Abstract
Objectives.
The association of alcohol consumption with performance in different cognitive domains has not been well studied.
Methods.
The Johns Hopkins Precursors Study was used to examine associations between prospectively collected information about alcohol consumption ascertained on multiple occasions starting at age 55 years on average with domain-specific cognition at age 72 years. Cognitive variables measured phonemic and semantic fluency, attention, verbal memory, and global cognition.
Results.
Controlling for age, hypertension, smoking status, sex, and other cognitive variables, higher average weekly quantity and frequency of alcohol consumed in midlife were associated with lower phonemic fluency. There were no associations with four other measures of cognitive function. With respect to frequency of alcohol intake, phonemic fluency was significantly better among those who drank three to four alcoholic beverages per week as compared with daily or almost daily drinkers. A measure of global cognition was not associated with alcohol intake at any point over the follow-up.
Discussion.
Results suggest that higher alcohol consumption in midlife may impair some components of executive function in late life.
Keywords: Alcohol, Cognition, Epidemiology, Older adults
THE physical health implications of alcohol consumption have been characterized extensively among older adults, but less is known about effects of alcohol on cognitive abilities. Moderate levels of alcohol consumption are generally associated with better physical health, whereas excessive use is associated with worse outcomes (McCaul et al., 2010; Mukamal et al., 2003; Peters et al., 2008; Simons et al., 2000; Stuck et al., 1999). Characterizing the physical as well as cognitive effects of alcohol intake informs clinicians’ care for older persons. Current dietary guidelines recommend alcohol use in moderation unless contraindicated by preexisting conditions (Mann & Folts, 2004; Willet & Stampfer, 2003).
Evidence for an association of alcohol intake with cognition among older adults is mixed. Studies comparing drinkers and nondrinkers report lower cognitive functioning among nondrinkers (Britton, Singh-Manoux, & Marmot, 2004; McDougall, Becker, & Areheart, 2006; McDougall et al., 2007). Many cross-sectional studies in older populations show that those consuming approximately 7–14 drinks per week have higher cognitive function than abstainers or heavier drinkers, that is, a “j-shaped” or “u-shaped” relationship (den Heijer et al., 2004; Lang et al., 2007; Launer et al., 1996; Reid et al., 2006; Rodgers et al., 2005). Similar associations, though usually not statistically significant, have been reported in longitudinal studies (Anttila et al., 2004; Bond et al., 2005; Britton et al.; Cervilla et al., 2000; Elias et al., 1999; Flicker et al., 2005; Galanis et al., 2000; Kalmijn et al., 2002; Leroi, Sheppard, & Lyketsos, 2002; McGuire, Ajani, & Ford, 2007). Proposed biologic mediators for the association involve beneficial impacts of alcohol on lipid and lipoprotein levels, lowered risk for heart disease, and increased cerebral blood flow, all of which are good for cognitive health (Britton et al.; Fontana et al., 1999; Gaziano et al., 1993; Kalmijn et al.; Kiechl et al., 1998; Rabbia et al., 1995; Sano et al., 1993). Other cross-sectional (Anstey et al., 2005; Elwood et al., 1999; McDougall et al., 2006) and longitudinal (Espeland et al., 2005; Galanis et al.; Ganguli et al., 2005; Stampfer et al., 2005; Wright et al., 2006) studies show no significant relationship between alcohol intake and cognitive function, although there is evidence that the association may differ as a function of cognitive domain (Anstey et al.; Britton et al.; Elias et al.; Ganguli et al.; Hebert et al., 1993; Kalmijn et al.; McDougall et al., 2006, 2007; Reid et al.; Rodgers et al.). A recent meta-analysis of 23 longitudinal studies reported no evidence of protective effects of low levels of alcohol consumption on cognitive function in adults older than 65 years of age (Peters et al.). Even among longitudinal studies that have reported statistically significant associations between better cognition and moderate alcohol consumption, magnitudes of the associations are small (e.g., Cervilla et al.).
The inconsistencies in findings outlined earlier with respect to the association between alcohol and cognition may be partly attributable to how alcohol use is quantified. Measures of alcohol use should capture both how much an individual drinks and how often he or she drinks. Quantity and frequency of alcohol intake may influence cognitive ability in qualitatively different ways. For example, the quantity of alcohol consumption is likely a more accurate measure of alcohol dose than frequency of drinking. It is also conceivable that the amount an individual drinks at any single time may be less associated with cognitive ability than one’s trajectory or change in alcohol intake over time. Finally, “problem” drinkers may show particular cognitive deficits (Moselhy, Georgiou, & Kahn, 2001; Tapert et al., 2001).
Few prospective studies have addressed effects of alcohol consumption during midlife on cognition in later life. Prospective studies are important because cognitive decline may impair accurate recall of past intake. In addition, alcohol intake declines with age (Dufour & Fuller, 1995; Karlamangla et al., 2006), meaning that exposure assessment in midlife may be a more accurate measure of lifetime exposure. Furthermore, null or weak associations seen in many studies may be due to insufficient follow-up time between measurement of alcohol use and cognitive testing. Thus, exposures occurring years before measurement of cognition may be more predictive of future cognitive function than cross-sectional or recent measures of exposure.
