Abstract
Objective.
It is unclear whether implicit probabilistic learning, the acquisition of regularities without intent or explicit knowledge, declines with healthy aging.
Methods.
Because age differences in previous work might reflect motor or rule learning deficits, we used the implicit Triplets Learning Task with reduced motor sequencing and non–rule-based associations. Fifteen young and 15 old adults responded only to the last event in a series of discrete 3-event sequences or triplets. A randomly chosen set of triplets occurred with high frequency, so there was no underlying rule to be learned.
Results.
Both age groups learned associative regularities, but age differences in favor of the young emerged with practice.
Discussion.
Age differences may reflect the different neural regions that are involved as training progresses, which differ in the extent to which they are compromised by aging.
Keywords: Aging, Implicit learning, Probabilistic associations, Sequence learning
OUR world is largely stable, in that over time, people, places, and things show up in predictable sequences. Learning about such environmental regularities involves becoming sensitive to the order in which events typically occur, regardless of whether or not the sequences have rule-governed structure. For example, in structured language, people can learn different probabilities between phonemes to segment speech into word-like units (Kuhl, 2004), and in unstructured social interactions, people can learn that subtle sequences in facial expressions are associated with one emotional outcome more than another (Lieberman, 2000). Such implicit learning often occurs without intent and explicit knowledge of what has been learned (Frensch, 1998).
It is often thought that implicit learning is spared in healthy aging relative to explicit learning, but this claim treats implicit learning as if it is unitary (Zacks, Hasher, & Li, 2000). In fact, there are many kinds of implicit learning that differ in both the nature of the regularity to be learned and in their neural substrates (Forkstam & Petersson, 2005). Much of the research on aging and implicit learning has focused on learning deterministic sequential regularities (i.e., those where an event perfectly predicts subsequent events, with studies typically showing no age differences; Cherry & Stadler, 1995; Daselaar, Rombouts, Veltman, Raaijmakers, & Jonker, 2003; Dennis, Howard, & Howard, 2006, Experiments 1 and 2; Gaillard, Destrebecqz, Michiels, & Cleeremans, 2009; D. V. Howard & Howard, 1989, 1992; Salthouse, McGuthry, & Hambrick, 1999). However, most sequences we encounter in daily life are not deterministic, but rather are probabilistic, such that an event predicts subsequent events with some uncertainty. Fewer studies have investigated how aging influences probabilistic learning, and the available evidence is inconclusive. Although some studies suggest age invariance (Aizenstein et al., 2006; Fera et al., 2005), most studies reveal age-related deficits in learning, especially as training increases (Bennett, Howard, & Howard, 2007; J. H. Howard & Howard, 1997; J. H. Howard, Howard, Dennis, & Yankovich, 2007; J. H. Howard, Howard, Dennis, Yankovich, & Vaidya, 2004; D. V. Howard et al., 2004).
Here, we investigate implicit sequential probabilistic learning using the Triplets Learning Task (TLT; J. H. Howard, Howard, Dennis, & Kelly, 2008). The TLT was designed to mimic some characteristics of widely used serial reaction time (RT) tasks (J. H. Howard & Howard, 1997; Nissen & Bullemer, 1987) while omitting motor sequencing and enabling precise control over event timing. In the TLT, participants view four open circles that become solid red or green in discrete, sequentially ordered three-event trials or “triplets.” On each trial, participants observe two red cues and only respond to a third green target by pressing corresponding buttons. This provides a continuous performance-based measure of learning without motor sequencing, because responses are only made to the target, not to each event. Triplets were originally designed to incorporate the rule-based structure of a probabilistic serial RT task (see J. H. Howard & Howard, 1997) in that the location of the first or second cue predicted the location of the target (J. H. Howard et al., 2008). Furthermore, a given cue location always predicted only one highly likely target, and a given target was always highly predicted by only one cue location. As in the probabilistic serial RT task, learning is revealed in the TLT by people responding more quickly and accurately to high relative to low probability (LP) triplets with practice, without any explicit knowledge of what has been learned.
