outgrowth, it is not clear why NAV1, but not NAV2 is not similarly reduced schizophrenia in our cohort.
From our previous work, we expected to find reduced dysbindin expression in the DLPFC (20, 43). In the present study, with a larger cohort we failed to replicate this reduction in dysbindin mRNA in schizophrenia (Figure 2B), although some individuals with schizophrenia appear to have lower dysbindin expression than controls, as may be expected due to the fact that only a small subset of schizophrenia cases would carry the “at risk” genetic variant in dysbindin that is linked to mRNA down-regulation(44). However, when we genotyped our cohort we found that none of the dysbindin SNPs tested which were previously reported to alter dysbindin mRNA levels (20), were associated with a change in dysbindin mRNA expression in the present cohort (Table 3). This suggests that in our Australian cohort these SNPs do not directly relate to variation in gene expression and individual differences in dysbindin gene expression may be more saliently influenced by other genetic, epigenetic or environmental factors. Additionally, it is possible that laminar-specific alterations in dysbindin mRNA may be present in our cohort and may have escaped detection with our homogenate-based approach. Interestingly, while overall levels of synaptic-related transcripts are not significantly altered on a group basis, some correlations between measured synaptic/cytoskeletal genes did display varying relationships in the control cohort as compared to schizophrenia subjects. Positive correlations of dysbindin with plasticity associated genes (GAP43 and NAV1) occurred in the disease state but not in controls (Figure 3). Dysbindin has been associated with adaptive modulation of vesicle release and regulation of LTP (71, 72) and the fact that those individuals with reduced dysbindin also tended to have reduced GAP-43 and NAV1 suggests that dysregulation of dysbindin in schizophrenia may limit synaptic plasticity/cytoskeleton reorganisation in schizophrenia (71).
While we have globally surveyed only a small subset of synaptic protein mRNAs we have chosen those previously reported to be altered in schizophrenia and/or representative of different terminal types to address the question of whether inhibitory or excitatory terminals may be most affected in schizophrenia. We report a lack of change in overall synaptic mRNA levels which may reflect no change in synaptic density; however, at the current resolution we are unable to determine whether subsets of terminals are reduced in number or density along with compensatory increases in the number or size of other subsets of terminals. While studies of synaptic proteins may by more closely linked to synaptic density, measurement of synaptic proteins in homogenates would also miss selective changes in subsets of terminals. Techniques with higher anatomical resolution will be needed to examine questions about synaptic mRNAs in specific subsets of neurons, such as laser capture or in situ hybridization, which would allow more specific examination of different neuronal subtypes or distinct cortical layers. However, even these approaches may not be sensitive enough to pick up changes in a subset of terminals within a neuron. So, it is premature to conclude that synaptic terminal number or density is completely unchanged in schizophrenia.
In summary, our analysis of mRNAs encoding synaptic proteins, using one of the largest schizophrenia cohorts studied to date does not support an overall widespread reduction in synaptic marker mRNAs in the frontal cortex of patients with schizophrenia. This implies that synaptic changes in schizophrenia cortex may be more subtle, anatomically restricted, or heterogeneous. Although there are consistent reports of a GABAergic deficit in schizophrenia cohorts (23-29), we do not find evidence for an overall deficit in mRNAs encoding proteins found in inhibitory synaptic terminals nor do we find a compensatory/parallel alteration in mRNAs encoding excitatory terminal proteins in schizophrenia. Our study does lend support to the theory that there is a deficit in synaptic plasticity associated genes, and potentially a deficit in the modifiability of synaptic terminals, and we speculate that this loss of plasticity may be more important in the cortical pathology of schizophrenia than gross reductions in the overall terminal abundance.