Another limitation in many studies of the association of alcohol use with cognition is that domain-specific measures of cognition are not often employed. Cognitive ability is a multidimensional construct embracing multiple domains. Although each domain can be characterized as a conceptually distinct unit, their functions are highly interrelated (Moscovitch, 1992). Many studies use global or aggregated composite measures of cognition (Bond et al., 2005; Cervilla et al., 2000; den Heijer et al., 2004; Elwood et al., 1999; Espeland et al., 2005; Flicker et al., 2005; Galanis et al., 2000; Lang et al., 2007; Launer et al., 1996; Leroi et al., 2002; McGuire et al., 2007; Wright et al., 2006). Such measures of global mental status or tests of general intelligence show good reliability and encompass a variety of cognitive constructs but are not as sensitive to domain-specific measures of cognition (Leroi, Sheppard, & Lyketsos, 2002; Lyketsos, Chen, & Anthony, 1999). Additionally, global measures are subject to ceiling effects among cognitively intact older adults (Leroi et al.). If alcohol use is associated with different mental abilities to varying degrees, such an approach might mask associations. Finally, alcohol consumption, depending on the level, can be both a risk and a protective factor for cardiovascular conditions such as stroke and hypertension, which in turn are associated with declines in specific cognitive functions (e.g., Rafnsson et al., 2007). Alcohol use affects certain brain regions, such as frontal lobe structures, more than others (Moselhy et al., 2001; Tapert et al., 2001), which may lead to differential neuropsychological test performance in domains associated with the affected brain regions (Weissenborn & Duka, 2003). Indeed, different effect sizes comparing drinkers and nondrinkers have been reported depending on the cognitive measure used; one study has shown that memory measures that rely on executive abilities like the Rivermead Behaviour Memory Test (effect size: 0.85) and the Hopkins Verbal Learning Test (HVLT; effect size: 0.62) show larger effect sizes than for visuospatial ability (effect size: 0.27; McDougall et al., 2006). Other studies have also reported deleterious effects of alcohol on executive functions or on the brain’s frontal lobe region (Tapert et al.; Weissenborn & Duka).
The Johns Hopkins Precursors Study addresses these limitations in several ways. Alcohol intake was assessed prospectively on multiple occasions over 17 years from middle to old age. Alcohol intake was examined separately as an average weekly quantity, frequency of alcohol intake, annual rate of change in weekly consumption levels over 17 years, and problem drinking. Alcohol intake was characterized using these separate metrics to allow for different associations with cognitive measures. We are not aware of any other studies among older adults that have quantified alcohol use in all these ways. Additionally, a battery of neuropsychological tests was administered that assessed several domains of cognitive function, including global cognition, aspects of executive function including semantic and phonemic fluency, memory, and attention. Our objective was to examine associations between prospectively collected information about alcohol intake at various ages throughout midlife and domain-specific cognitive function measures later in life. We hypothesized that alcohol quantity and frequency are associated with cognitive measures that rely most on executive functions, namely semantic and phonemic verbal fluency.
METHODS
The Johns Hopkins Precursors Study was started in 1947 by the late Caroline Bedell Thomas as a prospective longitudinal study of medical students who graduated from The Johns Hopkins Medical School between 1948 and 1964. The cohort consisted of 1,216 men and 121 women, most of whom are White, have a medical education, and have been followed since their graduation (Klag et al., 2002). Questionnaires are mailed annually to update morbidity and exposure information. Follow-up rates are high with annual response rates all above 72% for the years used in this article; vital status of nonrespondents is known for more than 99% of the cohort (Klag et al., 2002). This analysis consists of 588 participants, which represents 60% of those who were alive and consented to cognitive testing over the telephone in 2005. Cognitive measures were obtained from the 2005 telephone survey, and other variables including alcohol use were collected from annual mailed questionnaires.
Measurement Strategy
Five cognitive measures are available from the telephone administered neuropsychological test battery in 2005. They include the Telephone Interview for Cognitive Status (TICS) total score, which correlates highly with the Mini-Mental State Examination and is designed for assessments of global cognitive status over the telephone (test–retest reliability: 0.96; Brandt, Spencer, & Folstein, 1988; Folstein, Folstein, & McHugh, 1975). The Verbal Fluency Test (VFT) measures the flexibility and speed of verbal thought processes, which can be interpreted as executive functions; two measures from this test were treated separately, semantic (count of animal words produced; test–retest reliability: 0.77) and phonemic (count of F, A, and S words produced; test–retest reliability: 0.82) fluency (Harrison et al., 2000). The HVLT is a verbal word list–learning memory test of semantically related words that assesses episodic memory recall (test–retest reliability: 0.74; Brandt & Benedict, 2001), and the Brief Test of Attention (BTA) is a measure of short-term attentional monitoring (Cronbach’s α: .91; Schretlen, 1989). The version of the BTA used here tested attention for both numbers and letters.
Self-reported alcohol intake was assessed with quantity–frequency measures in mailed surveys from 1986, 1989, 1993, 1997, and 2003. Questions were the same at each assessment. Separately for beer, wine, mixed drinks, and hard liquor, participants were asked for their typical weekly intake in the past year. Beverage types were combined to calculate an average total number of drinks per week consumed. Respondents also estimated the frequency with which they drank alcohol, with possible responses being “daily or almost everyday,” “3–4×/week,” “1–2×/week,” “1–2×/month,” or “rarely.” Quantity–frequency questionnaires for alcohol assessment have been shown to have good psychometric properties (Armor, Pohch, & Stambul, 1978; Midanik, 1982; Pernanen, 1974; Russell, Welte, & Barnes, 1991). In the Precursors cohort, self-reports of weight, height, blood pressure, and other variables have been shown to be valid (Klag et al., 1993), and so, self-reported alcohol consumption was also likely reported with validity. The CAGE was administered with the alcohol use questionnaire to assess problem drinking (Conigliaro, Kraemer, & McNeil, 2000; Ewing, 1984). CAGE is an acronym formed from the four items in the questionnaire, which asks if people have felt they should “cut” down on their drinking, been “annoyed” by others’ criticisms of their drinking, felt “guilty” about their drinking, or used alcohol as an “eye-opener” in the morning. Alcohol was also assessed during medical school and periodically afterward until 1984 using different questions that do not allow calculation of counts or frequency of alcohol use. Consequently, we excluded alcohol information collected prior to 1986.
Other covariates, included in all models unless otherwise specified, were age in the year alcohol consumption was reported (coded continuously), hypertension (yes and no), sex, and smoking status (current smoker and current nonsmoker). These variables were selected a priori because each is associated with cognitive abilities and alcohol intake. Incidence of hypertension and other cardiovascular diseases was assessed by a committee of internists, trained in epidemiology, who reviewed annual questionnaires and medical records. Given that hypertension might mediate the relationship between alcohol consumption and cognition, all models described subsequently were estimated with and without hypertensive status to see if any inferences changed. Each of these characteristics was time dependent and measured at the time of the alcohol intake assessment.