Only one published study has used the TLT to study aging (J. H. Howard et al., 2008). Results showed that older adults can learn the subtle, probabilistic sequential regularities embedded in the TLT, but not as well as young. This age-related deficit in learning could not be due to age-related declines in fine motor finger movements (Smith et al., 1999) because participants did not perform motor sequences. Nor could the age deficit be due to age differences in event timing that occur in serial RT tasks as the result of older adults’ slower and more variable response times (see Salthouse, 2000). Finally, the age deficit in learning in the TLT could not be due to known age differences in explicit learning (e.g., Dixon et al., 2004) because sensitive recognition tests indicated that participants did not gain declarative knowledge of the regularities. Instead, age deficits in this task were attributed to declines in implicit probabilistic learning, such that older adults are impaired in their ability to form associations among events that are probabilistically related (J. H. Howard et al., 2008).
However, an alternative interpretation of previous findings is that age-related declines in learning may result from the presence of rule-based regularities within the triplets. Because age-related declines have previously been observed in second language acquisition, which calls on such rule-based structured probabilistic learning (Hakuta, Bialystok, & Wiley, 2003), age differences in the TLT may have been due to declines in nonconscious sensitivity to this rule-governed structure, rather than to a general deficit in learning probabilistic associations.
To examine this possibility, the present study used a version of the TLT in which all rule-governed structure was eliminated by randomly selecting a subset of triplets to occur more frequently than others. Thus, the predictive relationships between the cue locations and target were arbitrary. If previously observed age differences in probabilistic learning were due to age-related deficits in sensitivity to rule-based structure, then old adults should learn as well as young in this version of the TLT. If instead, as we predict, previously observed age differences reflect generalized age-related declines in probabilistic associative learning, then age differences favoring young adults should persist.
METHODS
Participants
Fifteen young (M = 19.0 ± 0.9 years; range 18–21 years; 6 male) and 15 healthy old adults (M = 71.3 ± 6.0 years; range 66–87 years; 5 male) were recruited from Georgetown University and the community by advertisements in the Washington Post Health Section. Participants received either monetary compensation or course credit. The Georgetown University Institutional Review Board approved the experimental procedures, and all participants gave informed consent. To screen for dementia, older participants completed the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975), and all scored ≥27, though scores were not obtained for two old adults.
Materials and Procedure
Stimuli were programmed, generated, and presented using E-Prime (Psychological Software Tools, Inc., Pittsburgh, PA). Participants viewed four open circles on a computer screen. Each trial or triplet consisted of two consecutive cue events (circles filled in red) followed by the target (a circle filled in green). Participants were asked to observe the first two events and respond to only the third, target event location, by pressing one of four corresponding buttons with their dominant hand. Red cues were displayed for 120 ms and the green target remained in view until participants made a correct response to its location, with 650 ms separating the correct response and the first cue on the following trial.
The version of the TLT used here has three important differences compared with earlier experiments. First, we used a shortened version of the TLT, reducing the original version from 6,000 to 750 trials (J. H. Howard et al., 2008). Second, repetitions (e.g., 111, 222) and trills (e.g., 141, 232) were not presented during the learning trials because previous studies have shown that responses to these stimuli reflect preexisting response tendencies (i.e., perceptual and motor priming; Cleeremans & McClelland, 1991) or negative recency (Boyer, Destrebecqz, & Cleeremans, 2005), in addition to learning. Finally, a randomly chosen set of 16 triplets occurred with high probability (HP) and the 32 remaining triplets occurred with LP. In other words, rather than selecting the HP triplets to conform to rules reflecting first- or second-order sequential structure, as in previous TLT studies (Bennett, Romano, Howard, & Howard, 2008; J. H. Howard et al., 2008), here the 16 HP triplets were chosen at random. As a result of this random selection, the four possible target positions did not occur equally often. For counterbalancing purposes, each participant received one of five different random assignments of HP triplets and the two age groups received the same assignments. The frequency of HP to LP triplets was approximately 9-to-1 throughout training.
Participants were not informed of any regularities; their only instruction was to respond as quickly and accurately as possible to the target event on each trial. Participants completed three epochs of 250 trials that were divided into five blocks of 50 trials each, with breaks provided intermittently (total task ∼30 min). Mean RT and accuracy were displayed to participants at each break, in an attempt to match the two age groups’ overall accuracy at approximately 92%; based on accuracy, participants were instructed to “focus more on speed,” “focus more on accuracy,” or “speed and accuracy are about right.”