Supplementary Material
Acknowledgments
We would like to thank Dr Maree Webster for her contribution to the drafting of this manuscript. We would also like to thank Shan Yuan Tsai and Duncan Sinclair for technical support. This work was supported by the Schizophrenia Research Institute, utilising infrastructure funding from NSW Health, the Macquarie Group Foundation, Neuroscience Research Australia and the University of New South Wales. Tissues were received from the Australian Brain Donor Programs NSW Tissue Resource Centre, which is supported by The University of Sydney, National Health and Medical Research Council of Australia, Schizophrenia Research Institute, National Institute of Alcohol Abuse and Alcoholism, NIH and NSW Department of Health.
Footnotes
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 2.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 3.Mirnics K, Middleton FA, Lewis DA, Levitt P. Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci. 2001;24:479–486. doi: 10.1016/s0166-2236(00)01862-2. [DOI] [PubMed] [Google Scholar]
- 4.Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, et al. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56:537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- 6.Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Feiner D, et al. Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry. 2000;57:471–480. doi: 10.1001/archpsyc.57.5.471. [DOI] [PubMed] [Google Scholar]
- 7.Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. discussion 819-820. [DOI] [PubMed] [Google Scholar]
- 8.Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- 9.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 10.Masliah E, Terry RD, Alford M, DeTeresa R. Quantitative immunohistochemistry of synaptophysin in human neocortex: an alternative method to estimate density of presynaptic terminals in paraffin sections. J Histochem Cytochem. 1990;38:837–844. doi: 10.1177/38.6.2110586. [DOI] [PubMed] [Google Scholar]
- 11.Eastwood SL, Burnet PW, McDonald B, Clinton J, Harrison PJ. Synaptophysin gene expression in human brain: a quantitative in situ hybridization and immunocytochemical study. Neuroscience. 1994;59:881–892. doi: 10.1016/0306-4522(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 12.Eastwood SL, Burnet PW, Harrison PJ. Altered synaptophysin expression as a marker of synaptic pathology in schizophrenia. Neuroscience. 1995;66:309–319. doi: 10.1016/0306-4522(94)00586-t. [DOI] [PubMed] [Google Scholar]
- 13.Eastwood SL, Cairns NJ, Harrison PJ. Synaptophysin gene expression in schizophrenia. Investigation of synaptic pathology in the cerebral cortex. Br J Psychiatry. 2000;176:236–242. doi: 10.1192/bjp.176.3.236. [DOI] [PubMed] [Google Scholar]
- 14.Glantz LA, Austin MC, Lewis DA. Normal cellular levels of synaptophysin mRNA expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2000;48:389–397. doi: 10.1016/s0006-3223(00)00923-9. [DOI] [PubMed] [Google Scholar]
- 15.Gray LJ, Dean B, Kronsbein HC, Robinson PJ, Scarr E. Region and diagnosis-specific changes in synaptic proteins in schizophrenia and bipolar I disorder. Psychiatry Res. 178:374–380. doi: 10.1016/j.psychres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Halim ND, Weickert CS, McClintock BW, Hyde TM, Weinberger DR, Kleinman JE, Lipska BK. Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Mol Psychiatry. 2003;8:797–810. doi: 10.1038/sj.mp.4001319. [DOI] [PubMed] [Google Scholar]
- 17.Honer WG, Falkai P, Chen C, Arango V, Mann JJ, Dwork AJ. Synaptic and plasticity-associated proteins in anterior frontal cortex in severe mental illness. Neuroscience. 1999;91:1247–1255. doi: 10.1016/s0306-4522(98)00679-4. [DOI] [PubMed] [Google Scholar]