Analysis Plan
We first examined associations between consumption of specific beverage types and relations between alcohol and potential confounders. Next, generalized estimating equations (GEE) methods were fit to the data to describe relationships between self-reported alcohol quantity across midlife and cognition in older age because each neuropsychological measure assesses distinct but correlated domain-specific aspects of cognition within each person (Liang & Zeger, 1986). The GEE approach tests for differences in the magnitude of associations between alcohol use for each year that alcohol was measured and cognitive variables, while also accounting for nonindependence of cognitive scores. Cognitive outcomes were global cognitive status (TICS), semantic fluency (from VFT), phonemic fluency (from VFT), verbal memory (HVLT), and attention (BTA). All five cognitive scores were regressed simultaneously on the year’s consumption variable, an indicator variable for the neuropsychological test, interactions between the two, and age, hypertension, sex, and smoking status. Coefficients with Wald confidence intervals (CIs) for the linear combination between each cognitive indicator and alcohol quantity provided estimates for the association between alcohol consumption in each year for each neuropsychological test, after adjusting for correlations with other cognitive measures and potential confounders. All GEE analyses were specified with an exchangeable correlation structure and an identity link function after reviewing autocorrelation plots; inferences did not change when unstructured or autoregressive correlation matrices were specified.
Presence of quadratic relationships between amount of alcohol consumed and each cognitive variable was assessed by adding quadratic terms to models. Because some evidence indicates that cognitive abilities may be lower among those with cardiovascular disease (Launer et al., 1996), interaction terms were added between cardiovascular disease and each year’s alcohol intake. Next, we explored whether any association between amount of alcohol consumed and cognition differs at different ages by adding interaction terms to models and inspecting nonparametric lowess plots (Cleveland, 1979). Basic regression assumptions were assessed using variance inflation factors and assorted graphical displays, including quantile–quantile plots, residuals plots, and nonparametric lowess plots.
A second set of GEE models considered frequency of alcohol use. Drinking frequency was categorized as daily or almost daily drinkers, one to two drinks per week, three to four drinks per week, and monthly drinkers (2×/month or less). The two least frequent drinking categories (“1–2×/month” and “rarely”) were consolidated because of the small numbers of respondents in these categories. The group of daily or almost daily drinkers served as the reference category. To see if the frequency of alcohol use was associated with cognition in later age, each cognitive outcome was regressed onto indicator variables for frequency of use with adjustment for age, hypertension, sex, and smoking status.
Third, to see if the rate of change over the years in average reported weekly alcohol consumption was associated with cognitive outcomes, individual linear slopes were calculated using random effects models to summarize the rate of change for each individual over the course of 17 years (Laird & Ware, 1982). Each neuropsychological test score was then regressed on these change slopes. These models adjusted for age, current smoking status, sex, hypertensive status, and baseline (i.e., 1986) alcohol quantity.
A final set of models evaluated the association of problem drinking, as defined by a CAGE score greater than 1, on cognitive outcomes. The CAGE has been shown to be a reliable predictor of alcoholism (Bernadt, Mumford, Taylor, Smith, & Murray, 1982; Wallace & Haines, 1985). Each neuropsychological test score was regressed on the presence of problem drinking separately in each year, controlling for covariates, using linear regression methods.
RESULTS
Table 1 shows characteristics at baseline in 1986 and at subsequent follow-up. Most participants in the sample were White men. The sample age at the time of cognitive testing ranged from 60 to 86 years. Alcohol consumption was consistently high, with the mean weekly consumption ranging between seven and nine drinks per week (Table 1). Between 1986 and 2003, members of the cohort decreased their intake of beer and hard liquor (p = .007 and p = .002, respectively) but increased wine consumption (p = .002). Consumption of mixed drinks and overall consumption did not change over follow-up (p = .45 and p = .29, respectively). Consequently, overall mean quantities of alcohol consumed per week did not change over time (Table 1). The Spearman's r for intake assessed by frequency compared with quantity is approximately .65 in all years alcohol use was measured.
Table 1.
Demographic, Alcohol, and Cognitive Measures From 1986 Through 2003 in 588 Men and Women Who Participated in Cognitive Function Assessments in 2005: Data From The Johns Hopkins Precursors Study
| 1986 | 1989 | 1993 | 1997 | 2003 | 2005 | |
| Number of participants, n (% of 588) | 487 (83) | 484 (82) | 489 (83) | 499 (85) | 508 (86) | 588 (100) |
| Age in years, mean (SD) | 55.5 (5.1) | 58.5 (5.1) | 62.5 (5.1) | 66.5 (5.1) | 72.5 (5.1) | |
| Hypertension, n (% yes) | 116 (20) | 138 (23) | 170 (29) | 218 (37) | 299 (51) | |
| Male, n (%) | 544 (92) | |||||
| Smoking status, n (% yes) | 41 (8) | 31 (6) | 25 (5) | 26 (5) | 21 (4) | |
| Amount of alcohol consumption, drinks per week, mean (SD) | ||||||
| Total | 8.6 (7.9) | 8.0 (7.7) | 8.5 (8.5) | 8.7 (8.3) | 8.8 (8.1) | |
| Beer | 1.6 (3.3) | 1.5 (3.3) | 1.5 (3.7) | 1.4 (3.9) | 1.3 (3.5) | |
| Hard liquor | 0.5 (1.6) | 0.4 (1.5) | 0.4 (1.9) | 0.3 (1.2) | 0.2 (1.1) | |
| Mixed drinks | 3.3 (5.4) | 2.9 (5.0) | 2.9 (5.2) | 3.1 (4.9) | 3.3 (5.1) | |
| Wine | 3.3 (4.4) | 3.2 (4.6) | 3.8 (5.5) | 4.0 (5.0) | 4.2 (5.0) | |
| Frequency of alcohol consumption, n (%) | ||||||
| Daily/almost daily | 209 (44) | 198 (42) | 212 (44) | 235 (48) | 266 (53) | |
| 3–4×/wk | 80 (17) | 106 (22) | 98 (20) | 82 (18) | 87 (17) | |
| 1–2×/wk | 100 (21) | 79 (17) | 86 (18) | 85 (17) | 72 (14) | |
| 2×/month or less | 89 (19) | 93 (20) | 86 (18) | 87 (18) | 73 (15) | |
| CAGE, n positive (%) | 161 (27) | 66 (12) | 51 (9) | 54 (10) | 66 (11) | |
| Cognitive scores, mean (SD) | ||||||
| Semantic fluency, VFT | 18.3 (4.7) | |||||
| Attention, BTA | 13.1 (4.1) | |||||
| Phonemic fluency, VFT | 42.5 (14.2) | |||||
| Verbal memory, HVLT | 21.9 (5.9) | |||||
| Global cognition, TICS | 34.3 (3.6) | |||||
Note: BTA = Brief Test of Attention; HVLT = Hopkins Verbal Learning Test; TICS = Telephone Interview for Cognitive Status; VFT = Verbal Fluency Test.