A sensitive measure of explicit awareness followed completion of the TLT. Participants viewed a random sample from the 64 possible triplets that included HP and LP triplets, in addition to novel triplet combinations (i.e., trills and repetitions). Participants were presented with 64 trials that included, on average, 15 HP triplets (M: 14.96 ± 3.37), 31 LP triplets (M: 31.12 ± 3.69), 12 trills (M: 12.00 ± 3.03), and 6 repetitions (M: 5.93 ± 2.39). Triplets were presented one at a time on the computer screen with the same within-triplet timing rate as in training. Participants were asked to judge whether each triplet had occurred “more often” or “less often” by responding with a button press of “2” or “1,” respectively, and to guess if unsure.
We matched the recognition task to the learning phase by displaying each of the triplets in colored events that simulated the testing phase (i.e., two red and one green event) as compared with earlier work, which displayed triplets in the recognition task as a series of black events (Bennett et al., 2008; J. H. Howard et al., 2008). This ensures that any inability to distinguish between high- and low-frequency triplets in the present study cannot be due to changes in perceptual processes between the TLT and recognition tasks.
RESULTS
Implicit Probabilistic Learning
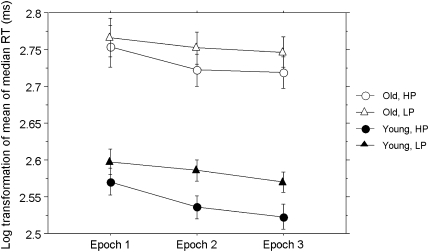
Median RT were determined for correct responses for each triplet type for each participant in each block. Overall accuracy was high (∼94%), so few trials were omitted. These medians were then averaged across blocks to obtain a single mean RT for each participant for HP and LP triplets for each of the three epochs, as displayed in Figure 1. An analysis of variance (ANOVA) was performed on logarithmic transformations of these values to control for the effect of general slowing in older adults. A similar procedure was used to calculate the mean accuracy for each participant for each triplet type.
Figure 1.
Log-transformed mean of median reaction times (RT). The log transformations of mean of median RT, in milliseconds, over epochs for high probability (HP) and low probability (LP) triplets by age group. Error bars represent the standard error of the mean.
To examine implicit learning, Group (young and old) × Triplet Type (HP and LP) × Epoch (1–3) mixed-design ANOVAs were conducted separately for accuracy and log-transformed RT measures. For accuracy, learning of triplet frequency was revealed as a main effect of triplet type, F(1, 28) = 25.5, p < .0001, reffect = .69, with more accurate responses to HP (95.5 ± 0.03%) versus LP (91.9 ± 0.07%) triplets. The only other effect to approach significance was that of age group; old adults were only marginally more accurate than young adults, F(1, 28) = 3.77, p = .06, reffect = .34, showing that, as intended, the feedback provided after every block resulted in similar overall accuracy for the two age groups.
The lack of significant main effects (p’s > .06) or interactions (p’s > .13) with age for accuracy simplified interpretation of the RT data (e.g., no speed–accuracy trade offs). As is typical for RT, a main effect of group, F(1, 28) = 44.32, p < .0001, reffect = .78, revealed that young adults responded significantly faster overall than old adults. Skill learning was revealed by a main effect of Epoch, with overall RT decreasing across epochs, F(2, 56) = 11.72 p < .0001, reffect = .54. Learning of probabilistic triplet frequencies was also revealed via a main effect of triplet type, F(1, 28) = 64.30, p < .0001, reffect = .83, as well as a Triplet Type × Epoch interaction, F(1, 28) = 5.73, p < .01 reffect = .43, with faster responses to HP versus LP triplets that increased over epochs. A significant Group × Triplet Type interaction, F(1, 28) = 4.88, p < .05 reffect = .41, showed that this difference in responding to HP and LP triplets was greater for young than for old adults. No other interactions were significant, p’s > .72.
Given the lack of a three-way interaction on the analysis of the RT data, the Group × Triplet Type interaction provides inconclusive evidence that three are age differences in probabilistic, associative learning. Moreover, two aspects of the task further limit the interpretation of this result. First, the large overall group difference in RT makes it problematic to directly compare the magnitude of the difference between high and low events across the ages (see Curran, 1997). Second, and more importantly, in using arbitrary non–rule-based regularities, it was not possible to equate target frequency without adding additional constraints on the triplets. So, the previous interaction may reflect frequency-based responding to the target events, in addition to probabilistic associative learning. In other words, because the four possible target positions did not occur equally often for each participant, faster RTs for HP triplets versus LP triplets, and any age-related differences therein, may be influenced by target frequency as well as by triplet frequency.