- 18.Karson CN, Mrak RE, Schluterman KO, Sturner WQ, Sheng JG, Griffin WS. Alterations in synaptic proteins and their encoding mRNAs in prefrontal cortex in schizophrenia: a possible neurochemical basis for ‘hypofrontality’. Mol Psychiatry. 1999;4:39–45. doi: 10.1038/sj.mp.4000459. [DOI] [PubMed] [Google Scholar]
- 19.Scarr E, Gray L, Keriakous D, Robinson PJ, Dean B. Increased levels of SNAP-25 and synaptophysin in the dorsolateral prefrontal cortex in bipolar I disorder. Bipolar Disord. 2006;8:133–143. doi: 10.1111/j.1399-5618.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- 20.Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, Herman MM, et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry. 2004;61:544–555. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- 21.Gla ntz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 1997;54:943–952. doi: 10.1001/archpsyc.1997.01830220065010. [DOI] [PubMed] [Google Scholar]
- 22.Perrone-Bizzozero NI, Sower AC, Bird ED, Benowitz LI, Ivins KJ, Neve RL. Levels of the growth-associated protein GAP-43 are selectively increased in association cortices in schizophrenia. Proc Natl Acad Sci U S A. 1996;93:14182–14187. doi: 10.1073/pnas.93.24.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan C, Webster M, Rothmond D, Bahn S, Elashoff M, Shannon Weickert C. Prefrontal GABA receptor alpha subunit expression in normal postnatal human development and schizophrenia. Journal of Psychiatric Research. 2010 doi: 10.1016/j.jpsychires.2009.12.007. epub ahead of print Jan 23. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- 26.Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- 27.Volk D, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- 28.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 29.Fung S, Webster M, Sivagnanasundaram S, Duncan C, Elashoff M, Shannon Weickert C. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010 doi: 10.1176/appi.ajp.2010.09060784. accepted July 2010. [DOI] [PubMed] [Google Scholar]
- 30.Sawada K, Young CE, Barr AM, Longworth K, Takahashi S, Arango V, Mann JJ, et al. Altered immunoreactivity of complexin protein in prefrontal cortex in severe mental illness. Mol Psychiatry. 2002;7:484–492. doi: 10.1038/sj.mp.4000978. [DOI] [PubMed] [Google Scholar]
- 31.Eastwood SL, Harrison PJ. Decreased expression of vesicular glutamate transporter 1 and complexin II mRNAs in schizophrenia: further evidence for a synaptic pathology affecting glutamate neurons. Schizophr Res. 2005;73:159–172. doi: 10.1016/j.schres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 33.Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weickert CS, Webster MJ, Hyde TM, Herman MM, Bachus SE, Bali G, Weinberger DR, et al. Reduced GAP-43 mRNA in dorsolateral prefrontal cortex of patients with schizophrenia. Cereb Cortex. 2001;11:136–147. doi: 10.1093/cercor/11.2.136. [DOI] [PubMed] [Google Scholar]
- 35.Chambers JS, Thomas D, Saland L, Neve RL, Perrone-Bizzozero NI. Growth-associated protein 43 (GAP-43) and synaptophysin alterations in the dentate gyrus of patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:283–290. doi: 10.1016/j.pnpbp.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Eastwood SL, Harrison PJ. Hippocampal and cortical growth-associated protein-43 messenger RNA in schizophrenia. Neuroscience. 1998;86:437–448. doi: 10.1016/s0306-4522(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 37.Tian SY, Wang JF, Bezchlibnyk YB, Young LT. Immunoreactivity of 43 kDa growth-associated protein is decreased in post mortem hippocampus of bipolar disorder and schizophrenia. Neuroscience letters. 2007;411:123–127. doi: 10.1016/j.neulet.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 38.Webster MJ, Shannon Weickert C, Herman MM, Hyde TM, Kleinman JE. Synaptophysin and GAP-43 mRNA levels in the hippocampus of subjects with schizophrenia. Schizophr Res. 2001;49:89–98. doi: 10.1016/s0920-9964(00)00052-9. [DOI] [PubMed] [Google Scholar]