Means and standard deviations for cognitive outcomes administered over the telephone are also displayed in Table 1. Cohort members who did not undergo cognitive testing in 2005 did not differ from those who were tested in terms of alcohol consumption (data not shown; p = .11). Likewise, respondents to the telephone cognitive assessment who did not complete all cognitive tests did not differ by age, sex, smoking status, or alcohol consumption at any point in time compared with those who completed the interview (all p > .13). Persons with incomplete data did have a higher prevalence of hypertension (odds ratio [OR] = 3.5, p = .01).
Quantity of Alcohol Intake and Cognition
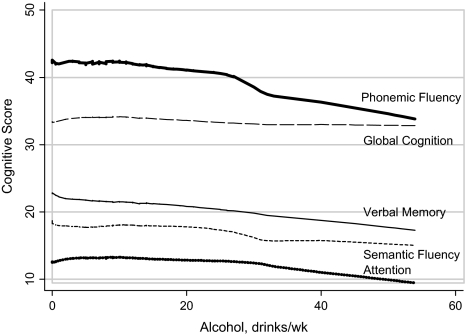
Figure 1 shows a lowess plot of cognitive scores, assessed in 2005, by level of alcohol consumption in 1993. All cognitive measures tended to be lower at higher levels of alcohol intake. This decrease was most pronounced for phonemic fluency, and in fact, parametric analysis showed this to be the only reliable effect. Analogous graphs for alcohol assessed in other years showed similar linear patterns, but the drop in phonemic fluency was less pronounced than for alcohol intake assessed in 1993.
Figure 1.
Nonparametric plot of cognitive scores by amount of alcohol consumption assessed in 1993 in 588 men and women: data from The Johns Hopkins Precursors Study.
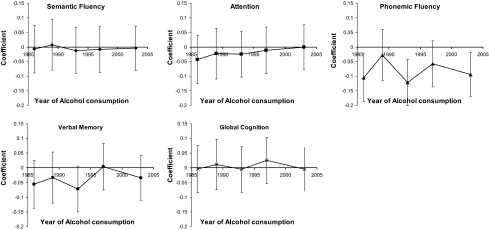
Parametric analysis yielded similar inferences. The results of five separate GEE analyses, one for each year of alcohol consumption, are shown in Figure 2. Greater quantity of alcohol intake was associated with lower mean adjusted phonemic fluency, but no associations were detected for other cognitive scores. In every year of assessment, a one drink per week greater alcohol intake was associated with lower phonemic fluency assessed in 2005. This inverse association was statistically significant in 1986 (p = .02), 1993 (p = .004), and 2003 (p = .03). The effect was strongest for alcohol intake in 1993, with a 0.12 point lower phonemic fluency score assessed 12 years later; the magnitude of this association relative to other cognitive measures is apparent from the slope of the lowess plot for phonemic fluency in Figure 1. Estimates of verbal memory were also lower for three of the five alcohol intake assessments, with the greatest difference seen in 1993 (β = −.068; p = .09), but none of the differences were statistically significant.
Figure 2.
Adjusted (all models were adjusted for age, hypertension, sex, and smoking status. Vertical bars represent 95% pointwise confidence intervals) difference in cognitive scores for a one drink per week greater alcohol consumption by year of alcohol use assessment in 588 men and women: data from the Johns Hopkins Precursors Study.
In analyses to determine the presence of quadratic relationships, attention scores in 2005 were lower both at lower and at greater quantities of alcohol intake assessed in 1986 (p = .05). No evidence of a quadratic relationship was seen for any other cognitive score. Age and cardiovascular disease status did not significantly modify the relationship between any cognitive outcome and alcohol consumption from any year (results not shown). No inferences changed when hypertensive status was not adjusted for in GEE models.
Frequency of Alcohol Intake and Cognition
In a second set of GEE models designed to assess relationships between frequency of alcohol use and cognition, cognitive scores were regressed on alcohol drinking frequency adjusting for potential confounders (Table 2). As in the analyses using quantity of alcohol intake, higher frequency of alcohol intake was associated with lower phonemic fluency. Participants who drank more than four times per week had statistically significantly lower scores than those in other drinking categories. Frequency of alcohol intake was not associated with other measures of cognition.
Table 2.
Adjusteda Differences in Cognitive Scores Associated With Frequency of Alcohol Consumption in 588 Men and Women: Data From The Johns Hopkins Precursors Study
| Difference, compared with persons drinking more than four times per week (n = 492) | |
| β (95% CI) | |
| Semantic fluency, VFT | |
| 3–4×/week | −0.81 (−2.65 to 1.02) |
| 1–2×/week | 0.10 (−1.60 to 1.80) |
| 2×/month or less | 0.41 (−1.38 to 2.19) |
| Attention, BTA | |
| 3–4×/week | 0.43 (−1.34 to 2.20) |
| 1–2×/week | −0.21 (−2.14 to 1.72) |
| 2×/month or less | 0.36 (−1.47 to 2.20) |
| Phonemic fluency, VFT | |
| 3–4×/week | 3.04* (1.23–4.86) |
| 1–2×/week | 1.96* (0.13–3.80) |
| 2×/month or less | 2.03* (0.15–3.91) |
| Verbal memory, HVLT | |
| 3–4×/week | −0.57 (−2.45 to 1.30) |
| 1–2×/week | 1.11 (−0.74 to 2.95) |
| 2×/month or less | 0.55 (−1.30 to 2.41) |
| Global cognition, TICS | |
| 3–4×/week | 0.15 (–1.63 to 1.93) |
| 1–2×/week | −0.10 (–2.07 to 1.87) |
| 2×/month or less | −0.09 (−1.99 to 1.81) |
Note: BTA = Brief Test of Attention; HVLT = Hopkins Verbal Learning Test; TICS = Telephone Interview for Cognitive Status; VFT = Verbal Fluency Test.