Therefore, similar to J. H. Howard and colleagues (2008), we used a measure of sequence-specific associative learning that was not influenced by overall RT. Namely, for each participant on each epoch, we determined the median RT for each unique triplet (e.g., 134) and we then correlated those RT with the number of times that each individual triplet actually occurred (i.e., the triplets’ actual frequency of occurrence). To remove any contribution of simple target frequency, partial correlations were computed between triplet frequency and median RT, with target frequency as a covariate. If a participant had learned nothing about triplet probabilities, this correlation would be 0, whereas if (s)he had learned a lot, the correlation would be highly negative (i.e., triplets occurring with HP would be responded to more quickly; J. H. Howard et al., 2008). For ease of interpreting these values, we multiplied each correlation by −1 to obtain an associative learning score, such that higher scores reflect greater sequence learning.
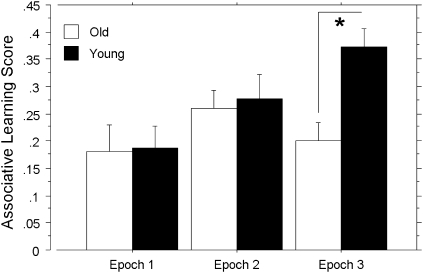
The means of the associative learning scores for each age group for each of the three epochs are shown in Figure 2. A mixed-design Group × Epoch ANOVA revealed main effects of epoch, showing that the associative learning scores increased with practice, F(2, 56) = 4.39, p < .05, reffect = .47, and a marginal effect of group, in that young adults tended to have higher associative learning scores than the old, F(1, 28) = 3.45, p = .07, reffect = .33. Most important, a Group × Epoch interaction revealed that age differences in favor of the young varied across epochs, F(2, 56) = 3.16, p = .05, reffect = .38. Post hoc t tests revealed that the associative learning scores were significantly greater for the young than for the old only on Epoch 3, t(28) = 3.67, p < .01, reffect = .33. Subsequent single sample t tests indicated that associative learning scores were significantly greater than 0 for all three epochs in both young, Epoch 1: t(14) = 4.66, p < .001, reffect = .61; Epoch 2: t(14) = 6.48, p < .0001, reffect = .75; Epoch 3: t(14) = 11.10, p < .0001, reffect = .90, and old adults, Epoch 1: t(14) = 3.75, p < .005, reffect = .50; Epoch 2: t(14) = 7.60, p < .0001, reffect = .80; Epoch 3: t(14) = 6.10, p < .0001, reffect = .73), indicating associative learning in both groups.
Figure 2.
Associative learning scores by epoch and age group. Mean partial correlations between triplet frequency and median reaction time, after controlling for target frequency, for Epochs 1–3 collapsed across individuals in each age group. Error bars represent the standard error of the mean.
Implicitness
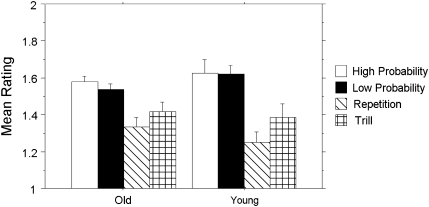
Because our purpose is to study implicit learning, it is important to show no evidence of explicit knowledge about HP versus LP frequencies. To assess explicit judgments of triplet frequencies on the recognition paradigm, a Group (young, old) × Triplet Type (HP, LP, repetitions and trills) mixed-design ANOVA was conducted on mean recognition ratings for each triplet type. Recognition data were lost for 1 young and 1 old participant, leaving 14 participants in each group for this analysis. As shown in Figure 3, there was a significant main effect of triplet type, F(3, 78) = 15.74, p < .0001, reffect = .97, with no age group differences, F(1, 26) = .009, p > .92, reffect = .01, and no interactions, F(3, 78) = 1.03, p > .39, reffect = .19. Most important, post hoc analysis showed that HP and LP triplet ratings did not differ from each other, t(27) = −.62, p > .54, reffect = .01. This is strong evidence for the implicitness of learning, in that people responded faster to HP than to LP during training but did not give higher recognition ratings to one than the other during recognition.
Figure 3.
Mean recognition ratings by triplet type and age group. A rating of 2 indicated that a triplet was believed to occur more often, whereas a rating of 1 indicated that a triplet was believed to occur less often. Error bars represent the standard error of the mean.