- 39.Blennow K, Bogdanovic N, Gottfries CG, Davidsson P. The growth-associated protein GAP-43 is increased in the hippocampus and in the gyrus cinguli in schizophrenia. J Mol Neurosci. 1999;13:101–109. doi: 10.1385/JMN:13:1-2:101. [DOI] [PubMed] [Google Scholar]
- 40.Sower AC, Bird ED, Perrone-Bizzozero NI. Increased levels of GAP-43 protein in schizophrenic brain tissues demonstrated by a novel immunodetection method. Mol Chem Neuropathol. 1995;24:1–11. doi: 10.1007/BF03160108. [DOI] [PubMed] [Google Scholar]
- 41.Weickert CS, Sheedy D, Rothmond DA, Dedova I, Fung S, Garrick T, Wong J, et al. Selection of reference gene expression in a schizophrenia brain cohort. Aust N Z J Psychiatry. 2010;44:59–70. doi: 10.3109/00048670903393662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo AY, Sun J, Riley BP, Thiselton DL, Kendler KS, Zhao Z. The dystrobrevin-binding protein 1 gene: features and networks. Mol Psychiatry. 2009;14:18–29. doi: 10.1038/mp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weickert CS, Rothmond DA, Hyde TM, Kleinman JE, Straub RE. Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophr Res. 2008;98:105–110. doi: 10.1016/j.schres.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bray NJ, Preece A, Williams NM, Moskvina V, Buckland PR, Owen MJ, O'Donovan MC. Haplotypes at the dystrobrevin binding protein 1 (DTNBP1) gene locus mediate risk for schizophrenia through reduced DTNBP1 expression. Hum Mol Genet. 2005;14:1947–1954. doi: 10.1093/hmg/ddi199. [DOI] [PubMed] [Google Scholar]
- 45.Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, Hahn CG, et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113:1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Fung S, Rothwell A, Tianmei S, Shannon Weickert C. ((submitted)) Increased Interstitial White Matter Neuron Density in the DLPFC of People with Schizophrenia. Biol Psych. doi: 10.1016/j.biopsych.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGuigan FE, Ralston SH. Single nucleotide polymorphism detection: allelic discrimination using TaqMan. Psychiatr Genet. 2002;12:133–136. doi: 10.1097/00041444-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Bragina L, Giovedi S, Barbaresi P, Benfenati F, Conti F. Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex: analysis of synaptogyrin, vesicle-associated membrane protein, and syntaxin. Neuroscience. 2010;165:934–943. doi: 10.1016/j.neuroscience.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Bennett MR. Synapse formation and regression in the cortex during adolescence and in schizophrenia. Med J Aust. 2009;190:S14–16. doi: 10.5694/j.1326-5377.2009.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 51.Hayashi-Takagi A, Sawa A. Disturbed synaptic connectivity in schizophrenia: Convergence of genetic risk factors during neurodevelopment. Brain Res Bull. 2010 doi: 10.1016/j.brainresbull.2010.04.007. ePub, April 28th. [DOI] [PubMed] [Google Scholar]
- 52.Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 53.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 54.Brose N. Altered complexin expression in psychiatric and neurological disorders: cause or consequence? Mol Cells. 2008;25:7–19. [PubMed] [Google Scholar]
- 55.Eastwood SL, Burnet PW, Harrison PJ. Expression of complexin I and II mRNAs and their regulation by antipsychotic drugs in the rat forebrain. Synapse. 2000;36:167–177. doi: 10.1002/(SICI)1098-2396(20000601)36:3<167::AID-SYN2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 56.Harrison PJ, Eastwood SL. Preferential involvement of excitatory neurons in medial temporal lobe in schizophrenia. Lancet. 1998;352:1669–1673. doi: 10.1016/S0140-6736(98)03341-8. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi S, Yamamoto H, Matsuda Z, Ogawa M, Yagyu K, Taniguchi T, Miyata T, et al. Identification of two highly homologous presynaptic proteins distinctly localized at the dendritic and somatic synapses. FEBS Lett. 1995;368:455–460. doi: 10.1016/0014-5793(95)00713-j. [DOI] [PubMed] [Google Scholar]