All models were adjusted for age, hypertension status, sex, and current smoking status. The reference group for alcohol frequency coefficients is daily or almost daily drinkers.
p < .05.
Change in Alcohol Consumption Over Time and Aognition
In random effects models, average alcohol intake increased by 0.02 drinks per week annually over the 17 years of follow-up (95% CI: −0.013 to 0.053). There was significant interindividual variability (p < .001). We regressed cognitive scores on individual linear trajectories of change in alcohol consumption over the 17-year period estimated from the random effects model, adjusting for age, hypertension in 2003, smoking status in 2003, sex, and 1986 alcohol consumption. The random slopes represent annual changes in alcohol consumption. In linear regressions, none of the measures of cognition was associated with change in overall alcohol consumption over the 17 years ranging between midlife and later life: semantic fluency, β = −0.43 (95% CI: −3.29 to 2.44); attention, β = 1.43 (95% CI: −1.01 to 3.88); phonemic fluency, β = −2.72 (95% CI: −11.75 to 6.30); verbal memory, β = −0.7 (95% CI: −4.61 to 3.22); and global cognition, β = −0.12 (95% CI: −2.14 to 1.91).
Problem Drinking and Cognition
In analyses testing the association between problem drinking, defined by the CAGE at each assessment of alcohol use, with cognitive ability adjusted for age, hypertension, sex, and smoking status, no associations were seen for any of the cognitive scores (all p > .13).
DISCUSSION
Our objective was to test the prospective association of alcohol consumption, measured repeatedly in a longitudinal study, with domain-specific cognitive abilities assessed up to 17 years after the first measure of alcohol intake. We found a prospective association between higher alcohol consumption, both quantity and frequency, and lower phonemic fluency for alcohol intake assessed 2, 12, and 17 years prior to the cognitive assessment. Our data suggest that alcohol intake three to four times per week or low levels of drinks per week through midlife and into later life confer the best cognitive outcomes in old age, as defined by word-finding ability in late life, a measure of executive function. These relationships were independent of age, smoking status, hypertension, sex, and correlations with other cognitive test scores. Change in alcohol use and problem drinking were not related to any measures of cognitive ability.
We found that the heaviest drinkers (those who drank more than four times per week) had the lowest phonemic fluency scores. This result is consistent with previous findings using the VFT (Britton et al., 2004; Elias et al., 1999) and other tests of executive function (e.g., Reid et al., 2006). Our results suggest that higher alcohol consumption in midlife may impair elements of executive function in late life but that semantic fluency is unaffected by alcohol consumption. Similar associations have been found using the HVLT and other tests of memory that also rely on executive abilities (McDougall et al., 2006; Reid et al.; Rodgers et al., 2005). The HVLT is a measure of verbal episodic memory, or the acquisition of new information (Albert, 2008), but because its items are semantically related words, some degree of executive control is required to strategically organize words and perform well (Woods et al., 2005). Phonemic fluency is similar in that it taps memory functions and also requires executive information processing functions to initiate and maintain systematic search strategies for information given an ambiguous cue. Impaired phonemic and semantic fluency are both predictive of dementia. Among dementia patients, semantic fluency is more impaired than phonemic fluency, although it is relatively invariant with age and relies less on executive control because the category needed for recall is provided to the respondent (Henry, Crawford, & Phillips, 2004). Alcohol consumption was not associated with semantic fluency in our study.
The meta-analysis by Peters and colleagues (2008) reported that alcohol intake of one to six drinks per week may be protective against Alzheimer’s disease. Our study included only six Alzheimer’s disease cases who underwent a cognitive assessment in 2005, which is not enough to detect meaningful differences in alcohol consumption levels by dementia status. The association of drinking with lower phonemic fluency in our study is consistent with an increased risk for dementia because although episodic memory distinguishes dementia from normal age-related cognitive decline, reduced executive functioning is also apparent and may precede declines in memory (Carlson, Xue, Zhou, & Fried, 2009).
This study has a number of strengths. First, the Precursors cohort has been followed for several decades and is well characterized. In the present analysis, cognitive abilities were assessed at around age 72 years, 17 years after the first assessment of alcohol use, a longer duration than most other prospective studies that have addressed this question. Second, the Precursors Study is comprised of mainly White men who attended medical school. Thus, participants were selected at baseline for a high and homogenous level of cognitive ability. In contrast, most studies try to account for baseline differences in cognitive abilities by statistically adjusting for years of education, social status, reading ability, or test scores collected at the start of a study (Britton et al., 2004; Elias et al., 1999; Krahn et al., 2003), which offer incomplete metrics of one’s cognitive function. A third strength of our sample is a comparatively higher level of drinking than is found in population-based samples, providing a more normally distributed and wider range of alcohol consumption. In many studies among population-dwelling older adults in the United States, less than half of samples report any drinking (Bond et al., 2005; McDougall et al., 2006; Stampfer et al., 2005). Fourth, we used a battery of cognitive measures to assess domain-specific associations between alcohol and cognition.