In addition, using 2 × 2 chi-square analyses conducted separately for each person, we found that no participants revealed awareness of triplet frequency. HP and LP triplets were equally sorted as occurring more often (p > .11 in all cases), indicating implicit learning. Moreover, associative learning was independent of recognition task judgments, in that across subjects, triplet ratings on the recognition task (i.e., HP–LP) did not correlate with associative learning scores in any of the three epochs (Epoch 1: r = .122, Epoch 2: r = .262, Epoch 3: r = −.199; p’s > .18).
Finally, the main effect of triplet frequency in the ANOVA reported above was due to the fact that both HP and LP triplets were judged as occurring more frequently than trills, HP: t(27) = −3.47, p < .005, reffect = .31; LP: t(27) = −3.77, p < .005, reffect = .34, and repetitions, HP: t(27) = −5.53, p < .0001, reffect = .53; LP:, t(27) = −5.80, p < .0001, reffect = .55. The fact that participants did differentiate trills and repetitions from HP and LP triplets suggests that they understood the recognition task and were not responding randomly; thus, this recognition task is sensitive to explicit knowledge when it is present.
DISCUSSION
The present study examined age differences in implicit learning of probabilistic non–rule-based sequences, using a modified version of the TLT. Results revealed that both young and old adults learned but that the old learned less than the young, particularly at the end of training. As discussed below, this difference cannot be attributed to age-related motor impairments, such as slower and/or more variable responding. Nor can it be attributed to declarative knowledge of the sequences or to deficits in learning new rule-governed sequences. Instead, contrary to the commonly held view that implicit learning and memory are spared in aging (Hedden & Gabrieli, 2004; Zacks et al., 2000), our findings support the view that healthy aging is accompanied by a decrease in at least one form of implicit learning, namely in the ability to learn sequential probabilistic associations. We argue below that these age differences may reflect age-related declines in a striatal-based learning system.
The present results join earlier findings using the TLT in enabling us to rule out three alternative interpretations for the age deficits observed. First, age differences in learning found here and in the earlier TLT study (J. H. Howard et al., 2008) are not due to general age-related declines in motor movements (Smith et al., 1999). The absence of motor sequences reduces motor demands and variability, which not only minimizes confounds associated with motor impairments but also enables cognitive contributions to sequence learning to be differentiated from motor ones (see Lungu, Wachter, Liu, Willingham, & Ashe, 2004). Second, age differences cannot be due to age differences in event timing. Older adults are often slower and often experience different event timing in motor learning tasks because each event typically follows the preceding response by a fixed interval (J. H. Howard et al., 2007). However, event timing within a trial is fixed in the TLT and, therefore, identical for the two age groups. Finally, age differences cannot be due to deficits in explicit declarative learning. Results from our sensitive posttraining recognition measure and subsequent nonsignificant correlations between ratings of triplet frequency and associative learning scores indicated that learning is implicit, consistent with previous TLT studies (Bennett et al., 2008; J. H. Howard et al., 2008). This is important because explicit contamination, such as the application of deliberate strategies, may confound age differences found on tasks that are intended to measure implicit learning (D. V. Howard & Howard, 2001).
The present results go beyond earlier findings with the TLT in that they also enable us to rule out effects of age differences in rule-governed learning. We eliminated all rule-governed structure in the task used here and thereby dissociated rule-based implicit learning from more general implicit association-based processes (Cleeremans, 1993). As a result, the present study demonstrated that both young and old adults are able to learn non–rule-based sequential structure, in addition to the rule-based first- or second-order structure demonstrated in previous studies (Bennett et al., 2008; J. H. Howard & Howard, 1997; D. V. Howard et al., 2004; J. H. Howard et al., 2008). However, the fact that age differences in learning probabilistic sequences emerged with practice, points to a fundamental deficit in older adults’ ability to learn subtle sequential associative regularities over time.
The present findings also go beyond earlier work by revealing more about the time course of implicit probabilistic learning and the age differences therein. For example, the earlier TLT study only examined associative learning scores for the second half of testing, after 3,000 trials of training. In contrast, we examined the first 750 trials of training separated into three epochs. Though several theories have proposed distinct associative learning stages, with stimulus representations being formed in early trials and habit learning occurring in later trials (Anderson, 1982; Karni, 1996), few have examined how aging influences these separate learning phases. Here, we did not observe age deficits in associative learning during the first epoch, but by the third and final epoch, older adults revealed less learning than their younger counterparts. This finding is consistent with previous implicit sequence learning studies that have shown the greatest divergence between age groups after extensive practice (Bennett et al., 2007; Dennis, Howard, & Howard, 2003; J. H. Howard & Howard, 1997; D. V. Howard et al., 2004; J. H. Howard et al., 2004; J. H. Howard et al., 2007; J. H. Howard et al., 2008; Negash, Howard, Japikse, & Howard, 2003). Interestingly, the two studies that revealed age invariance in probabilistic learning had relatively little training (Aizenstein et al., 2006; Fera et al., 2005). Both had fewer trials than the present study and reported only small learning effects that would make detecting age differences challenging. Thus, one possible explanation for the lack of age differences in those studies is that training was insufficient to reveal age deficits in learning.