- 58.Eastwood SL, Harrison PJ. Hippocampal synaptic pathology in schizophrenia, bipolar disorder and major depression: a study of complexin mRNAs. Mol Psychiatry. 2000;5:425–432. doi: 10.1038/sj.mp.4000741. [DOI] [PubMed] [Google Scholar]
- 59.Eastwood SL, Harrison PJ. Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull. 2001;55:569–578. doi: 10.1016/s0361-9230(01)00530-5. [DOI] [PubMed] [Google Scholar]
- 60.Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 61.Sawada K, Barr AM, Nakamura M, Arima K, Young CE, Dwork AJ, Falkai P, et al. Hippocampal complexin proteins and cognitive dysfunction in schizophrenia. Arch Gen Psychiatry. 2005;62:263–272. doi: 10.1001/archpsyc.62.3.263. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt KL, Marcus-Gueret N, Adeleye A, Webber J, Baillie D, Stringham EG. The cell migration molecule UNC-53/NAV2 is linked to the ARP2/3 complex by ABI-1. Development. 2009;136:563–574. doi: 10.1242/dev.016816. [DOI] [PubMed] [Google Scholar]
- 63.Dean B, Keriakous D, Scarr E, Thomas EA. Gene expression profiling in Brodmann's area 46 from subjects with schizophrenia. Aust N Z J Psychiatry. 2007;41:308–320. doi: 10.1080/00048670701213245. [DOI] [PubMed] [Google Scholar]
- 64.Gronborg M, Pavlos NJ, Brunk I, Chua JJ, Munster-Wandowski A, Riedel D, Ahnert-Hilger G, et al. Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein. J Neurosci. 30:2–12. doi: 10.1523/JNEUROSCI.4074-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Denny JB. Molecular Mechanisms, Biological Actions, and Neuropharmacology of the Growth-Associated Protein GAP-43. Curr Neuropharmacol. 2006;4:293–304. doi: 10.2174/157015906778520782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinez-Lopez MJ, Alcantara S, Mascaro C, Perez-Branguli F, Ruiz-Lozano P, Maes T, Soriano E, et al. Mouse neuron navigator 1, a novel microtubule-associated protein involved in neuronal migration. Mol Cell Neurosci. 2005;28:599–612. doi: 10.1016/j.mcn.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 67.Maes T, Barcelo A, Buesa C. Neuron navigator: a human gene family with homology to unc-53, a cell guidance gene from Caenorhabditis elegans. Genomics. 2002;80:21–30. doi: 10.1006/geno.2002.6799. [DOI] [PubMed] [Google Scholar]
- 68.van Haren J, Draegestein K, Keijzer N, Abrahams JP, Grosveld F, Peeters PJ, Moechars D, et al. Mammalian Navigators are microtubule plus-end tracking proteins that can reorganize the cytoskeleton to induce neurite-like extensions. Cell Motil Cytoskeleton. 2009;66:824–838. doi: 10.1002/cm.20370. [DOI] [PubMed] [Google Scholar]
- 69.Muley PD, McNeill EM, Marzinke MA, Knobel KM, Barr MM, Clagett-Dame M. The atRA-responsive gene neuron navigator 2 functions in neurite outgrowth and axonal elongation. Dev Neurobiol. 2008;68:1441–1453. doi: 10.1002/dneu.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merrill RA, Plum LA, Kaiser ME, Clagett-Dame M. A mammalian homolog of unc-53 is regulated by all-trans retinoic acid in neuroblastoma cells and embryos. Proc Natl Acad Sci U S A. 2002;99:3422–3427. doi: 10.1073/pnas.052017399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dickman DK, Davis GW. The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science. 2009;326:1127–1130. doi: 10.1126/science.1179685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang TT, Yang F, Chen BS, Lu Y, Ji Y, Roche KW, Lu B. Dysbindin regulates hippocampal LTP by controlling NMDA receptor surface expression. Proc Natl Acad Sci U S A. 2009;106:21395–21400. doi: 10.1073/pnas.0910499106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.