In view of the strengths of this study, several limitations should be highlighted. First, the Precursors Study is an observational cohort study, not a clinical trial. Therefore, participants choose how much alcohol to drink. Changes in executive function or memory ability might lead to changes in patterns of alcohol use, creating either a spurious association or a bias toward the null. Such a bias is most likely to be seen in cross-sectional analyses, when alcohol use and cognition are assessed at the same time, but less likely in a prospective study because alcohol intake is assessed long before the determination of cognitive function. A second limitation is that detected associations might be the product of differential loss to follow-up. However, this is probably not the case in this study as 69% of those who answered the alcohol information in 1986 received neuropsychological testing in 2005. Third, some evidence suggests that alcohol consumption may have a stronger effect in older women than in older men (Elias et al., 1999; Kalmijn et al., 2002; Lang et al., 2007; Mann & Folts, 2004; Rodgers et al., 2005). Our study contains too few women to test for effect modification by sex. When we limited our analyses to men, however, coefficient magnitudes and directions did not differ considerably (analyses not shown). Fourth, we were unable to compare long-term cognitive outcomes among never-drinkers versus drinkers because there were very few lifetime abstainers (n = 5). Fifth, we were unable to study cognitive change or decline because cognitive abilities were measured only once in this cohort. Sixth, our findings may have limited generalizability to non-White, female, or less-educated persons or to groups with different patterns and levels of alcohol intake. However, our findings are consistent with other studies, and we found no reports suggesting that effect of alcohol on cognition is modified by level of education. A seventh potential limitation is that cognitive assessments were collected over a telephone; however, studies indicate that telephone cognitive assessments are valid (Crooks, Parsons, & Buckwalter, 2007) and some cognitive instruments are designed specifically for telephone use (e.g., Brandt et al., 1988). Eighth, we did not model a cumulative dose of alcohol to test associations with cognition. In regard to the associations found over time, however, it appeared that the magnitude of the effect of alcohol with phonemic fluency did not vary too considerably by length of follow-up. Finally, the power in our sample to detect significant associations was limited due to sample size and may be a reason we found few associations between alcohol intake and different cognitive ability measures. However, we were able to find significant associations between alcohol on phonemic fluency despite the sizable variability in that cognitive variable (Table 1).
This study has several important implications. Our data suggest that alcohol use in midlife should be maintained at low to moderate levels to support cognitive health in older age. Clinicians should assess alcohol intake in persons who present with impaired phonemic fluency. From an observational study such as ours, it is not possible to know for certain that decreasing alcohol intake will maintain or improve phonemic fluency. Given the near impossibility of carrying out long-term clinical trials of alcohol intake with cognitive outcomes, however, it is advisable for individuals with impaired phonemic fluency who drink more than three to four drinks per week to decrease their alcohol intake. Future research examining alcohol and cognition should take advantage of domain-specific measures of cognition, particularly verbal fluency, rather than of global or summary measures.
FUNDING
This work was supported by the National Institute on Aging (AG01760), the National Institute of Diabetes and Digestive and Kidney Diseases (K24 DK02856 to M.J.K.), and National Center for Research Resources (UL1 RR025005 to N.-Y.W.).
CONFLICT OF INTEREST
None of the authors have any financial, commercial, or other conflict of interests or connections, direct or indirect, that influenced the preparation of this manuscript. Human participant protection: All study procedures were approved by the Institutional Review Board (Johns Hopkins Medical Institution, Baltimore, Maryland).
References
- Albert M. The neuropsychology of the development of Alzheimer's disease. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 4th ed. London: Academic Press; 2008. pp. 97–132. [Google Scholar]
- Anstey KJ, Windsor TD, Rodgers B, Jorm AF, Christensen H. Lower cognitive test scores observed in alcohol abstainers are associated with demographic, personality, and biological factors: The PATH Through Life Project. Addiction. 2005;100:1291–1301. doi: 10.1111/j.1360-0443.2005.01159.x. [DOI] [PubMed] [Google Scholar]
- Anttila T, Helkala EL, Viitanen M, Kåreholt I, Fratiglioni L, Winblad B, Soininen H, Tuomilehto J, Nissinen A, Kivipelto M. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: A prospective population based study. British Medical Journal. 2004;329:539. doi: 10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armor D, Pohch J, Stambul H. Reliability and validity of self-reported drinking behavior. In: Armor D, Polich J, Stumble H, editors. Alcoholism and treatment. New York: John Wiley; 1978. pp. 173–211. [Google Scholar]
- Bernadt MW, Mumford J, Taylor C, Smith B, Murray RM. Comparison of questionnaire and laboratory tests in the detection of excessive drinking and alcoholism. Lancet. 1982;1:325–328. doi: 10.1016/s0140-6736(82)91579-3. [DOI] [PubMed] [Google Scholar]
- Bond GE, Burr RL, McCurry SM, Rice MM, Borenstein AR, Larson EB. Alcohol and cognitive performance: A longitudinal study of older Japanese Americans. The Kame project. International Psychogeriatrics. 2005;17:1–16. doi: 10.1017/S1041610205001651. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict R. Hopkins verbal learning test—Revised: Professional manual. Odessa, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1988;1:111–117. [Google Scholar]
- Britton A, Singh-Manoux A, Marmot M. Alcohol consumption and cognitive function in the Whitehall II Study. American Journal of Epidemiology. 2004;160:240–247. doi: 10.1093/aje/kwh206. [DOI] [PubMed] [Google Scholar]