A possible explanation for the changing patterns of age differences with training is that different brain structures may be involved as training progresses. Studies of probabilistic associative learning in young adults and animals show that the medial temporal lobe governs responding in the early stages of learning, whereas performance becomes increasingly dependent on the striatum over the course of training (Poldrack & Packard, 2003; Schendan, Searl, Melrose, & Stern, 2003). Similarly, preliminary functional neuroimaging data of the TLT in young adults revealed early learning-related hippocampal activation, whereas the caudate was recruited with more practice (Simon, Barnes, Vaidya, Howard, & Howard, 2008). The striatum typically comes to dominate probabilistic associative learning because these structures are good at integrating probabilistic information gradually over time (Packard & Knowlton, 2002; Seger, 2006; Shohamy, Myers, Kalanithi, & Gluck, 2008). However, this brain region shows substantial age-related changes in structure and function in healthy older adults (Backman, Nyberg, Lindenberger, Li, & Farde, 2006; Gunning-Dixon, Head, McQuain, Acker, & Raz, 1998; Raz et al., 2003) that may compromise learning in tasks that rely on this system (Cabeza, Nyberg, & Park, 2005), such as implicit probabilistic associative learning. Thus, age differences observed during the third training epoch of the present study may be due to greater age-related declines in the striatum relative to the medial temporal lobe in healthy older adults (Jernigan et al., 1991, 2001; Raz et al., 2005). In other words, when responding relies on the relatively intact medial temporal systems early on, there is age invariance in performance, but when responding gradually shifts to the age-impaired striatal system, age differences emerge. This is consistent with findings that striatal-based information integration learning reveals age deficits (Filoteo & Maddox, 2004). Though this conclusion may, at first, seem paradoxical, given that older adults did demonstrate learning in all three training epochs, several studies of older adults have revealed increased reliance on extrastriatal brain regions during implicit associative learning when striatal processes are impaired (Aizenstein et al., 2006; Fera et al., 2005; Rieckmann, Fischer, & Backman, 2010).
In summary, the present experiment revealed that both young and old adults are sensitive to repeating unstructured, non–rule-based sequences. However, there are age differences in this sort of implicit probabilistic learning, in that older adults revealed less learning than the younger group with age differences being carried by later training, perhaps reflecting age-related deficits in the striatal associative learning system. Of note, these age differences were observed with only 30 min of testing, using an abbreviated version of the TLT. Shorter training is more practical and is often preferred in functional imaging studies or behavioral studies of older adults or patient groups. The exact mechanisms underlying this age-related deficit in implicit probabilistic learning are yet to be determined, but such an understanding is important for building and testing theories and for developing interventions for older adults that maximize learning.
FUNDING
This work was supported by grants R37AG15450, R01AG036863, and F31AG034691 from the National Institute on Aging/National Institutes of Health; and a dissertation grant to J.R.S. from the American Psychological Association.
Acknowledgments
The authors want to thank Alison Lenet and Meghan Shapiro for assisting with data collection. Preliminary findings from this project were presented at the 13th Biennial Meeting of the Cognitive Aging Conference in Atlanta, GA, in April 2010.