- Carlson MC, Xue Q, Zhou J, Fried LP. Executive decline and dysfunction precedes declines in memory: The Women's Health and Aging Study II. Journal of Gerontology: Series A: Biological and Medical Sciences. 2009;64A:110–117. doi: 10.1093/gerona/gln008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervilla JA, Prince M, Joels S, Lovestone S, Mann A. Long-term predictors of cognitive outcome in a cohort of older people with hypertension. British Journal of Psychiatry. 2000;177:66–71. doi: 10.1192/bjp.177.1.66. [DOI] [PubMed] [Google Scholar]
- Cleveland WS. Robust locally weighted regression and smoothing scatterplots. Journal of the American Statistical Association. 1979;74:829–836. [Google Scholar]
- Conigliaro J, Kraemer K, McNeil M. Screening and identification of older adults with alcohol problems in primary care. Journal of Geriatric Psychology and Neurology. 2000;13:106–114. doi: 10.1177/089198870001300303. [DOI] [PubMed] [Google Scholar]
- Crooks VC, Parsons TD, Buckwalter JG. Validation of the Cognitive Assessment of Later Life Status (CALLS) instrument: A computerized telephonic measure. BMC Neurology. 2007;7:10. doi: 10.1186/1471-2377-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, van Duijn CM, Hofman A, Breteler MM. Alcohol intake in relation to brain magnetic resonance imaging findings in older persons without dementia. American Journal of Clinical Nutrition. 2004;80:992–997. doi: 10.1093/ajcn/80.4.992. [DOI] [PubMed] [Google Scholar]
- Dufour M, Fuller RK. Alcohol in the elderly. Annual Review of Medicine. 1995;46:123–132. doi: 10.1146/annurev.med.46.1.123. [DOI] [PubMed] [Google Scholar]
- Elias PK, Elias MF, D’Agnostino RB, Silbershatz H, Wolf PA. Alcohol consumption and cognitive performance in the Framingham Heart Study. American Journal of Epidemiology. 1999;150:580–589. doi: 10.1093/oxfordjournals.aje.a010056. [DOI] [PubMed] [Google Scholar]
- Elwood PC, Gallacher JEJ, Hopkinson CA, Pickering J, Rabbitt P, Stollery B, Brayne C, Huppert FA, Bayer A. Smoking, drinking, and other life style factors and cognitive function in men in the Caerphilly cohort. Journal of Epidemiological Community Health. 1999;53:9–14. doi: 10.1136/jech.53.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Gu L, Masaki KH, Langer RD, Coker LH, Stefanick ML, Ockene J, Rapp SR. Association between reported alcohol intake and cognition: Results from the Women's Health Initiative Memory Study. American Journal of Epidemiology. 2005;161:228–238. doi: 10.1093/aje/kwi043. [DOI] [PubMed] [Google Scholar]
- Ewing JA. Detecting alcoholism: The CAGE questionnaire. Journal of the American Medical Association. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- Flicker L, Almeida OP, Acres J, Le MT, Tuohy RJ, Jamrozik K, Hankey G, Norman P. Predictors of impaired cognitive function in men over the age of 80 years: Results from the Health in Men Study. Age and Ageing. 2005;34:77–80. doi: 10.1093/ageing/afh235. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical guide for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fontana P, Mooser V, Bovet P, Shamlaye C, Burnand B, Lenain V, Marcovina SM, Riesen W, Darioli R. Dose-dependent inverse relationship between alcohol consumption and serum Lp(a) levels in black African males. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:1075–1082. doi: 10.1161/01.atv.19.4.1075. [DOI] [PubMed] [Google Scholar]
- Galanis DJ, Joseph C, Masaki KH, Petrovitch H, Ross GW, White L. A longitudinal study of drinking and cognitive performance in elderly Japanese American men: The Honolulu-Asia Aging Study. American Journal of Public Health. 2000;90:1254–1259. doi: 10.2105/ajph.90.8.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M, VanderBilt J, Saxton JA, Shen C, Dodge HH. Alcohol consumption and cognitive function in late life: A longitudinal community study. Neurology. 2005;65:1210–1217. doi: 10.1212/01.wnl.0000180520.35181.24. [DOI] [PubMed] [Google Scholar]
- Gaziano KM, Buring JE, Breslow JL, Goldhaber SZ, Rosner B, VanDenburgh M, Willett W, Hennekens CH. Moderate alcohol intake, increased levels of high density lipoprotein and its subfraction, and decreased risk of myocardial infarction. New England Journal of Medicine. 1993;329:1829–1834. doi: 10.1056/NEJM199312163292501. [DOI] [PubMed] [Google Scholar]
- Harrison JE, Buxton P, Husain M, Wise R. Short test of semantic and phonological fluency: Normal performance, validity and test–retest reliability. British Journal of Clinical Psychology. 2000;39:181–191. doi: 10.1348/014466500163202. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Beckett LA, Albert MS, Rosner B, Taylor JO, Evans DA. Relation of smoking and low-to-moderate alcohol consumption to change in cognitive function: A longitudinal study in a defined community of older persons. American Journal of Epidemiology. 1993;137:881–891. doi: 10.1093/oxfordjournals.aje.a116749. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer's type: A meta-analysis. Neuropsychologia. 2004;42:1212–1222. doi: 10.1016/j.neuropsychologia.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, van Boxtel MPJ, Verschuren MWM, Jolles J, Launer LJ. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. American Journal of Epidemiology. 2002;156:936–944. doi: 10.1093/aje/kwf135. [DOI] [PubMed] [Google Scholar]
- Karlamangla A, Zhou K, Reuben D, Greendale G, Moore A. Longitudinal trajectories of heavy drinking in adults in the United States of America. Addiction. 2006;101:91–99. doi: 10.1111/j.1360-0443.2005.01299.x. [DOI] [PubMed] [Google Scholar]
- Kiechl S, Willeit J, Rungger G, Egger G, Oberhollenzer F, Bonora E. Alcohol consumption and atherosclerosis: What is the relation? Stroke. 1998;29:900–907. doi: 10.1161/01.str.29.5.900. [DOI] [PubMed] [Google Scholar]
- Klag MJ, He J, Mead LA, Ford DE, Pearson TA, Levine DM. Validity of physicians’ self-reports of cardiovascular disease risk factors. Annals of Epidemiology. 1993;3:442–447. doi: 10.1016/1047-2797(93)90074-e. [DOI] [PubMed] [Google Scholar]
- Klag MJ, Wang NY, Meoni LA, Brancati FL, Cooper LA, Liang KY, Young JH, Ford DE. Coffee intake and risk of hypertension: The Johns Hopkins Precursors Study. Archives of Internal Medicine. 2002;162:657–662. doi: 10.1001/archinte.162.6.657. [DOI] [PubMed] [Google Scholar]
- Krahn D, Freese J, Hauser R, Barry K, Goodman B. Alcohol use and cognition at mid-life: The importance of adjusting for baseline cognitive ability and educational attainment. Alcoholism: Clinical and Experimental Research. 2003;27:1162–1166. doi: 10.1097/01.ALC.0000078060.18662.C1. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random effects models for longitudinal data: An overview of recent results. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lang I, Wallace RB, Huppert FA, Melzer D. Moderate alcohol consumption in older adults is associated with better cognition and well-being than abstinence. Age and Ageing. 2007;36:256–261. doi: 10.1093/ageing/afm001. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Feskens EJM, Kalmijn S, Kromhout D. Smoking, drinking, and thinking: The Zutphen Elderly Study. American Journal of Epidemiology. 1996;143:219–227. doi: 10.1093/oxfordjournals.aje.a008732. [DOI] [PubMed] [Google Scholar]