References
- Aizenstein HJ, Butters MA, Clark KA, Figurski JL, Andrew Stenger V, Nebes RD, Reynolds C. F. 3rd, Carter CS. Prefrontal and striatal activation in elderly subjects during concurrent implicit and explicit sequence learning. Neurobiology of Aging. 2006;27:741–751. doi: 10.1016/j.neurobiolaging.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Anderson JR. Acquisition of cognitive skill. Psychological Review. 1982;89:369–406. [Google Scholar]
- Backman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neuroscience & Biobehavioral Reviews. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Howard JH, Jr., Howard DV. Age-related differences in implicit learning of subtle third-order sequential structure. Journal of Gerontology. Series B, Psychological Sciences and Social Sciences. 2007;62:98–103. doi: 10.1093/geronb/62.2.p98. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Romano JC, Howard JHJ, Howard DV. Two forms of implicit learning in young adults with dyslexia. Annals of the New York Academy of Sciences. 2008;1145:184–198. doi: 10.1196/annals.1416.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer M, Destrebecqz A, Cleeremans A. Processing abstract sequence structure: learning without knowing, or knowing without learning? Psychological Research. 2005;69:383–398. doi: 10.1007/s00426-004-0207-4. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L, Park D. Cognitive Neuroscience of Aging: Linking cognitive and cerebral aging. New York, NY: Oxford University Press: 2005. [Google Scholar]
- Cherry KE, Stadler MA. Implicit learning of a nonverbal sequence in younger and older adults. Psychology and Aging. 1995;10:379–394. doi: 10.1037//0882-7974.10.3.379. [DOI] [PubMed] [Google Scholar]
- Cleeremans A. Mechanisms of implicit learning: Connectionist models of sequence processing. Cambridge, MA: MIT press; 1993. [Google Scholar]
- Cleeremans A, McClelland JL. Learning the structure of event sequences. Journal of Experimental Psychology: General. 1991;120:235–253. doi: 10.1037//0096-3445.120.3.235. [DOI] [PubMed] [Google Scholar]
- Curran T. Effects of aging on implicit sequence learning: accounting for sequence structure and explicit knowledge. Psychological Research. 1997;60:24–41. doi: 10.1007/BF00419678. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SA, Veltman DJ, Raaijmakers JG, Jonker C. Similar network activated by young and old adults during the acquisition of a motor sequence. Neurobiology of Aging. 2003;24:1013–1019. doi: 10.1016/s0197-4580(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Howard JH, Jr., Howard DV. Age deficits in learning sequences of spoken words. Journal of Gerontology: Psychological Sciences. 2003;58:P224–P227. doi: 10.1093/geronb/58.4.p224. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Howard JH, Jr., Howard DV. Implicit sequence learning without motor sequencing in young and old adults. Experimental Brain Research. 2006;175:153–164. doi: 10.1007/s00221-006-0534-3. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Wahlin A, Maitland SB, Hultsch DF, Hertzog C, Backman L. Episodic memory change in late adulthood: Generalizability across samples and performance indices. Memory and Cognition. 2004;32:768–778. doi: 10.3758/bf03195867. [DOI] [PubMed] [Google Scholar]
- Fera F, Weickert TW, Goldberg TE, Tessitore A, Hariri A, Das S, Lee S, Zoltick B, Meeter M, Myers C. E.,, et al. Neural mechanisms underlying probabilistic category learning in normal aging. Journal of Neuroscience. 2005;25:11340–11348. doi: 10.1523/JNEUROSCI.2736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoteo JV, Maddox WT. A quantitative model-based approach to examining aging effects on information-integration category learning. Psychology and Aging. 2004;19:171–182. doi: 10.1037/0882-7974.19.1.171. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forkstam C, Petersson KM. Towards an explicit account of implicit learning. Current Opinions in Neurology. 2005;18:435–441. doi: 10.1097/01.wco.0000171951.82995.c4. [DOI] [PubMed] [Google Scholar]
- Frensch PA. One concept, multiple meanings: On how to define the concept of implicit learning. In: Stadler MA, Frensch PA, editors. Handbook of implicit learning. Thousand Oaks, CA: Sage Publications; 1998. pp. 47–104. [Google Scholar]
- Gaillard V, Destrebecqz A, Michiels S, Cleeremans A. Effects of age and practice in sequence learning: A graded account of ageing, learning, and control. European Journal of Cognitive Psychology. 2009;21:255–282. [Google Scholar]
- Gunning-Dixon FM, Head D, McQuain J, Acker JD, Raz N. Differential aging of the human striatum: A prospective MR imaging study. American Journal of Neuroradiology. 1998;19:1501–1507. [PMC free article] [PubMed] [Google Scholar]