- Leroi I, Sheppard JM, Lyketsos CG. Cognitive function after 11.5 years of alcohol use: Relation to alcohol use. American Journal of Epidemiology. 2002;156:747–752. doi: 10.1093/aje/kwf107. [DOI] [PubMed] [Google Scholar]
- Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lyketsos CG, Chen LS, Anthony JC. Cognitive decline in adulthood: An 11.5-year follow-up of the Baltimore Epidemiologic Catchment Area study. American Journal of Psychiatry. 1999;156:58–65. doi: 10.1176/ajp.156.1.58. [DOI] [PubMed] [Google Scholar]
- Mann LB, Folts JD. Effects of ethanol and other constituents of alcoholic beverages on coronary heart disease: A review. Pathophysiology. 2004;10:105–112. doi: 10.1016/j.pathophys.2003.10.011. [DOI] [PubMed] [Google Scholar]
- McCaul KA, Almeida OP, Hankey GJ, Jamrozik K, Byles JE, Flicker L. Alcohol use and mortality in older men and women. Addiction. 2010;105:1391–1400. doi: 10.1111/j.1360-0443.2010.02972.x. [DOI] [PubMed] [Google Scholar]
- McDougall GJ, Jr., Becker H, Areheart KL. Older males, cognitive function, and alcohol consumption. Issues in Mental Health Nursing. 2006;27:337–353. doi: 10.1080/01612840600569609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ, Jr., Becker H, Delville CL, Vaughan PW, Acee TW. Alcohol use and older adults–A little goes a long way. International Journal of Disability and Human Development. 2007;6:431–440. doi: 10.1901/jaba.2007.6-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire LC, Ajani UA, Ford ES. Cognitive functioning in late life: The impact of moderate alcohol consumption. Annals of Epidemiology. 2007;17:93–99. doi: 10.1016/j.annepidem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Midanik L. The validity of self-reported alcohol consumption and alcohol problems: A literature review. Addiction. 1982;77:357–382. doi: 10.1111/j.1360-0443.1982.tb02469.x. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: A review of the literature. Alcoholism and Alcoholism. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. Journal of the American Medical Association. 2003;289:1405–1413. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- Pernanen K. Validity of survey data on alcohol use. In: Gibbins RJ, Israel Y, Kalant H, Popham RE, Schmidt W, Smart RG, editors. Research advances in alcohol and drug problems. New York: John Wiley; 1974. pp. 355–374. [Google Scholar]
- Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: A systematic review. Age and Ageing. 2008;37:505–512. doi: 10.1093/ageing/afn095. [DOI] [PubMed] [Google Scholar]
- Rabbia F, Veglio F, Russo R, Schiavone D, Oliva S, Chiandussi L. Role of alcoholic beverages in essential hypertensive patients. Alcohol and Alcoholism. 1995;30:433–439. [PubMed] [Google Scholar]
- Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Fowkes FG. Cardiovascular diseases and decline in cognitive function in an elderly community population: The Edinburgh Artery Study. Psychosomatic Medicine. 2007;69:425–434. doi: 10.1097/psy.0b013e318068fce4. [DOI] [PubMed] [Google Scholar]
- Reid MC, Van Ness PH, Hawkins KA, Towle V, Concato J, Guo Z. Light to moderate alcohol consumption is associated with better cognitive function among older male veterans receiving primary care. Journal of Geriatric Psychiatry and Neurology. 2006;19:98–105. doi: 10.1177/0891988706286513. [DOI] [PubMed] [Google Scholar]
- Rodgers B, Windsor TD, Anstey KJ, Dear KB, Jorm A, Christensen H. Non-linear relationships between cognitive function and alcohol consumption in young, middle-aged and older adults: The PATH Through Life Project. Addiction. 2005;100:1280–1290. doi: 10.1111/j.1360-0443.2005.01158.x. [DOI] [PubMed] [Google Scholar]
- Russell M, Welte JW, Barnes GM. Quantity-frequency measures of alcohol consumption: Beverage-specific vs global questions. British Journal of Addiction. 1991;86:409–417. doi: 10.1111/j.1360-0443.1991.tb03418.x. [DOI] [PubMed] [Google Scholar]
- Sano M, Wendt PE, Wirsen A, Stenberg G, Risberg J, Ingvar DH. Acute effects of alcohol on regional cerebral blood flow in man. Journal of Studies on Alcohol. 1993;54:369–376. doi: 10.15288/jsa.1993.54.369. [DOI] [PubMed] [Google Scholar]
- Schretlen D. Brief test of attention. Odessa, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- Simons LA, McCallum J, Friedlander Y, Ortiz M, Simons J. Moderate alcohol intake is associated with survival in the elderly: The Dubbo study. Medical Journal of Australia. 2000;173:121–123. doi: 10.5694/j.1326-5377.2000.tb125562.x. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F. Effects of moderate alcohol consumption on cognitive function in women. New England Journal of Medicine. 2005;352:245–253. doi: 10.1056/NEJMoa041152. [DOI] [PubMed] [Google Scholar]
- Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: A systematic literature review. Social Science & Medicine. 1999;48:445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical, and Experimental Research. 2001;25:236–245. [PubMed] [Google Scholar]
- Wallace P, Haines A. Use of a questionnaire in general practice to increase the recognition of patients with excessive alcohol consumption. British Medical Journal. 1985;290:1949–1953. doi: 10.1136/bmj.290.6486.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn R, Duka T. Acute alcohol effects on frontal lobe function in social drinkers: Their relationship to personality traits and drinking habits. Psychopharmacology. 2003;165:306–312. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]
- Willet WC, Stampfer MJ. Rebuilding the food pyramid. Scientific American. 2003;288:64–69. doi: 10.1038/scientificamerican0103-64. [DOI] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Conover E, Marcotte TD, Heaton RK, Grant I, HIV Neurobehavioral Research Center Group Test–retest reliability of component process variables within the Hopkins Verbal Learning Test–Revised. Assessment. 2005;12:96–100. doi: 10.1177/1073191104270342. [DOI] [PubMed] [Google Scholar]
- Wright CB, Elkind MS, Luo X, Paik MC, Sacco RL. Reported alcohol consumption and cognitive decline: The northern Manhattan study. Neuroepidemiology. 2006;27:201–207. doi: 10.1159/000096300. [DOI] [PMC free article] [PubMed] [Google Scholar]