- Hakuta K, Bialystok E, Wiley E. Critical evidence: A test of the critical-period hypothesis for second-language acquisition. Psychological Science. 2003;14:31–38. doi: 10.1111/1467-9280.01415. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: A view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH., Jr. Age differences in learning serial patterns: Direct versus indirect measures. Psychology and Aging. 1989;4:357–364. doi: 10.1037//0882-7974.4.3.357. [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH., Jr. Adult age differences in the rate of learning serial patterns: Evidence from direct and indirect tests. Psychology and Aging. 1992;7:232–241. doi: 10.1037//0882-7974.7.2.232. [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH., Jr. When it does hurt to try: Adult age differences in the effects of instructions on implicit pattern learning. Psychonomic Bulletin and Review. 2001;8:798–805. doi: 10.3758/bf03196220. [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH, Jr., Japikse K, DiYanni C, Thompson A, Somberg R. Implicit sequence learning: Effects of level of structure, adult age, and extended practice. Psychology and Aging. 2004;19:79–92. doi: 10.1037/0882-7974.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JH, Jr., Howard DV. Age differences in implicit learning of higher order dependencies in serial patterns. Psychology and Aging. 1997;12:634–656. doi: 10.1037//0882-7974.12.4.634. [DOI] [PubMed] [Google Scholar]
- Howard JH, Jr., Howard DV, Dennis NA, Kelly AJ. Implicit learning of predictive relationships in three-element visual sequences by young and old adults. Journal of Experimental Psychology: Learning, Memory and Cognition. 2008;34:1139–1157. doi: 10.1037/a0012797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JH, Jr., Howard DV, Dennis NA, Yankovich H. Event Timing and age deficits in higher-order sequence learning. Aging, Neuropsychology and Cognition. 2007;14:647–668. doi: 10.1080/13825580601186635. [DOI] [PubMed] [Google Scholar]
- Howard JH, Jr., Howard DV, Dennis NA, Yankovich H, Vaidya CJ. Implicit spatial contextual learning in healthy aging. Neuropsychology. 2004;18:124–134. doi: 10.1037/0894-4105.18.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, Part I: Localization of age-related changes. Biological Psychiatry. 1991;29:55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Karni A. The acquisition of perceptual and motor skills: A memory system in the adult human cortex. Brain Research. Cognitive Brain Research. 1996;5:39–48. doi: 10.1016/s0926-6410(96)00039-0. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Early language acquisition: Cracking the speech code. Nature Reviews Neuroscience. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Intuition: A social cognitive neuroscience approach. Psychological Bulletin. 2000;126:109–137. doi: 10.1037/0033-2909.126.1.109. [DOI] [PubMed] [Google Scholar]
- Lungu OV, Wachter T, Liu T, Willingham DT, Ashe J. Probability detection mechanisms and motor learning. Experimental Brain Research. 2004;159:135–150. doi: 10.1007/s00221-004-1945-7. [DOI] [PubMed] [Google Scholar]
- Negash S, Howard DV, Japikse KC, Howard JH., Jr. Age-related differences in implicit learning of non-spatial sequential patterns. Aging, Neuropsychology and Cognition. 2003;10:108–121. [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annual Review of Neuroscience. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, Acker JD. Differential aging of the human striatum: Longitudinal evidence. American Journal of Neuroradiology. 2003;24:1849–1856. [PMC free article] [PubMed] [Google Scholar]
- Rieckmann A, Fischer H, Backman L. Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: Relations to performance. Neuroimage. 2010;50:1303–1312. doi: 10.1016/j.neuroimage.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biological Psychology. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, McGuthry KE, Hambrick DZ. A framework for analyzing and interpreting differential aging patterns: Application to three measures of implicit learning. Aging, Neuropsychology and Cognition. 1999;6:1–18. [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Seger CA. The basal ganglia in human learning. Neuroscientist. 2006;12:285–290. doi: 10.1177/1073858405285632. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal ganglia and dopamine contributions to probabilistic category learning. Neuroscience and Biobehavioral Reviews. 2008;32:219–236. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR, Barnes KA, Vaidya CJ, Howard JH, Howard DV. Neural basis of implicit sequence learning in a probabilistic Triplets Learning Task. 2008. Paper presented at the Cognitive Neuroscience Society, San Francisco, CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Umberger GH, Manning EL, Slevin JT, Wekstein DR, Schmitt FA, Markesbery WR, Zhang Z, Gerhardt GA, Kryscio RJ. Critical decline in fine motor hand movements in human aging. Neurology. 1999;53:1458–1461. doi: 10.1212/wnl.53.7.1458. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Hasher L, Li KZH. Human memory. In: Craik FI, Salthouse TA, editors. The handbook of aging and cognition. 2nd ed. Mahwah, NJ: Lawrence Erlbaum; 2000. pp. 293–357. [Google Scholar]